Correlation between Time to Hyperbaric Oxygen Therapy and Delayed Neurological Sequelae in Acute Carbon Monoxide Poisoning Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Variables and Definitions
2.3. HBOT Protocol
2.4. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics
3.2. Clinical Characteristics according to DNS Status
3.3. Risk Factors for DNS in Cases of Acute CO Poisoning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bleecker, M.L. Carbon monoxide intoxication. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 131, pp. 191–203. [Google Scholar]
- Lippi, G. Worldwide epidemiology of carbon monoxide poisoning. Hum. Exp. Toxicol. 2020, 39, 387–392. [Google Scholar]
- Huang, C.C.; Ho, C.H.; Chen, Y.C.; Lin, H.J.; Hsu, C.C.; Wang, J.J.; Su, S.B.; Guo, H.R. Hyperbaric oxygen therapy is associated with lower short-and long-term mortality in patients with carbon monoxide poisoning. Chest 2017, 152, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Shie, H.G.; Li, C.Y. Population-based case-control study of risk factors for unintentional mortality from carbon monoxide poisoning in Taiwan. Inhal. Toxicol. 2007, 19, 905–912. [Google Scholar] [CrossRef]
- Ernst, A.; Zibrak, J.D. Carbon monoxide poisoning. N. Engl. J. Med. 1998, 339, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.K. Carbon monoxide poisoning. Crit Care Clin. 1999, 15, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Choi, S.C. Acute carbon monoxide poisoning and delayed neurological sequelae: A potential neuroprotection bundle therapy. Neural Regen Res. 2015, 10, 36–38. [Google Scholar]
- Buckley, N.A.; Isbister, G.K.; Stokes, B.; Juurlink, D.N. Hyperbaric oxygen for carbon monoxide poisoning: A systematic review and critical analysis of the evidence. Toxicol. Rev. 2005, 24, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Castelli, M.; Nazerian, P.; Vanni, S.; Del Panta, M.; Gambassi, F.; Botti, P.; Missanelli, A.; Grifoni, S. Delayed neuropsychological sequelae after carbon monoxide poisoning: Predictive risk factors in the Emergency Department. A retrospective study. Scand J. Trauma Resusc. Emerg. Med. 2011, 19, 16. [Google Scholar] [CrossRef]
- Choi, I.S. Delayed neurologic sequelae in carbon monoxide intoxication. Arch. Neurol. 1983, 40, 433–435. [Google Scholar] [CrossRef]
- Mimura, K.; Harada, M.; Sumiyoshi, S.; Tohya, G.; Takagi, M.; Fujita, E.; Takata, A.; Tatetsu, S. Long-term follow-up study on sequelae of carbon monoxide poisoning; serial investigation 33 years after poisoning. Seishin Shinkeigaku Zasshi. 1999, 101, 592–618. [Google Scholar]
- Jay, G.D.; McKindley, D.S. Alterations in pharmacokinetics of carboxyhemoglobin produced by oxygen under pressure. Undersea Hyperb. Med. 1997, 24, 165–173. [Google Scholar]
- Weaver, L.K.; Hopkins, R.O.; Chan, K.J.; Churchill, S.; Elliott, C.G.; Clemmer, T.P.; Orme, J.F., Jr.; Thomas, F.O.; Morris, A.H. Hyperbaric oxygen for acute carbon monoxide poisoning. N. Engl. J. Med. 2002, 347, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Taber, R.L.; Mendiguren, I.I.; Clark, J.M.; Hardy, K.R.; Fisher, A.B. Delayed neuropsychologic sequelae after carbon monoxide poisoning: Prevention by treatment with hyperbaric oxygen. Ann. Emerg. Med. 1995, 25, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Nah, S.; Choi, S.; Kim, H.B.; Lee, J.; Lee, S.U.; Lee, Y.H.; Kim, G.W.; Han, S. Cerebral White Matter Lesions on Diffusion-Weighted Images and Delayed Neurological Sequelae after Carbon Monoxide Poisoning: A Prospective Observational Study. Diagnostics 2020, 10, 698. [Google Scholar] [CrossRef]
- Ducasse, J.; Celsis, P.; Marc-Vergnes, J.P. Non-comatose patients with acute carbon monoxide poisoning: Hyperbaric or normobaric oxygenation? Undersea Hyperb. Med. 1995, 22, 9–15. [Google Scholar]
- Lee, Y.; Cha, Y.S.; Kim, S.H.; Kim, H. Effect of Hyperbaric Oxygen Therapy Initiation Time in Acute Carbon Monoxide Poisoning. Crit Care Med. 2021, 49, e910–e919. [Google Scholar] [CrossRef]
- Liao, S.C.; Mao, Y.C.; Yang, K.J.; Wang, K.C.; Wu, L.Y.; Yang, C.C. Targeting optimal time for hyperbaric oxygen therapy following carbon monoxide poisoning for prevention of delayed neuropsychiatric sequelae: A retrospective study. J. Neurol. Sci. 2019, 396, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, H.; Ko, B.S.; Oh, J.; Lim, T.H.; Cho, Y. Initial creatine kinase level as predictor for delayed neuropsychiatric sequelae associated with acute carbon monoxide poisoning. Am. J. Emerg. Med. 2021, 43, 195–199. [Google Scholar] [CrossRef]
- Jeon, S.B.; Sohn, C.H.; Seo, D.W.; Oh, B.J.; Lim, K.S.; Kang, D.W.; Kim, W.Y. Acute brain lesions on magnetic resonance imaging and delayed neurological sequelae in carbon monoxide poisoning. JAMA Neurol. 2018, 75, 436–443. [Google Scholar] [CrossRef]
- Akinwande, M.O.; Dikko, H.G.; Samson, A. Variance inflation factor: As a condition for the inclusion of suppressor variable (s) in regression analysis. Open J. Stat. 2015, 5, 754. [Google Scholar] [CrossRef]
- Song, Y.Y.; Ying, L. Decision tree methods: Applications for classification and prediction. Shanghai Arch. Psychiatry 2015, 27, 130. [Google Scholar] [PubMed]
- Weaver, L.K. Clinical practice. Carbon monoxide poisoning. N. Engl. J. Med. 2009, 360, 1217–1225. [Google Scholar] [CrossRef]
- Choi, S.; Han, S.; Nah, S.; Lee, Y.H.; Cho, Y.S.; Lim, H.; Kim, M.S.; Kim, G.W. Effect of ethanol in carbon monoxide poisoning and delayed neurologic sequelae: A prospective observational study. PLoS ONE 2021, 16, e0245265. [Google Scholar] [CrossRef] [PubMed]
- Scheinkestel, C.D.; Myles, P.S.; Cooper, D.J.; Millar, I.L.; Tuxen, D.V.; Bailey, M.; Jones, K. Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: A randomised controlled clinical trial. Med. J. Aust. 1999, 170, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.J.; Im, Y.G.; Park, E.; Min, Y.G.; Choi, S.C. Treatment of acute carbon monoxide poisoning with induced hypothermia. Clin. Exp. Emerg. Med. 2016, 3, 100–104. [Google Scholar] [CrossRef]
- Pace, N.; Strajman, E.; Walker, E.L. Acceleration of carbon monoxide elimination in man by high pressure oxygen. Science 1950, 111, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Piantadosi, C.A. Reversal of carbon monoxide-cytochrome c oxidase binding by hyperbaric oxygen in vivo. Adv. Exp. Med. Biol. 1989, 248, 747–754. [Google Scholar]
- Brown, S.D.; Piantadosi, C.A. Recovery of energy metabolism in rat brain after carbon monoxide hypoxia. J. Clin. Investig. 1992, 89, 666–672. [Google Scholar] [CrossRef]
- Thom, S.R. Functional inhibition of leukocyte B2 integrins by hyperbaric oxygen in carbon monoxide-mediated brain injury in rats. Toxicol. Appl. Pharmacol. 1993, 123, 248–256. [Google Scholar] [CrossRef]
- Thom, S.R.; Mendiguren, I.; Hardy, K.; Bolotin, T.; Fisher, D.; Nebolon, M.; Kilpatrick, L. Inhibition of human neutrophil beta2-integrin-dependent adherence by hyperbaric O2. Am. J. Physiol. 1997, 272 Pt 1, C770–C777. [Google Scholar] [CrossRef]
- Oter, S.; Korkmaz, A.; Topal, T.; Ozcan, O.; Sadir, S.; Ozler, M.; Ogur, R.; Bilgic, H. Correlation between hyperbaric oxygen exposure pressures and oxidative parameters in rat lung, brain, and erythrocytes. Clin. Biochem. 2005, 38, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Oter, S.; Sadir, S.; Topal, T.; Uysal, B.; Ozler, M.; Ay, H.; Akin, A. Exposure time related oxidative action of hyperbaric oxygen in rat brain. Neurochem. Res. 2008, 33, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Raphael, J.C.; Elkharrat, D.; Jars-Guincestre, M.C.; Chastang, C.; Chasles, V.; Vercken, J.B.; Gajdos, P. Trial of normobaric and hyperbaric oxygen for acute carbon monoxide intoxication. Lancet 1989, 2, 414–419. [Google Scholar] [CrossRef]
- Annane, D.; Chadda, K.; Gajdos, P.; Jars-Guincestre, M.C.; Chevret, S.; Raphael, J.C. Hyperbaric oxygen therapy for acute domestic carbon monoxide poisoning: Two randomized controlled trials. Intensive Care Med. 2011, 37, 486–492. [Google Scholar] [CrossRef]
- Pan, K.T.; Shen, C.H.; Lin, F.G.; Chou, Y.C.; Croxford, B.; Leonardi, G.; Huang, K. Prognostic factors of carbon monoxide poisoning in Taiwan: A retrospective observational study. BMJ Open 2019, 9, e031135. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, Y.; Cao, L.; Huang, W.; Zhang, J.; Gao, X.; Lin, C. Glasgow Coma Scale is a better delayed neurological sequelae risk factor than neurological examination abnormalities in carbon monoxide poisoning. Am. J. Emerg. Med. 2020, 38, 2468–2469. [Google Scholar] [CrossRef]
- Weaver, L.K.; Valentine, K.J.; Hopkins, R.O. Carbon monoxide poisoning: Risk factors for cognitive sequelae and the role of hyperbaric oxygen. Am. J. Respir. Crit Care Med. 2007, 176, 491–497. [Google Scholar] [CrossRef]
- Han, S.; Nah, S.; Choi, S.; Lee, Y.H.; Kim, G.W.; Cho, Y.S. Hyperbaric Oxygen Therapy Did Not Prevent Delayed Neuropsychiatric Sequelae in a Prospective Observational Study with Propensity Score Matching in 224 Patients with Acute Carbon Monoxide Toxicity. J. Emerg. Med. 2021, 60, 498–505. [Google Scholar] [CrossRef]


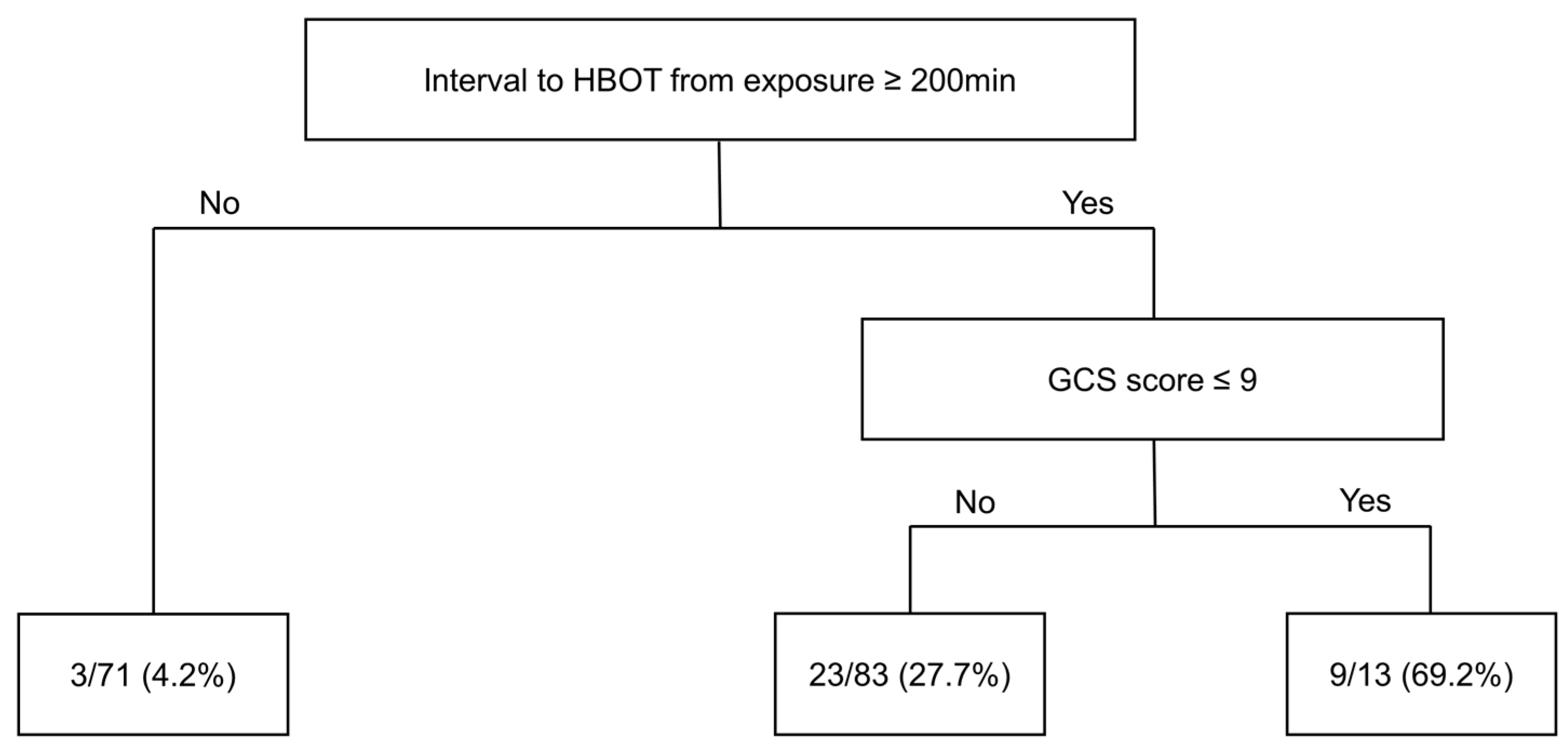
| Total (N = 167) | |
|---|---|
| Age, years | 42 [31–52.5] |
| Males, n (%) | 117 (70.1) |
| BMI, kg/m2 | 24.0 [21.3–26.3] |
| Comorbidities, n (%) | |
| Hypertension | 18 (10.8) |
| Diabetes mellitus | 13 (7.8) |
| Current smoker, n (%) | 78 (46.7) |
| Initial GCS score | 15 [13–15] |
| Intentionality (%) | 125 (74.9) |
| CO exposure time, min | 99 [50, 150] |
| Interval between HBOT and exposure, min | 239 [145.5–300] |
| Length of hospital stay, days | 3 [2–4] |
| Rate of DNS (%) | 35 (21.0) |
| Non-DNS | DNS | p-Value | |
|---|---|---|---|
| (n = 132) | (n = 35) | ||
| Age, years | 41 [30–52.3] | 44 [37–52.5] | 0.376 |
| Male, n (%) | 94 (71.2) | 23 (65.7) | 0.672 * |
| BMI, kg/m2 | 23.9 [21.3–26.2] | 24 [21.3–26.6] | 0.594 |
| Initial GCS score | 15 [14–15] | 13 [9–15] | <0.001 |
| Initial GCS score ≤ 9, n (%) | 9 (6.8) | 10 (28.6) | 0.001 ** |
| Vital signs | |||
| Systolic blood pressure, mmHg | 130 [120–140] | 130 [111–145] | 0.893 |
| Diastolic blood pressure, mmHg | 80 [73–90] | 80 [70–94.5] | 0.986 |
| Heart rate, beats/min | 90 [80–102.3] | 90 [78–100] | 0.795 |
| Respiratory rate, breaths/min | 20 [18.8–20] | 20 [18–20] | 0.208 |
| Body temperature, °C | 36.8 [36.5–37.1] | 36.8 [36.5–37.3] | 0.833 |
| Oxygen saturation, % | 98 [96–99] | 98 [96–99] | 0.704 |
| Comorbidities, n (%) | |||
| Hypertension | 11 (8.3) | 7 (20) | 0.064 ** |
| Diabetes mellitus | 11 (8.3) | 2 (5.7) | >0.99 ** |
| Current smoker, n (%) | 63 (47.7) | 15 (42.9) | 0.747 * |
| Exposure time of CO, min | 90 [44.5, 126.3] | 144 [89, 229.5] | <0.001 |
| Interval to HBOT from exposure, min | 197.5 [132.3–295.3] | 281 [241.5–370] | <0.001 |
| Intentionality, n (%) | 96 (72.7) | 29 (82.9) | 0.313 * |
| Symptoms, n (%) | |||
| Headache | 13 (9.9) | 3 (8.6) | >0.99 ** |
| Loss of consciousness | 35 (26.5) | 9 (25.7) | >0.99 * |
| Dyspnea | 11 (8.3) | 1 (2.9) | 0.463 ** |
| Chest pain | 5 (3.8) | 1 (2.9) | >0.99 ** |
| Laboratory findings | |||
| COHb, % | 12.3 [6.2–19.0] | 12.7 [11.3–17.6] | 0.160 |
| White blood cells, ×103/mm3 | 11.4 [8.2–14.9] | 11.6 [8.3–16.4] | 0.803 |
| Blood urea nitrogen, mg/dL | 12.6 [10.8–16.6] | 14.3 [11.3–21.0] | 0.137 |
| Creatinine, mg/dL | 1 [0.8–1.1] | 1 [0.9–1.2] | 0.399 |
| Creatine kinase, U/L | 121 [83.8–220.3] | 203 [100–1,199.5] | 0.016 |
| Arterial pH | 7.41 [7.37–7.43] | 7.41 [7.38–7.44] | 0.969 |
| C-reactive protein, mg/dL | 0.11 [0.04–0.34] | 0.2 [0.09–0.6] | 0.050 |
| Lactate, mmol/L | 1.9 [1.5–2.4] | 2.1 [1.5–2.4] | 0.534 |
| Troponin I, ng/mL | 0.1 [0.1–0.24] | 0.1 [0.1–−0.63] | 0.615 |
| Length of hospital stay, days | 3 [2–3] | 5 [3–11] | <0.001 |
| OR (95% CI) | p-Value | VIF | |
|---|---|---|---|
| Initial GCS score ≤ 9 | 5.059 (1.602–15.976) | 0.006 | 1.046 |
| Interval between HBOT and CO exposure ≥ 200 min | 18.971 (4.310–83.508) | <0.001 | 1.307 |
| COHb, % | 1.043 (0.995–1.093) | 0.079 | 1.323 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Nah, S.; Han, S. Correlation between Time to Hyperbaric Oxygen Therapy and Delayed Neurological Sequelae in Acute Carbon Monoxide Poisoning Patients. Diagnostics 2024, 14, 186. https://doi.org/10.3390/diagnostics14020186
Choi S, Nah S, Han S. Correlation between Time to Hyperbaric Oxygen Therapy and Delayed Neurological Sequelae in Acute Carbon Monoxide Poisoning Patients. Diagnostics. 2024; 14(2):186. https://doi.org/10.3390/diagnostics14020186
Chicago/Turabian StyleChoi, Sungwoo, Sangun Nah, and Sangsoo Han. 2024. "Correlation between Time to Hyperbaric Oxygen Therapy and Delayed Neurological Sequelae in Acute Carbon Monoxide Poisoning Patients" Diagnostics 14, no. 2: 186. https://doi.org/10.3390/diagnostics14020186
APA StyleChoi, S., Nah, S., & Han, S. (2024). Correlation between Time to Hyperbaric Oxygen Therapy and Delayed Neurological Sequelae in Acute Carbon Monoxide Poisoning Patients. Diagnostics, 14(2), 186. https://doi.org/10.3390/diagnostics14020186








