Diagnosing Progression in Glioblastoma—Tackling a Neuro-Oncology Problem Using Artificial-Intelligence-Derived Volumetric Change over Time on Magnetic Resonance Imaging to Examine Progression-Free Survival in Glioblastoma
Abstract
:1. Introduction
2. Methods
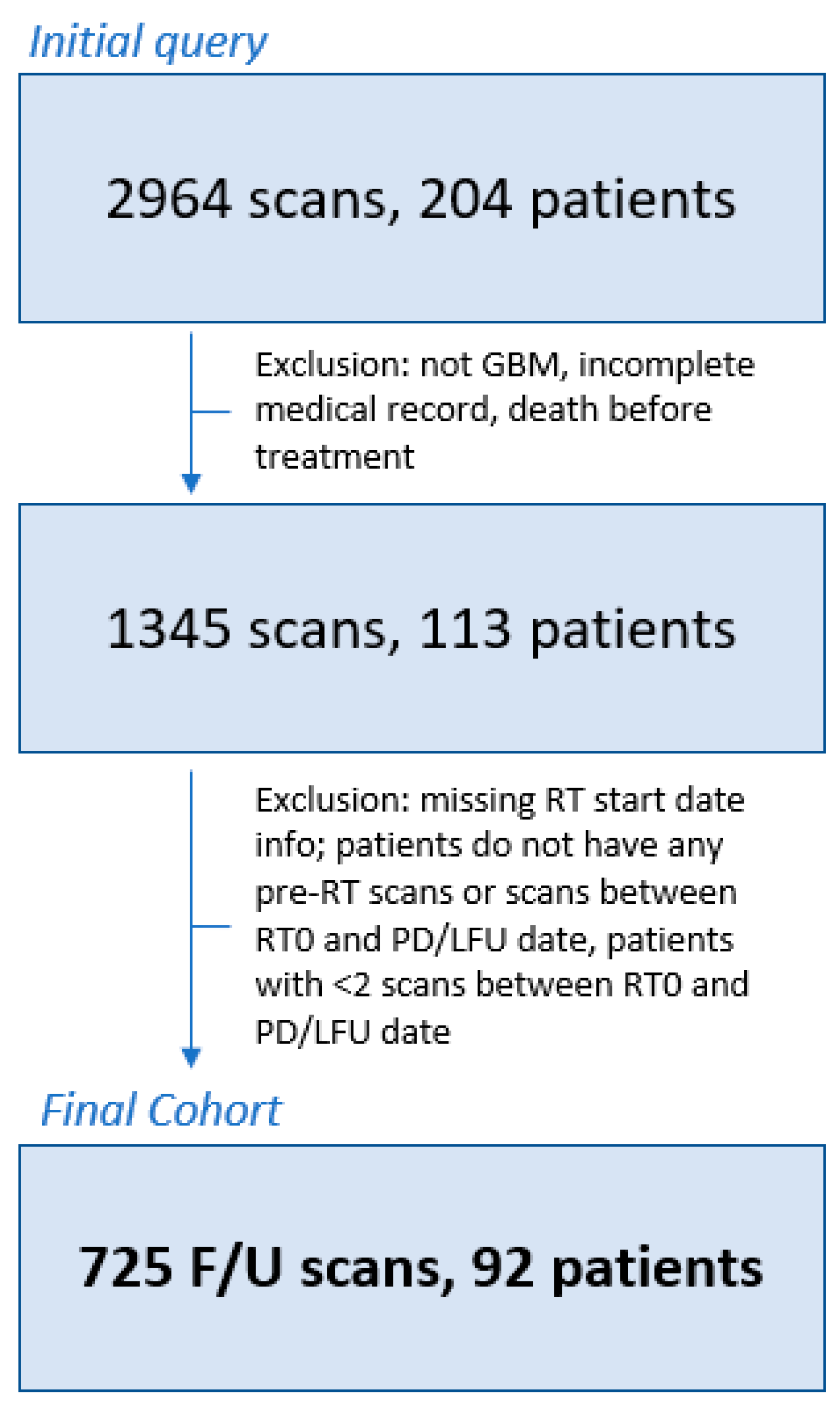
2.1. Patient Population and Clinical Assessment
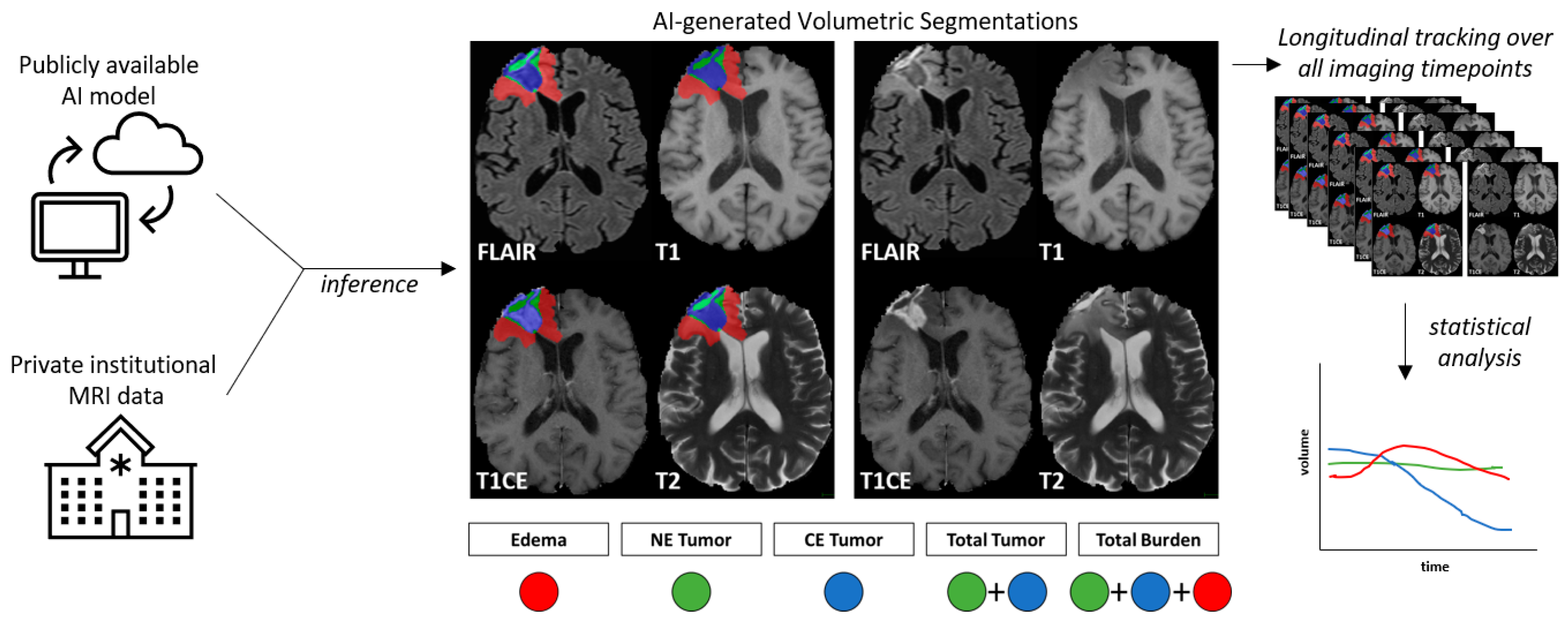
2.2. Preprocessing and AI-Based Volume Estimation
2.3. Tumor Dynamics Calculation
2.4. Statistical Analysis
3. Results
3.1. Clinical Cohort
3.2. AI-Based Volume Quantification and Change over Time
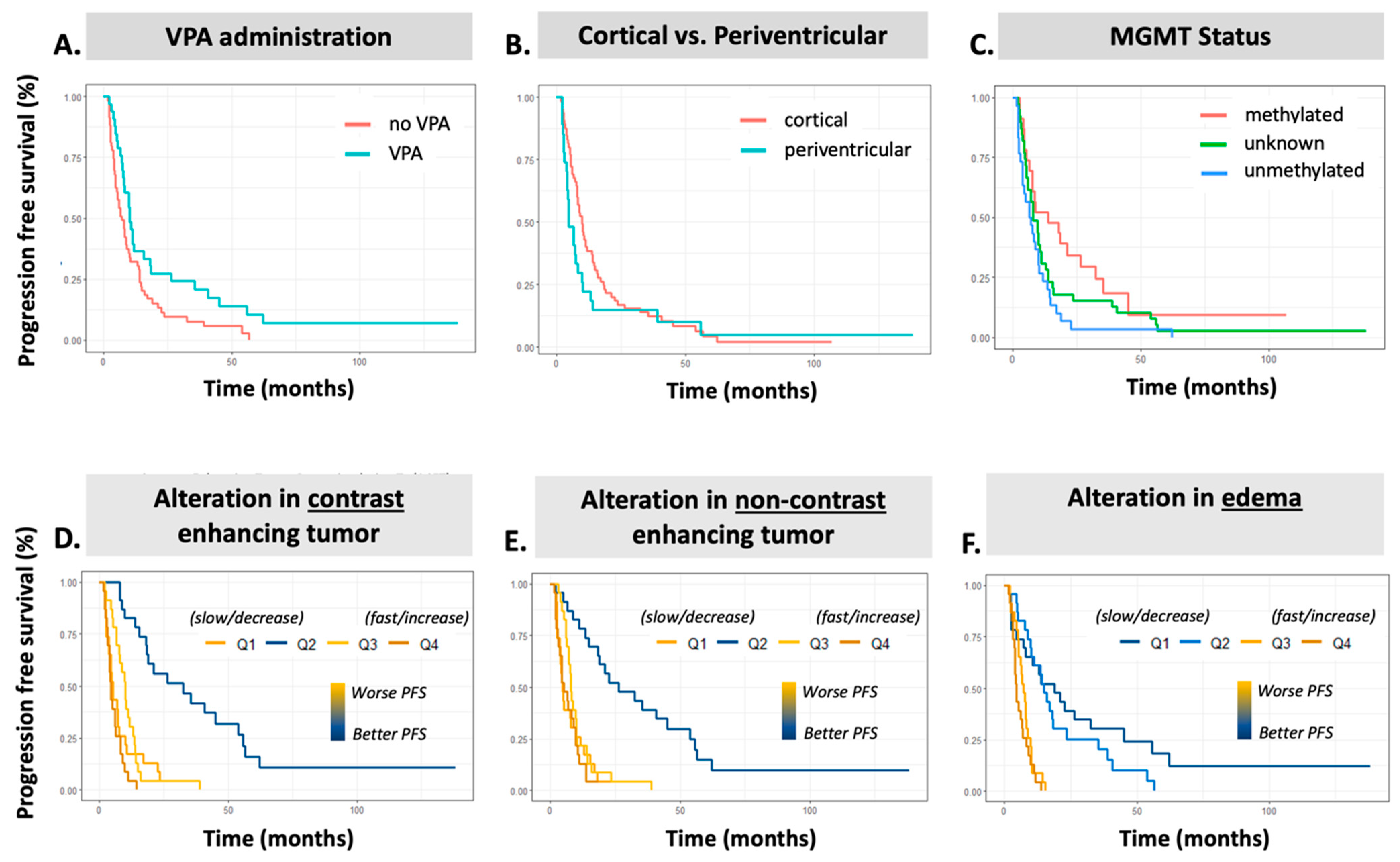
3.3. Association of Clinical Features with PFS
3.4. Association of AI-Based Features with PFS
3.5. Association of AI-Based Volumes with RT Treatment Volumes and MGMT Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| BraTS | Brain Tumor Segmentation |
| CaPTk | Cancer Imaging Phenomics Toolkit |
| CET | Contrast Enhancing Tumor |
| CNS | Central Nervous System |
| CRT | Chemoirradiation |
| FLAIR | Fluid-Attenuated Inversion Recovery |
| GBM | Glioblastoma |
| GTR | Gross Total Resection |
| GTV T1 | Gross Tumor Volume on T1 Gadolinium-enhanced MRI sequence |
| GTV T2 | Gross Tumor Volume on T2 FLAIR signal sequence |
| IMRT | Intensity Modulated Radiation Therapy |
| MRI | Magnetic Resonance Imaging |
| NET | Non-contrast enhancing volume as summation of NET and CET regions |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| RANO | Response Assessment in Neuro-Oncology |
| RPA | Recursive partitioning analysis score |
| RT | Radiation Therapy |
| SOC | Standard of Care |
| STR | Subtotal Resection |
| TB | Total Volumetric Burden |
| TMZ | Temozolomide |
| TT | Total Tumor |
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22 (Suppl. S2), iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Chinot, O.L.; Macdonald, D.R.; Abrey, L.E.; Zahlmann, G.; Kerloëguen, Y.; Cloughesy, T.F. Response assessment criteria for glioblastoma: Practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr. Neurol. Neurosci. Rep. 2013, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Weller, M.; Huang, R.; Finocchiaro, G.; Gilbert, M.R.; Wick, W.; Ellingson, B.M.; Hashimoto, N.; Pollack, I.F.; Brandes, A.A.; et al. Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol. 2015, 16, e534–e542. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Wefel, J.S.; Schiff, D.; Taphoorn, M.J.; Jaeckle, K.; Junck, L.; Armstrong, T.; Choucair, A.; Waldman, A.D.; Gorlia, T.; et al. Response assessment in neuro-oncology (a report of the RANO group): Assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011, 12, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.L.; Li, C.; Boonzaier, N.R.; Fountain, D.M.; Larkin, T.J.; Matys, T.; van der Hoorn, A.; Price, S.J. Multimodal MRI characteristics of the glioblastoma infiltration beyond contrast enhancement. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419844664. [Google Scholar] [CrossRef] [PubMed]
- Würtemberger, U.; Diebold, M.; Erny, D.; Hosp, J.A.; Schnell, O.; Reinacher, P.C.; Rau, A.; Kellner, E.; Reisert, M.; Urbach, H.; et al. Diffusion Microstructure Imaging to Analyze Perilesional T2 Signal Changes in Brain Metastases and Glioblastomas. Cancers 2022, 14, 1155. [Google Scholar] [CrossRef] [PubMed]
- Zanfardino, M.; Pane, K.; Mirabelli, P.; Salvatore, M.; Franzese, M. TCGA-TCIA Impact on Radiogenomics Cancer Research: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 6033. [Google Scholar] [CrossRef]
- Soffietti, R.; Bettegowda, C.; Mellinghoff, I.K.; Warren, K.E.; Ahluwalia, M.S.; De Groot, J.F.; Galanis, E.; Gilbert, M.R.; Jaeckle, K.A.; Le Rhun, E.; et al. Liquid biopsy in gliomas: A RANO review and proposals for clinical applications. Neuro Oncol. 2022, 24, 855–871. [Google Scholar] [CrossRef]
- Singh, G.; Manjila, S.; Sakla, N.; True, A.; Wardeh, A.H.; Beig, N.; Vaysberg, A.; Matthews, J.; Prasanna, P.; Spektor, V. Radiomics and radiogenomics in gliomas: A contemporary update. Br. J. Cancer 2021, 125, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, S.; Reddy, K.; Midya, A.; Pandav, K.B.; Madabhushi, A.; Abedalthagafi, M. Artificial intelligence in neuro-oncology: Advances and challenges in brain tumor diagnosis, prognosis, and precision treatment. npj Precis. Oncol. 2024, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Ghandour, F.; Squassina, A.; Karaky, R.; Diab-Assaf, M.; Fadda, P.; Pisanu, C. Presenting Psychiatric and Neurological Symptoms and Signs of Brain Tumors before Diagnosis: A Systematic Review. Brain Sci. 2021, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.S.; Jo, Y.; Yoo, R.E.; Choi, S.H.; Nam, S.J.; Kim, J.H. Radiomics prognostication model in glioblastoma using diffusion- and perfusion-weighted MRI. Sci. Rep. 2020, 10, 4250. [Google Scholar] [CrossRef] [PubMed]
- Babaei Rikan, S.; Sorayaie Azar, A.; Naemi, A.; Bagherzadeh Mohasefi, J.; Pirnejad, H.; Wiil, U.K. Survival prediction of glioblastoma patients using modern deep learning and machine learning techniques. Sci. Rep. 2024, 14, 2371. [Google Scholar] [CrossRef] [PubMed]
- Samartha, M.V.S.; Dubey, N.K.; Jena, B.; Maheswar, G.; Lo, W.C.; Saxena, S. AI-driven estimation of O6 methylguanine-DNA-methyltransferase (MGMT) promoter methylation in glioblastoma patients: A systematic review with bias analysis. J. Cancer Res. Clin. Oncol. 2024, 150, 57. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Li, J.; Quarles, C.C.; Fonkem, E.; Wu, E. Assessment and prediction of glioblastoma therapy response: Challenges and opportunities. Brain 2023, 146, 1281–1298. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Zhang, P.; Bi, Y.; Yang, C.; Wu, M.; He, D.; Huang, S.; Yang, K.; Qi, S.; Wang, J. MRI radiomic features of peritumoral edema may predict the recurrence sites of glioblastoma multiforme. Front. Oncol. 2022, 12, 1042498. [Google Scholar] [CrossRef]
- Kelly, C.; Majewska, P.; Ioannidis, S.; Raza, M.H.; Williams, M. Estimating progression-free survival in patients with glioblastoma using routinely collected data. J. Neurooncol. 2017, 135, 621–627. [Google Scholar] [CrossRef]
- Isensee, F.; Jäger, P.F.; Full, P.M.; Vollmuth, P.; Maier-Hein, K.H. nnU-Net for Brain Tumor Segmentation; Springer: Cham, Switzerland, 2021; pp. 118–132. [Google Scholar]
- Kocher, M.; Ruge, M.I.; Galldiks, N.; Lohmann, P. Applications of radiomics and machine learning for radiotherapy of malignant brain tumors. Strahlenther. Onkol. 2020, 196, 856–867. [Google Scholar] [CrossRef]
- Lohmann, P.; Elahmadawy, M.A.; Gutsche, R.; Werner, J.M.; Bauer, E.K.; Ceccon, G.; Kocher, M.; Lerche, C.W.; Rapp, M.; Fink, G.R.; et al. FET PET Radiomics for Differentiating Pseudoprogression from Early Tumor Progression in Glioma Patients Post-Chemoradiation. Cancers 2020, 12, 3835. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.E.; Jo, Y.; Shim, W.H.; Nam, S.J.; Kim, J.H.; Yoo, R.E.; Choi, S.H.; Kim, H.S. Incorporating diffusion- and perfusion-weighted MRI into a radiomics model improves diagnostic performance for pseudoprogression in glioblastoma patients. Neuro Oncol. 2019, 21, 404–414. [Google Scholar] [CrossRef]
- Ismail, M.; Hill, V.; Statsevych, V.; Huang, R.; Prasanna, P.; Correa, R.; Singh, G.; Bera, K.; Beig, N.; Thawani, R.; et al. Shape Features of the Lesion Habitat to Differentiate Brain Tumor Progression from Pseudoprogression on Routine Multiparametric MRI: A Multisite Study. AJNR Am. J. Neuroradiol. 2018, 39, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Menze, B.H.; Jakab, A.; Bauer, S.; Kalpathy-Cramer, J.; Farahani, K.; Kirby, J.; Burren, Y.; Porz, N.; Slotboom, J.; Wiest, R.; et al. The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS). IEEE Trans. Med. Imaging 2015, 34, 1993–2024. [Google Scholar] [CrossRef] [PubMed]
- Palantir Foundry—The NIH Integrated Data Analysis Platform (NIDAP); NCI Center for Biomedical Informatics & Information Technology (CBIIT); Software Provided by Palantir Technologies Inc. Available online: https://www.palantir.com (accessed on 5 May 2024).
- Mirimanoff, R.O.; Gorlia, T.; Mason, W.; Van den Bent, M.J.; Kortmann, R.D.; Fisher, B.; Reni, M.; Brandes, A.A.; Curschmann, J.; Villa, S.; et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: Recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J. Clin. Oncol. 2006, 24, 2563–2569. [Google Scholar] [CrossRef]
- Hodapp, N. The ICRU Report 83: Prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlenther. Onkol. 2012, 188, 97–99. [Google Scholar] [CrossRef]
- Davatzikos, C.; Rathore, S.; Bakas, S.; Pati, S.; Bergman, M.; Kalarot, R.; Sridharan, P.; Gastounioti, A.; Jahani, N.; Cohen, E.; et al. Cancer imaging phenomics toolkit: Quantitative imaging analytics for precision diagnostics and predictive modeling of clinical outcome. J. Med. Imaging 2018, 5, 011018. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Singh, A.; Rathore, S.; Gastounioti, A.; Bergman, M.; Ngo, P.; Ha, S.M.; Bounias, D.; Minock, J.; Murphy, G.; et al. The Cancer Imaging Phenomics Toolkit (CaPTk): Technical Overview. Brainlesion 2020, 11993, 380–394. [Google Scholar] [CrossRef]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods 2021, 18, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Isensee, F. nnU-Net Model Weights for BraTS 2020 Submission (1.0). 25 March 2021. Available online: https://zenodo.org/records/4635763 (accessed on 17 June 2024).
- Baid, U.; Ghodasara, S.; Mohan, S.; Bilello, M.; Calabrese, E.; Colak, E.; Farahani, K.; Kalpathy-Cramer, J.; Kitamura, F.C.; Pati, S. The rsna-asnr-miccai brats 2021 benchmark on brain tumor segmentation and radiogenomic classification. arXiv 2021, arXiv:2107.02314. [Google Scholar]
- Bakas, S.; Reyes, M.; Jakab, A.; Bauer, S.; Rempfler, M.; Crimi, A.; Shinohara, R.T.; Berger, C.; Ha, S.M.; Rozycki, M. Identifying the best machine learning algorithms for brain tumor segmentation, progression assessment, and overall survival prediction in the BRATS challenge. arXiv 2018, arXiv:1811.02629. [Google Scholar]
- Binabaj, M.M.; Bahrami, A.; ShahidSales, S.; Joodi, M.; Joudi Mashhad, M.; Hassanian, S.M.; Anvari, K.; Avan, A. The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials. J. Cell. Physiol. 2018, 233, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.L.; Stewart, J.; Sahgal, A.; Soliman, H.; Tseng, C.L.; Detsky, J.; Chen, H.; Ho, L.; Das, S.; Maralani, P.; et al. Predictors of tumour dynamics over a 6-week course of concurrent chemoradiotherapy for glioblastoma and the impact on survival. Int. J. Radiat. Oncol. Biol. Phys. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Krauze, A.V.; Zhao, Y.; Li, M.C.; Shih, J.; Jiang, W.; Tasci, E.; Cooley Zgela, T.; Sproull, M.; Mackey, M.; Shankavaram, U.; et al. Revisiting Concurrent Radiation Therapy, Temozolomide, and the Histone Deacetylase Inhibitor Valproic Acid for Patients with Glioblastoma-Proteomic Alteration and Comparison Analysis with the Standard-of-Care Chemoirradiation. Biomolecules 2023, 13, 1499. [Google Scholar] [CrossRef] [PubMed]
- Bernchou, U.; Arnold, T.S.T.; Axelsen, B.; Klüver-Kristensen, M.; Mahmood, F.; Harbo, F.S.G.; Asmussen, J.T.; Hansen, O.; Bertelsen, A.S.; Hansen, S.; et al. Evolution of the gross tumour volume extent during radiotherapy for glioblastomas. Radiother. Oncol. 2021, 160, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Stewart, J.; Ruschin, M.; Wang, M.H.; Myrehaug, S.; Tseng, C.L.; Detsky, J.; Husain, Z.; Chen, H.; Sahgal, A.; et al. Inter-fraction dynamics during post-operative 5 fraction cavity hypofractionated stereotactic radiotherapy with a MR LINAC: A prospective serial imaging study. J. Neurooncol. 2022, 156, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Gzell, C.E.; Wheeler, H.R.; McCloud, P.; Kastelan, M.; Back, M. Small increases in enhancement on MRI may predict survival post radiotherapy in patients with glioblastoma. J. Neurooncol. 2016, 128, 67–74. [Google Scholar] [CrossRef]
- Ly, K.I.; Vakulenko-Lagun, B.; Emblem, K.E.; Ou, Y.; Da, X.; Betensky, R.A.; Kalpathy-Cramer, J.; Duda, D.G.; Jain, R.K.; Chi, A.S.; et al. Publisher Correction: Probing tumor microenvironment in patients with newly diagnosed glioblastoma during chemoradiation and adjuvant temozolomide with functional MRI. Sci. Rep. 2019, 9, 8721. [Google Scholar] [CrossRef]
| Variable | Patients | |
|---|---|---|
| n (%) | ||
| Age (years) | Median (Range) or N (%) | |
| 56.79 (28.9–99.3) | ||
| Gender | ||
| Male | 64 | |
| Female | 28 | |
| Location | ||
| Temporal | 28 | |
| Frontal | 21 | |
| Parietal | 17 | |
| Frontotemporal | 8 | |
| Temporoparietal | 7 | |
| Occipitoparietal | 6 | |
| Frontoparietal | 2 | |
| Occipital | 2 | |
| Posterior fossa | 1 | |
| Region | ||
| Cortical | 68 | |
| Periventricular | 27 | |
| Resection Status | ||
| GTR | 32 | |
| STR | 53 | |
| Bx | 8 | |
| MGMT Methylation Status | ||
| Methylated | 23 | |
| Unmethylated | 30 | |
| Unknown | 39 | |
| Radiation Therapy Volumes | ||
| GTV T2 * | ||
| 10–50 cc | 23 | |
| 50–100 cc | 22 | |
| >100 cc | 32 | |
| GTV T1 * | ||
| <20 cc | 24 | |
| 20–40 cc | 32 | |
| >40 cc | 31 | |
| Technique | ||
| VMAT | 21 | |
| IMRT | 34 | |
| 3D | 33 | |
| Valproic Acid Administration | ||
| No | 59 | |
| Yes | 33 | |
| Feature | # Patients Evaluable | AI Volume Type | Median (Range) |
|---|---|---|---|
| Pre-RT volume (cm3) | 55 | Edema | 37.23 (0.09–111.4) |
| CET | 15.66 (0–63.9) | ||
| NET | 8.16 (0–41.5) | ||
| TT | 23.81 (0–102.8) | ||
| TB | 61.04 (0.09–163.3) | ||
| Final volume (cm3) | 92 | Edema | 59.04 (3.14–350.6) |
| CET | 8.43 (0–70.24) | ||
| NET | 6.39 (0–47.49) | ||
| TT | 15.29 (0–101.69) | ||
| TB | 77.44 (3.14–379.5) | ||
| Slope—all time (cm3/month) | 92 | ∆Edema | 3.72 (−66.8–266.6) |
| ∆CET | 0.061 (−27.6–15.8) | ||
| ∆NET | 0.078 (−4.45–14.09) | ||
| ∆TT | 0.234 (−29.05–24.33) | ||
| ∆TB | 5.04 (−89.95–265.5) | ||
| Slope—6 months (cm3/month) | 91 | ∆Edema | 44.43 (9–350.6) |
| ∆CET | 6.55 (0–54.98) | ||
| ∆NET | 5.01 (0–46.67) | ||
| ∆TT | −0.112 (−29.05–24.32) | ||
| ∆TB | 3.51 (−89.95–265.5) | ||
| Slope—12 months (cm3/month) | 92 | ∆Edema | 3.92 (−66.86–266.6) |
| ∆CET | −0.0018 (−27.58–15.84) | ||
| ∆NET | 0.05 (−4.45–14.09) | ||
| ∆TT | 0.496 (−29.95–24.32) | ||
| ∆TB | 4.31 (−89.95–265.5) | ||
| Slope—24 months (cm3/month) | 92 | ∆Edema | 3.93 (−66.86–266.6) |
| ∆CET | 0.055 (−27.58–15.85) | ||
| ∆NET | 0.091 (−4.45–14.09) | ||
| ∆TT | 0.209 (−29.05–25.33) | ||
| ∆TB | 5.04 (−89.95–265.5) |
| Variable | Category | PFS | ||
|---|---|---|---|---|
| HR | HR SE | p Value | ||
| Age | 1.025 | 0.009 | 0.005 | |
| Gender | Male | reference | ||
| Female | 0.757 | 0.236 | 0.238 | |
| Location | Frontal | reference | ||
| Frontoparietal | 0.662 | 0.744 | 0.579 | |
| Frontotemporal | 0.649 | 0.424 | 0.308 | |
| Occipital | 2.700 | 0.751 | 0.186 | |
| Occipitoparietal | 3.267 | 0.483 | 0.014 | |
| Parietal | 0.880 | 0.338 | 0.704 | |
| Posterior fossa | 5.580 | 1.054 | 0.103 | |
| Temporal | 0.770 | 0.301 | 0.384 | |
| Temporoparietal | 1.009 | 0.468 | 0.984 | |
| Region | Cortical | reference | ||
| Periventricular | 1.438 | 0.239 | 0.128 | |
| Resection Status | GTR | reference | ||
| STR | 1.149 | 0.236 | 0.555 | |
| Bx | 1.140 | 0.404 | 0.746 | |
| MGMT methylation status | Methylated | reference | ||
| Unknown | 1.499 | 0.283 | 0.153 | |
| Unmethylated | 2.122 | 0.298 | 0.012 | |
| GTV T2 | 10–50 cc | reference | ||
| 50–100 cc | 1.115 | 0.308 | 0.725 | |
| >100 cc | 1.258 | 0.280 | 0.414 | |
| GTV T1 | <20 cc | reference | ||
| 20–40 cc | 1.374 | 0.281 | 0.258 | |
| >40 cc | 1.769 | 0.291 | 0.050 | |
| Radiation Therapy Technique | VMAT | reference | ||
| IMRT | 0.972 | 0.291 | 0.923 | |
| 3D | 1.471 | 0.292 | 0.187 | |
| Valproic Acid Administration | No | reference | ||
| Yes | 0.585 | 0.232 | 0.021 | |
| Timepoint | Volume of Interest | Any PFS | |||
|---|---|---|---|---|---|
| HR | HR SE | p-Value | |||
| Volumes pre-chemoirradiation * | Edema | 1.04669 | 0.13939 | 0.74338 | |
| CET | 1.25459 | 0.12945 | 0.07977 | ||
| NET | 1.21473 | 0.12297 | 0.11366 | ||
| TT | 1.28723 | 0.12606 | 0.04519 | ||
| TB | 1.16816 | 0.13147 | 0.2371 | ||
| Volumes on final MR within analysis interval | Edema | 1.27066 | 0.10959 | 0.02884 | |
| CET | 1.35109 | 0.09957 | 0.00251 | ||
| NET | 1.21227 | 0.09862 | 0.05095 | ||
| TT | 1.33481 | 0.1008 | 0.00417 | ||
| TB | 1.33525 | 0.10828 | 0.00758 | ||
| Δ Continuous volumetric rate of change over time | Edema | 1.31578 | 0.09529 | 0.00398 | |
| CET | 1.0601 | 0.23054 | 0.80015 | ||
| NET | 1.29001 | 0.11954 | 0.03316 | ||
| TT | 1.2316 | 0.19914 | 0.29552 | ||
| TB | 1.35377 | 0.10303 | 0.00328 | ||
| Δ Quartile-based volumetric rate of change over time | ΔEdema | Q1 | Reference | ||
| Q2 | 1.48125 | 0.32367 | 0.22481 | ||
| Q3 | 5.14428 | 0.37068 | 9.94 × 10−6 | ||
| Q4 | 7.18473 | 0.37563 | 1.52 × 10−7 | ||
| ΔCET | Q1 | Reference | |||
| Q2 | 0.16377 | 0.36523 | 7.28 × 10−7 | ||
| Q3 | 0.77139 | 0.30524 | 0.39513 | ||
| Q4 | 1.93728 | 0.31451 | 0.03550 | ||
| ΔNET | Q1 | Reference | |||
| Q2 | 0.18179 | 0.36322 | 2.68 × 10−6 | ||
| Q3 | 0.76497 | 0.3005 | 0.37263 | ||
| Q4 | 1.19821 | 0.30532 | 0.55368 | ||
| ΔTT | Q1 | Reference | |||
| Q2 | 0.21242 | 0.37119 | 3.00 × 10−5 | ||
| Q3 | 1.21835 | 0.3119 | 0.52660 | ||
| Q4 | 1.9056 | 0.31299 | 0.03939 | ||
| ΔTB | Q1 | Reference | |||
| Q2 | 0.88524 | 0.31179 | 0.69584 | ||
| Q3 | 2.98905 | 0.34033 | 0.00129 | ||
| Q4 | 5.15277 | 0.3532 | 3.45 × 10−6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belue, M.J.; Harmon, S.A.; Chappidi, S.; Zhuge, Y.; Tasci, E.; Jagasia, S.; Joyce, T.; Camphausen, K.; Turkbey, B.; Krauze, A.V. Diagnosing Progression in Glioblastoma—Tackling a Neuro-Oncology Problem Using Artificial-Intelligence-Derived Volumetric Change over Time on Magnetic Resonance Imaging to Examine Progression-Free Survival in Glioblastoma. Diagnostics 2024, 14, 1374. https://doi.org/10.3390/diagnostics14131374
Belue MJ, Harmon SA, Chappidi S, Zhuge Y, Tasci E, Jagasia S, Joyce T, Camphausen K, Turkbey B, Krauze AV. Diagnosing Progression in Glioblastoma—Tackling a Neuro-Oncology Problem Using Artificial-Intelligence-Derived Volumetric Change over Time on Magnetic Resonance Imaging to Examine Progression-Free Survival in Glioblastoma. Diagnostics. 2024; 14(13):1374. https://doi.org/10.3390/diagnostics14131374
Chicago/Turabian StyleBelue, Mason J., Stephanie A. Harmon, Shreya Chappidi, Ying Zhuge, Erdal Tasci, Sarisha Jagasia, Thomas Joyce, Kevin Camphausen, Baris Turkbey, and Andra V. Krauze. 2024. "Diagnosing Progression in Glioblastoma—Tackling a Neuro-Oncology Problem Using Artificial-Intelligence-Derived Volumetric Change over Time on Magnetic Resonance Imaging to Examine Progression-Free Survival in Glioblastoma" Diagnostics 14, no. 13: 1374. https://doi.org/10.3390/diagnostics14131374
APA StyleBelue, M. J., Harmon, S. A., Chappidi, S., Zhuge, Y., Tasci, E., Jagasia, S., Joyce, T., Camphausen, K., Turkbey, B., & Krauze, A. V. (2024). Diagnosing Progression in Glioblastoma—Tackling a Neuro-Oncology Problem Using Artificial-Intelligence-Derived Volumetric Change over Time on Magnetic Resonance Imaging to Examine Progression-Free Survival in Glioblastoma. Diagnostics, 14(13), 1374. https://doi.org/10.3390/diagnostics14131374






