Microparticles in Human Perspiration as an Inflammatory Response Index
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Protocol
2.3. Materials
2.4. Blood Collection
2.5. Perspiration Collection
2.6. Extracellular Vesicle Isolation
2.7. MP Analysis
2.8. Exosome Analysis
2.9. IL-1beta
2.10. Statistical Analysis
3. Results
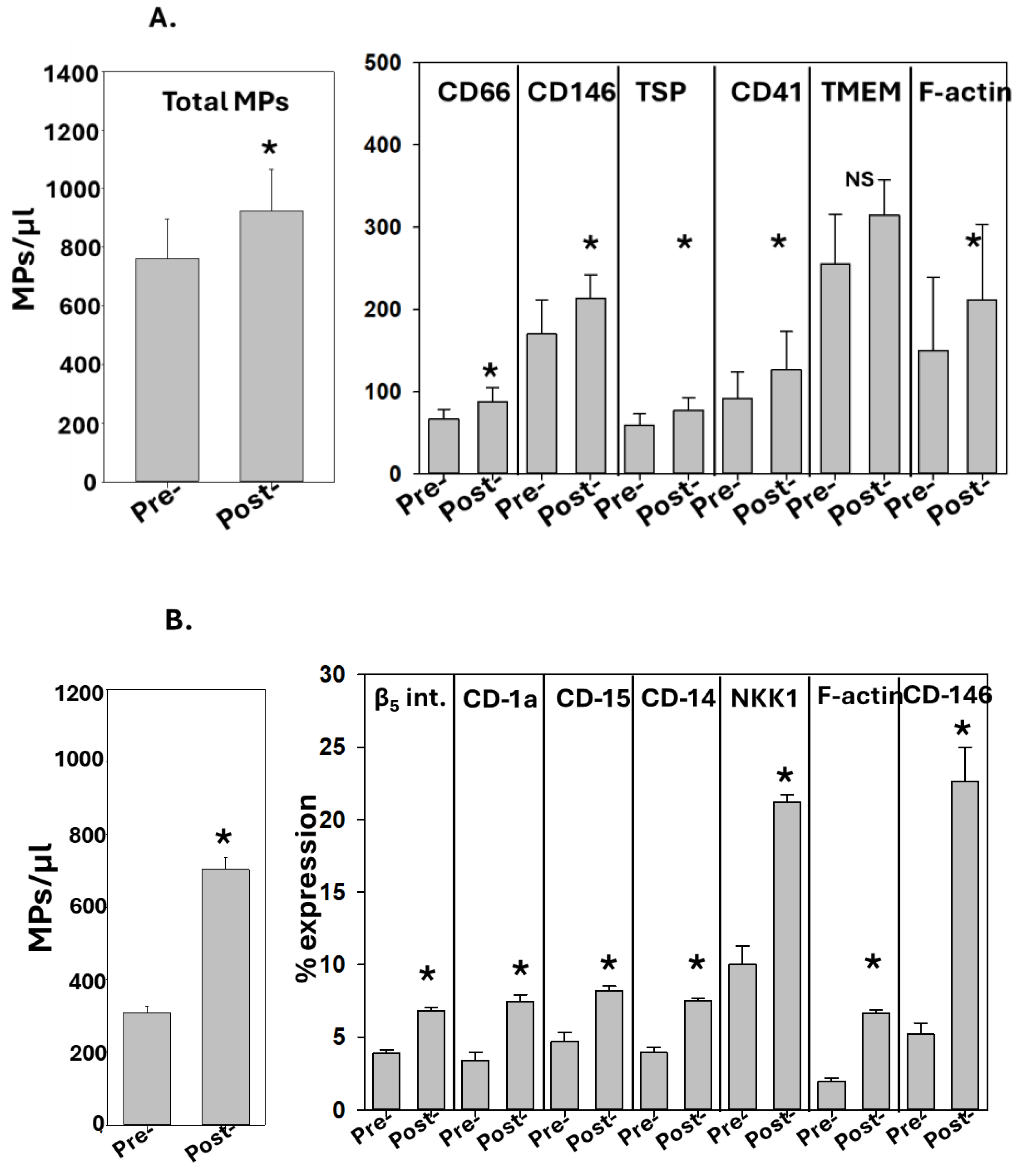
3.1. Correlation between Perspiration and Blood MPs
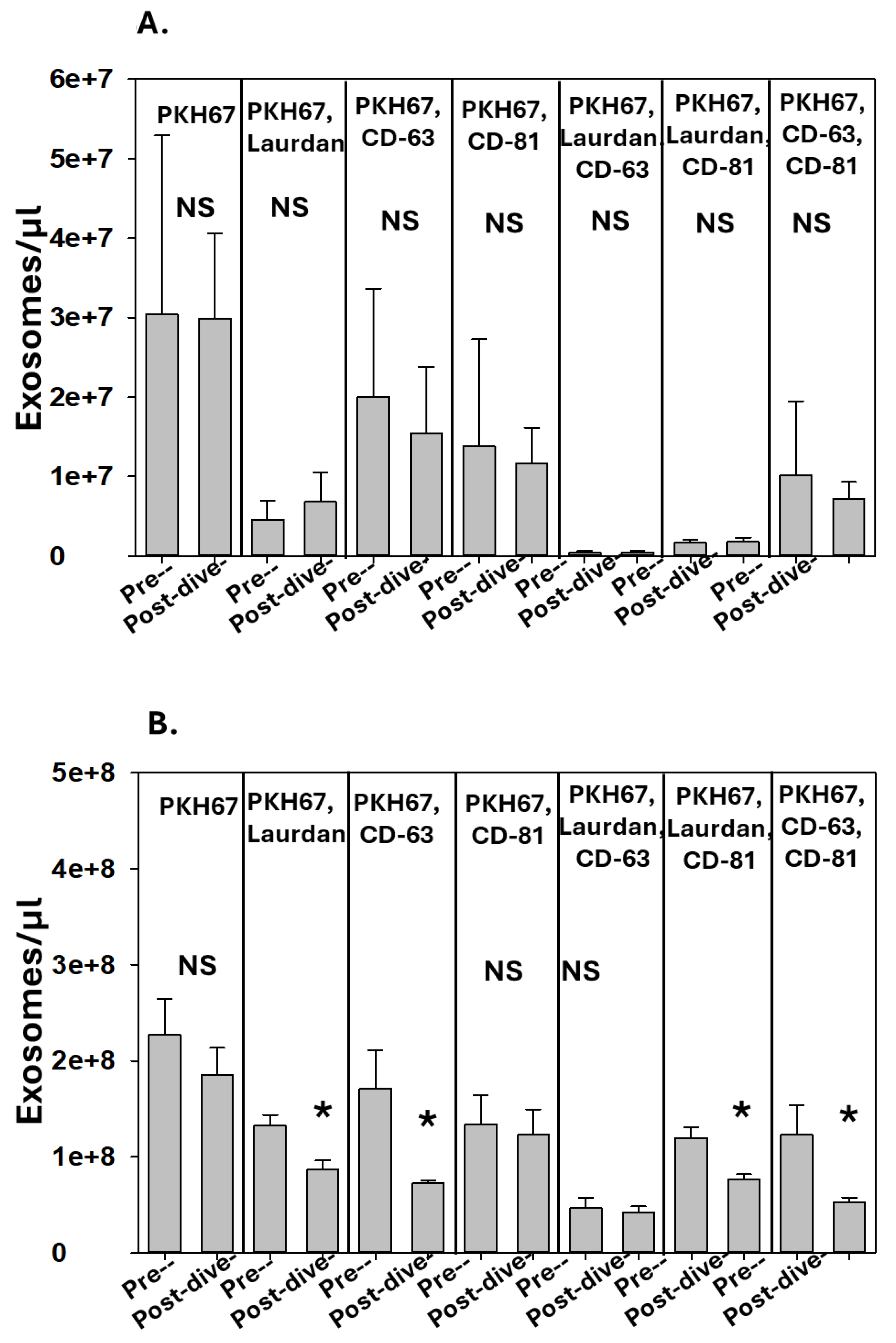
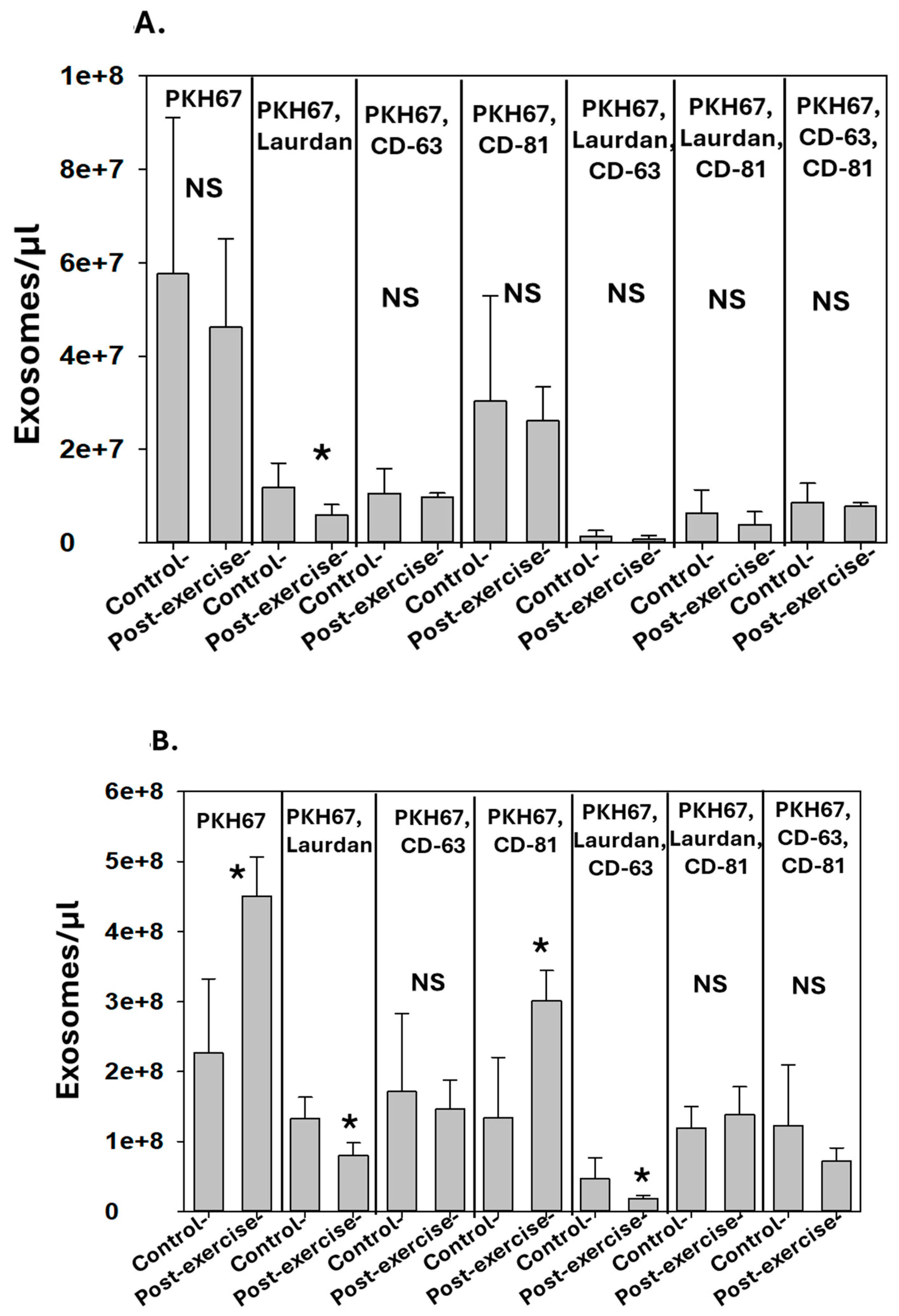
3.2. Correlation between Perspiration and Blood Exosomes
3.3. IL-1B in EVs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katchman, B.A.; Zhu, M.; Blain Christen, J.; Anderson, K.S. Eccrine Sweat as a Biofluid for Profiling Immune Biomarkers. Proteom. Clin. Appl. 2018, 12, e1800010. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.X.; Liu, Z.F. Proteomic Profiling of Sweat Exosome Suggests its Involvement in Skin Immunity. J. Investig. Dermatol. 2018, 138, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Okazaki, H.; Hanakawa, Y.; Murakami, M.; Tohyama, M.; Shirakata, Y.; Sayama, K. Eccrine sweat contains IL-1α, IL-1β and IL-31 and activates epidermal keratinocytes as a danger signal. PLoS ONE 2013, 8, e67666. [Google Scholar] [CrossRef] [PubMed]
- Cizza, G.; Marques, A.H.; Eskandari, F.; Christie, I.C.; Torvik, S.; Silverman, M.N.; Phillips, T.M.; Sternberg, E.M. Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: The POWER study. Biol. Psychiatry 2008, 64, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Raiszadeh, M.M.; Ross, M.M.; Russo, P.S.; Schaepper, M.A.; Zhou, W.; Deng, J.; Ng, D.; Dickson, A.; Dickson, C.; Strom, M.; et al. Proteomic analysis of eccrine sweat: Implications for the discovery of schizophrenia biomarker proteins. J. Proteome Res. 2012, 11, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Adewole, O.O.; Erhabor, G.E.; Adewole, T.O.; Ojo, A.O.; Oshokoya, H.; Wolfe, L.M.; Prenni, J.E. Proteomic profiling of eccrine sweat reveals its potential as a diagnostic biofluid for active tuberculosis. Proteom. Clin. Appl. 2016, 10, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Karvinen, S.; Sievänen, T.; Karppinen, J.E.; Hautasaari, P.; Bart, G.; Samoylenko, A.; Vainio, S.J.; Ahtiainen, J.P.; Laakkonen, E.K.; Kujala, U.M. MicroRNAs in Extracellular Vesicles in Sweat Change in Response to Endurance Exercise. Front. Physiol. 2020, 11, 676. [Google Scholar] [CrossRef] [PubMed]
- Ruhela, D.; Bhopale, V.M.; Yang, M.; Yu, K.; Weintraub, E.; Greenblatt, A.; Thom, S.R. Blood-borne and brain-derived microparticles in morphine-induced anti-nociceptive tolerance. Brain Behav. Immun. 2020, 87, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Bennett, M.; Banham, N.D.; Chin, W.; Blake, D.F.; Rosen, A.; Pollock, N.W.; Madden, D.; Barak, O.; Marroni, A.; et al. Association of microparticles and neutrophil activation with decompression sickness. J. Appl. Physiol. (1985) 2015, 119, 427–434. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Arya, A.K.; Ruhela, D.; Bhat, A.R.; Mitra, N.; Hoffstad, O.; Malay, D.S.; Mirza, Z.K.; Lantis, J.C.; et al. Blood-Borne Microparticles Are an Inflammatory Stimulus in Type 2 Diabetes Mellitus. Immunohorizons 2023, 7, 71–80. [Google Scholar] [CrossRef]
- Ruhela, D.; Bhopale, V.M.; Kalakonda, S.; Thom, S.R. Astrocyte-derived microparticles initiate a neuroinflammatory cycle due to carbon monoxide poisoning. Brain Behav. Immun. Health 2021, 18, 100398. [Google Scholar] [CrossRef]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J. Neuroinflamm. 2017, 14, 47. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Y.; Tian, Y.; Li, M.; Dong, J.F.; Zhang, J. Cellular microparticles and pathophysiology of traumatic brain injury. Protein Cell 2017, 8, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Bhopale, V.M.; Ruhela, D.; Brett, K.D.; Nugent, N.Z.; Fraser, N.K.; Levinson, S.L.; DiNubile, M.J.; Thom, S.R. Plasma gelsolin modulates the production and fate of IL-1β-containing microparticles following high-pressure exposure and decompression. J. Appl. Physiol. (1985) 2021, 130, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Cibrian, D.; de la Fuente, H.; Sánchez-Madrid, F. Metabolic Pathways That Control Skin Homeostasis and Inflammation. Trends Mol. Med. 2020, 26, 975–986. [Google Scholar] [CrossRef]
- Arya, A.K.; Balestra, C.; Bhopale, V.M.; Tuominen, L.J.; Räisänen-Sokolowski, A.; Dugrenot, E.; L’Her, E.; Bhat, A.R.; Thom, S.R. Elevations of Extracellular Vesicles and Inflammatory Biomarkers in Closed Circuit SCUBA Divers. Int. J. Mol. Sci. 2023, 24, 5969. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.J.; Bennett, M.H.; Moon, R.E. Decompression sickness and arterial gas embolism. N. Engl. J. Med. 2022, 386, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Yang, M.; Bhopale, V.M.; Milovanova, T.N.; Bogush, M.; Buerk, D.G. Intra-microparticle nitrogen dioxide is a bubble nucleation site leading to decompression-induced neutrophil activation and vascular injury. J. Appl. Physiol. 2013, 114, 550–558. [Google Scholar] [CrossRef]
- Pospichalova, V.; Svoboda, J.; Dave, Z.; Kotrbova, A.; Kaiser, K.; Klemova, D.; Ilkovics, L.; Hampl, A.; Crha, I.; Jandakova, E.; et al. Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J. Extracell. Vesicles 2015, 4, 25530. [Google Scholar] [CrossRef]
- MacKenzie, A.; Wilson, H.L.; Kiss-Toth, E.; Dower, S.K.; North, R.A.; Surprenant, A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 2001, 15, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Pizzirani, C.; Ferrari, D.; Chiozzi, P.; Adinolfi, E.; Sandonà, D.; Savaglio, E.; Di Virgilio, F. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood 2007, 109, 3856–3864. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Milovanova, T.N.; Bogush, M.; Bhopale, V.M.; Yang, M.; Bushmann, K.; Pollock, N.W.; Ljubkovic, M.; Denoble, P.; Dujic, Z. Microparticle production, neutrophil activation, and intravascular bubbles following open-water SCUBA diving. J. Appl. Physiol. (1985) 2012, 112, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Bhopale, V.M.; Yang, M. Neutrophils generate microparticles during exposure to inert gases due to cytoskeletal oxidative stress. J. Biol. Chem. 2014, 289, 18831–18845. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R. Carbon monoxide transport and actions in blood and tissues. Compr. Physiol. 2011, 1, 421–446. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Hu, J.; Yang, M. Increased carbon dioxide levels stimulate neutrophils to produce microparticles and activate the nucleotide-binding domain-like receptor 3 inflammasome. Free Radic. Biol. Med. 2017, 106, 406–416. [Google Scholar] [CrossRef]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imtiyaz, Z.; Bhopale, V.M.; Arya, A.K.; Bhat, A.R.; Thom, S.R. Microparticles in Human Perspiration as an Inflammatory Response Index. Diagnostics 2024, 14, 1293. https://doi.org/10.3390/diagnostics14121293
Imtiyaz Z, Bhopale VM, Arya AK, Bhat AR, Thom SR. Microparticles in Human Perspiration as an Inflammatory Response Index. Diagnostics. 2024; 14(12):1293. https://doi.org/10.3390/diagnostics14121293
Chicago/Turabian StyleImtiyaz, Zuha, Veena M. Bhopale, Awadhesh K. Arya, Abid R. Bhat, and Stephen R. Thom. 2024. "Microparticles in Human Perspiration as an Inflammatory Response Index" Diagnostics 14, no. 12: 1293. https://doi.org/10.3390/diagnostics14121293
APA StyleImtiyaz, Z., Bhopale, V. M., Arya, A. K., Bhat, A. R., & Thom, S. R. (2024). Microparticles in Human Perspiration as an Inflammatory Response Index. Diagnostics, 14(12), 1293. https://doi.org/10.3390/diagnostics14121293




