Liver Phantoms Cast in 3D-Printed Mold for Image-Guided Procedures
Abstract
1. Introduction
2. Materials and Methods
2.1. Making of the Mold
2.1.1. Virtual Mold
- After opening the STL file, the 3D model of the liver was selected and, using the “automatic repair” function, possible integrity problems of the model were checked and corrected.
- Using the “cut” and “delete” functions, the unwanted parts and overlapping geometries of the model were eliminated and adjusted.
- Later, the manual repair functions were used for any remaining problems (small holes or unfavorable geometry).
- Using the “measure” function, the correct dimensions of the model were checked and the number of triangles on the surface of the model was optimized using the “reduce” function (this function reduces the size of the file and improves its performance for 3D printing).
- Using the “extrude” function, a thickness of 5 mm was given to the outer surface (Figure 1A,B), and with the “cut” function, the 3D model was sectioned into 4 segments, so that demolding could be carried out without damaging the finished product, considering the irregular shape of the human liver (Figure 1C).
- Geometries were created on the 4 segments using the same “extrude” function.
- Using the “Boolean” function, assembly/fixing holes were created on these geometries through which screws could be mounted. The dimensions of the assembled mold were a length of 300 mm, a width of 200 mm, and a height of 200 mm (Figure 1D).
- Using the “cut” function, a casting hole was created on the quarter of the mold with the highest point, specifically in the quarter that reproduces the surface of segments VII and VIII of the liver; the cut region could be used as a cap for the casting hole (Figure 1E).
2.1.2. Physical Mold
2.2. Making of a Liver Phantom
- The 3 segments of the mold were assembled, with the exception of the segment with the casting hole (Figure 3), using screws for fixing and sanitary silicone for sealing.
- The tumor formations were placed inside, on the mold’s base (Figure 3).
- Vascular formations were also placed inside, around the tumors, on the mold’s base (Figure 3).
- The base liquid of the phantom (gelatin-based liquid or bi-component silicone) was poured inside the mold to the level allowed, with only 3 of the mold segments mounted.
- The last element of the mold was assembled in the same way as the first 3, and we continued to pour the base liquid through the pouring hole up to the level of the hole.
- The bi-component silicone phantom was left to solidify in the mold at room temperature for 6 h, and the gelatin-based phantom was left in the refrigerator at 2–4 °C for gelabased phantom tin-based phantom.
- After solidification, we proceeded to demold the liver phantoms by removing the fixing screws and carefully removing the mold segments one at a time (Figure 3).
- For the silicone liver, after the ZA13 transparent bi-component silicone solution was homogenized at room temperature, it was placed in a vacuum vessel for 10 min to extract the air bubbles inside the composition before it was poured inside the mold cavity. After demolding, a silicone liver phantom with semi-transparent parenchyma was obtained, through which the tumor formations and the simulated vascular axes could be seen (Figure 3A).
- For the gelatin-based liver phantom, after homogenization at 40 °C, the gelatin solution was allowed to cool for 15 min at room temperature, since pouring at a high temperature can lead to partial or total dissolution of already solidified tumors mounted inside the mold. After the liquid temperature dropped below 20 °C, it was slowly poured into the mold cavity as described above. After demolding, a gelatin-based liver phantom with transparent parenchyma was obtained, through which the tumor formations and vascular structures could be seen (Figure 3B,C).
2.3. Testing of the Gelatin-Based Liver Phantom
- World Congress for Endoscopic Surgery (WCES; Barcelona, 2021) [26]: 29 participants (13 participants attended the guided procedures and intraoperative modules);
- Congress of European Association for Endoscopic Surgery (EAES; Krakow, 2022) [27]: 22 participants (9 participants attended the guided procedures and intraoperative modules);
- Congress of the Romanian Association for Endoscopic Surgery (RAES; Timisoara, 2022) [28]: 12 participants (7 participants attended both the guided procedures and trauma US modules);
- EAES Winter Meeting (Malta, 2023) [29]: 13 participants (6 participants attended intraoperative module and 7 attended both guided procedures and trauma US modules).
- Q1: How real/natural did you think the gelatin liver phantoms used in the course were?
- Q2: What do you think about the general appearance of the anatomical gelatin liver phantoms?
- Q3: What do you think about the quality of the anatomical gelatin liver phantoms used in the course?
- Q4: What do you think about the consistency of the anatomical gelatin liver phantoms used in the course?
- Q5: How satisfied are you with regard to the durability of the anatomical gelatin liver phantoms for repeated ultrasound guided punctures?
- Q6: How satisfied are you with the ultrasound images produced by the anatomical gelatin liver phantoms?
- Q7: How well can you practice ultrasound-guided punctures on the anatomical gelatin liver phantoms used in the course?
- Q8: How useful do you think the use of anatomical gelatin liver phantoms is for ultrasound-guided puncture skill development?
- Q9: Which molds did you find more useful: the anatomical ones of the liver or the simple NON-anatomical ones?
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D.; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009, 49, 1017–1044. [Google Scholar] [CrossRef]
- Chmarra, M.K.; Hansen, R.; Mårvik, R.; Langø, T. Multimodal phantom of liver tissue. PLoS ONE 2013, 8, e64180. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, N.; Schwaiger, J.; Markert, M.; Flatz, W.; Lueth, T.C. Evaluation of a resectable ultrasound liver phantom for testing of surgical navigation systems. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 916–919. [Google Scholar] [CrossRef]
- Rethy, A.; Sæternes, J.O.; Halgunset, J.; Mårvik, R.; Hofstad, E.F.; Sánchez-Margallo, J.A.; Langø, T. Anthropomorphic liver phantom with flow for multimodal image-guided liver therapy research and training. Int. J. CARS 2018, 13, 61–72. [Google Scholar] [CrossRef] [PubMed]
- CIRS Inc. Computerized Imaging Reference Systems, Tissue Simulation & Phantom Technology. Triple Modality 3D Abdominal Phantom—Model 057A. 2021. Available online: https://www.cirsinc.com/products/ultrasound/zerdine-hydrogel/triple-modality-3d-abdominal-phantom/ (accessed on 2 August 2021).
- MDD Inc. Medical Device Depot: Triple Modality 3D Abdominal Phantom—Model 057A. Available online: https://www.medicaldevicedepot.com/Triple-Modality-3D-Abdominal-Phantom-p/057a.htm (accessed on 2 August 2021).
- Kyoto Kagaku Co., Ltd. Abdominal Intraoperative & Laparoscopic Ultrasound Phantom—IOUSFAN, Update 7 August 2020. Available online: https://www.kyotokagaku.com/en/products_data/us-3/ (accessed on 2 August 2021).
- Limbs&Things. IOUSFAN—Abdominal Intraoperative & Laparoscopic Ultrasound Phantom. Available online: https://limbsandthings.com/au/products/KKUS-3/kkus-3-iousfan-abdominal-intraoperative-laparoscopic-ultrasound-phantom (accessed on 2 August 2021).
- Bao, P.; Warmath, J.; Galloway, R.; Herline, A. Ultrasound-tocomputer-tomography registration for image-guided laparoscopic liver surgery. Surg. Endosc. 2005, 19, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Luddington, O.S.; Culleton, S.R.; Francis, R.J.; Boucek, J.A. A gelatin liver phantom of suspended 90Y resin microspheres to simulate the physiologic microsphere biodistribution of a postradioembolization liver. J. Nucl. Med. Technol. 2014, 42, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Banovac, F.; Tang, J.; Xu, S.; Lindisch, D.; Chung, H.Y.; Levy, E.B.; Chang, T.; McCullough, M.F.; Yaniv, Z.; Wood, B.J.; et al. Precision targeting of liver lesions using a novel electromagnetic navigation evice in physiologic phantom and swine. Med. Phys. 2005, 32, 2698–2705. [Google Scholar] [CrossRef]
- Joe, E.; Kim, S.H.; Lee, K.B.; Jang, J.-J.; Lee, J.Y.; Lee, J.M.; Han, J.K.; Choi, B.I. Feasibility and accuracy of dual-source dual-energy CT for noninvasive determination of hepatic iron accumulation. Radiology 2012, 262, 126–135. [Google Scholar] [CrossRef]
- Murotani, K.; Kazuhiro, M.; Kawai, N.; Sato, M.; Minamiguchi, H.; Nakai, M.; Sonomura, T.; Hosokawa, S.; Nishioku, T. Optimum CT reconstruction parameters for vascular and hepatocellular carcinoma models in a liver phantom with multi-level dynamic computed tomography with 64 detector rows: A basic study. Radiol. Phys. Technol. 2013, 6, 317–325. [Google Scholar] [CrossRef]
- Widmann, G.; Wallach, D.; Toporek, G.; Schullian, P.; Weber, S.; Bale, R. Angiographic C-arm CT- versus MDCT-guided stereotactic punctures of liver lesions: Nonrigid phantom study. Am. J. Roentgenol. 2013, 201, 1136–1140. [Google Scholar] [CrossRef]
- In, E.; Naguib, H.; Haider, M. Mechanical stability analysis of carrageenan-based polymer gel for magnetic resonance imaging liver phantom with lesion particles. J. Med. Imaging 2014, 1, 035502. [Google Scholar] [CrossRef]
- Rube, M.A.; Holbrook, A.B.; Cox, B.F.; Buciuc, R.; Melzer, A. Wireless mobile technology to improve workflow and feasibility of MR-guided percutaneous interventions. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 665–676. [Google Scholar] [CrossRef]
- Schwaiger, J.; Markert, M.; Shevchenko, N.; Lueth, T.C. The effects of real-time image navigation in operative liver surgery. Int. J. Comput. Assist. Radiol. Surg. 2011, 6, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Moriyasu, F.; Shiraishi, J.; Yamada, M.; Imai, Y. A phantom study comparing ultrasound-guided liver tumor puncture using newreal-time 3Dultrasound and conventional 2Dultrasound. Am. J. Roentgenol. 2011, 196, W753–W757. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, A.; Carbone, M.; Freschi, C.; Viglialoro, R.; Ferrari, V.; Ferrari, M. Patient-specific ultrasound liver phantom: Materials and fabricationmethod. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Witowski, J.S.; Pedziwiatr, M.; Major, P.; Budzynski, A. Cost-effective, personalized, 3D-printed liver model for preoperative planning before laparoscopic liver hemihepatectomy for colorectal cancer metastases. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Culjat, M.O.; Goldenberg, D.; Tewari, P.; Singh, R.S. A review of tissuesubstitutes for ultrasound imaging. Ultrasound Med. Biol. 2010, 36, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Defensible. Ballistic Gel 1kg (10kg Cast). Available online: https://www.defensible.co.uk/products/p/ballistic-gel-1kg (accessed on 2 August 2021).
- Bude, R.O.; Adler, R.S. An easily made, low-cost, tissue-like ultrasound phantom material. J. Clin. Ultrasound 1995, 23, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Andersen, V.; Sonne, J.; Sletting, S.; Prip, A. The volume of the liver in patients correlates to body weight and alcohol consumption. Alcohol Alcohol. 2000, 35, 531–532. [Google Scholar] [CrossRef]
- Agrawal, D.; Lalwani, R.; Asghar, A.; Sahai, A.; Sharma, P.; Singh, R. Assessment of liver volume with spiral computerized tomography scanning in north indian adults. Internet J. Radiol. 2009, 13, 1. Available online: https://ispub.com/IJRA/13/1/9978 (accessed on 2 August 2021).
- EAES. Available online: https://eaes.eu/wces2021/ (accessed on 2 August 2021).
- EAES. Available online: https://eaes.eu/eaes2022/programme-2022/ (accessed on 10 July 2022).
- ARCE. Minimal Invasive Surgery: Knowing Better, Doing Better. Available online: https://ralcom.eventsair.com/arce-2022/program-stiintific (accessed on 3 October 2022).
- EAES. Available online: https://eaes.eu/events/eaes-wintermeeting/ (accessed on 30 January 2023).
- Friedman, L.S. Controversies in liver biopsy: Who, where, when, how, why? Curr. Gastroenterol. Rep. 2004, 6, 30–36. [Google Scholar] [CrossRef]
- Poon, R.T.; Ng, K.K.; Lam, C.M.; Ai, V.; Yuen, J.; Fan, S.T.; Wong, J. Learning curve for Radiofrequency Ablation of Liver Tumors. Ann. Surg. 2004, 239, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Stratasys. F170. Available online: https://www.stratasys.com/3d-printers/f123-series (accessed on 3 October 2022).
- Stratasys. J850™ Digital Anatomy™ 3D Printer. Available online: https://www.stratasys.com/3d-printers/j750-digital-anatomy (accessed on 30 January 2023).
- Tong, F.; Zhou, Y.; Xu, Y.; Chen, Y.; Yudintceva, N.; Shevtsov, M.; Gao, H. Supramolecular nanomedicines based on host-guest interactions of cyclodextrins. Exploration 2023, 3, 20210111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, W.; Zhang, W.; Chen, J.; Zhou, J. Mono-functionalized pillar[n]arenes: Syntheses, host–guest properties and applications. Chin. Chem. Lett. 2024, 35, 108740. [Google Scholar] [CrossRef]
- Yan, M.; Wu, S.; Wang, Y.; Liang, M.; Wang, M.; Hu, W.; Yu, G.; Mao, Z.; Huang, F.; Zhou, J. Recent Progress of Supramolecular Chemotherapy Based on Host-Guest Interactions. Adv. Mater. 2024, 36, e2304249. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiong, M.; Rong, Q.; Zhang, M.; Zhang, X.B. Nucleic acid sensors in vivo: Challenges and opportunities. View 2023, 4, 20220064. [Google Scholar] [CrossRef]
- Aseni, P.; Santaniello, T.; Rizzetto, F.; Gentili, L.; Pezzotta, F.; Cavaliere, F.; Vertemati, M.; Milani, P. Hybrid Additive Fabrication of a Transparent Liver with Biosimilar Haptic Response for Preoperative Planning. Diagnostics 2021, 11, 1734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arm, R.; Shahidi, A.; Clarke, C.; Alabraba, E. Synthesis and characterisation of a cancerous liver for presurgical planning and training applications. BMJ Open Gastroenterol. 2022, 9, e000909. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaksa, L.; Aryeetey, O.J.; Hatamikia, S.; Nägl, K.; Buschmann, M.; Dieter, H.P.; Kronreif, G.; Lorenz, A. 3D-Printed multi-material liver model with simultaneous mechanical and radiological tissue-mimicking features for improved realism. Int. J. Bioprint. 2023, 9, 721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maneas, E.; Xia, W.; Nikitichev, D.I.; Daher, B.; Manimaran, M.; Wong, R.Y.J.; Chang, C.W.; Rahmani, B.; Capelli, C.; Schievano, S.; et al. Anatomically realistic ultrasound phantoms using gel wax with 3D printed moulds. Phys. Med. Biol. 2018, 63, 015033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raffaele, A.; Mauri, V.; Negrini, M.; Negrello, E.; Parigi, G.B.; Avolio, L.; Pietrabissa, A.; Auricchio, F.; Marconi, S. Elaboration and development of a realistic 3D printed model for training in ultrasound-guided placement of peripheral central venous catheter in children. J. Vasc. Access. 2023, 11297298231187005. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, A.L.; Mituś, J.W.; Sanchez Hurtado, M.A.; Sanchez Margallo, F.M. Porcine Model In The Laparoscopic Liver Surgery Training. Pol. Przegl Chir. 2015, 87, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Bredmose, P.P.; Stave, H.; Eriksen, M.; Osbakk, S.A.; Farstad, G.; Hagemo, J.S. Live Tissue Training on Anesthetized Pigs for Air Ambulance Crews. Air Med. J. 2021, 40, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, J.; Li, S.; Wang, S.; Zhou, W.; Jin, Z.; Wang, K. Validation of a novel swine model for training in EUS-FNA (with videos). Endosc. Ultrasound 2020, 9, 232–237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tucan, P.; Vaida, C.; Horvath, D.; Caprariu, A.; Burz, A.; Gherman, B.; Iakab, S.; Pisla, D. Design and Experimental Setup of a Robotic Medical Instrument for Brachytherapy in Non-Resectable Liver Tumors. Cancers 2022, 14, 5841. [Google Scholar] [CrossRef]
- Pisla, D.; Vaida, C.; Birlescu, I.; Hajjar, N.A.; Gherman, B.; Radu, C.; Plitea, N. Risk Management for the Reliability of Robotic Assisted Treatment of Non-resectable Liver Tumors. Appl. Sci. 2020, 10, 52. [Google Scholar] [CrossRef]





| Examination/ Procedure | Examination/Procedure on Gelatin Liver Phantom | Examination/Procedure on Real Liver | |
|---|---|---|---|
| 1 | US examination | 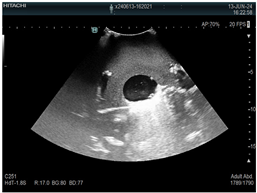 * * | 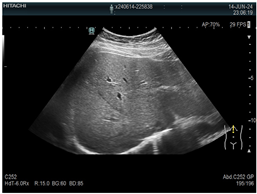 * * |
| 2 | Elastography | 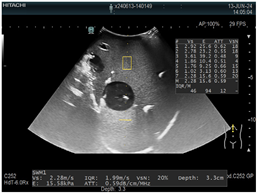 * * |  * * |
| 3 | Fibroscan |  * * |  ** ** |
| 4 | US-guided tumor puncture/biopsy | 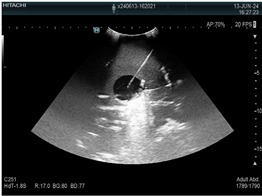 * * |  * * |
| 5 | RFA needle insertion into the tumor |  * * |  * * |
| 6 | US-guided Biliary drainage | 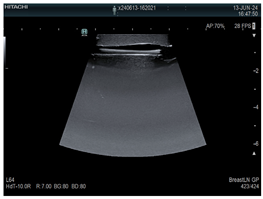 * * | 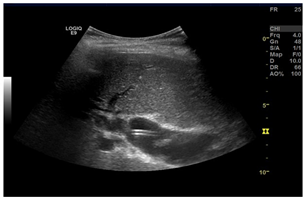 *** *** |
| 7 | Laparoscopic US-guided tumor puncture/biopsy |  * * |  * * |
| 8 | CT-scan examination | 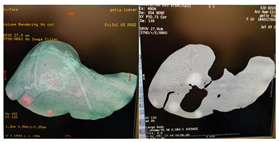 * * |  4* 4* |
| 9 | MRI examination |  * * |  4* 4* |
| 10 | US-CT fusion examination |  * * | 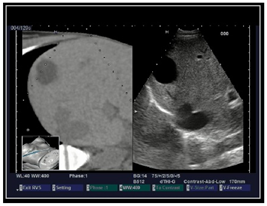 5* 5* |
| 11 | CT-guided tumor puncture/biopsy |  * * | 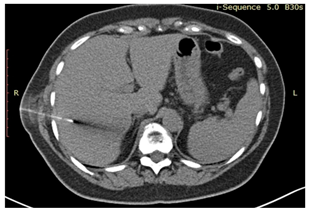 6* 6* |
| This Study | Witowski, J.S. et al. [20] | Pacioni, A. et al. [19] | |
|---|---|---|---|
| 3D printing technology/material | FDM/PLA (polyactid) | FDM/ABS | FDM/ABS |
| Layer thickness | 0.256 mm (high quality) | Not specified (lower quality) | Not specified (lower quality) |
| Mold parts thickness | 5 mm | 3 mm | Not specified |
| Mold parts size | 300/100/100 mm | <200 mm (largest dimension) | Not specified |
| Total 3D printing time/printing jobs | 42 h/2 print jobs | 72 h/6 print jobs | Not specified |
| Single use/multiple use mold | Multiple use | Single use | Multiple use |
| Liver phantom volume | 1534 cm3 | 1289 cm3 | Not specified |
| Type of material that can be poured into the mold | Gelatin based, silicon based | Silicon based | Silicon based |
| Cost of materials for the mold | EUR 270 | USD 45 | Not specified |
| Cost of a gelatin phantom | EUR 8–15 | Not applicable | Not applicable |
| Cost of a silicon phantom | EUR 85 | USD 150 (mold + phantom) | USD 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elisei, R.C.; Graur, F.; Melzer, A.; Moldovan, S.C.; Tiu, C.; Popa, C.; Mois, E.; Pisla, D.; Vaida, C.; Ștefănescu, H.; et al. Liver Phantoms Cast in 3D-Printed Mold for Image-Guided Procedures. Diagnostics 2024, 14, 1521. https://doi.org/10.3390/diagnostics14141521
Elisei RC, Graur F, Melzer A, Moldovan SC, Tiu C, Popa C, Mois E, Pisla D, Vaida C, Ștefănescu H, et al. Liver Phantoms Cast in 3D-Printed Mold for Image-Guided Procedures. Diagnostics. 2024; 14(14):1521. https://doi.org/10.3390/diagnostics14141521
Chicago/Turabian StyleElisei, Radu Claudiu, Florin Graur, Andreas Melzer, Sever Calin Moldovan, Calin Tiu, Calin Popa, Emil Mois, Doina Pisla, Calin Vaida, Horia Ștefănescu, and et al. 2024. "Liver Phantoms Cast in 3D-Printed Mold for Image-Guided Procedures" Diagnostics 14, no. 14: 1521. https://doi.org/10.3390/diagnostics14141521
APA StyleElisei, R. C., Graur, F., Melzer, A., Moldovan, S. C., Tiu, C., Popa, C., Mois, E., Pisla, D., Vaida, C., Ștefănescu, H., Coțe, A., & Al-Hajjar, N. (2024). Liver Phantoms Cast in 3D-Printed Mold for Image-Guided Procedures. Diagnostics, 14(14), 1521. https://doi.org/10.3390/diagnostics14141521









