Abstract
This study aimed to investigate the relationship between rosacea and headaches, focusing on different subtypes, as well as the associated clinical features and triggering factors. In this prospective study, 300 patients diagnosed with rosacea and 320 control subjects without rosacea or any connected mast cell activation illness were included. Patients with rosacea were assessed by a dermatologist according to the 2019 updated rosacea classification (ROSCO panel). Accordingly, patients were classified based on their predominant rosacea subtype as follows: erythematotelangiectatic (ETR), papulopustular (PPR), or phymatous (RhR). Patients experiencing headaches were assessed using the International Headache Classification. Headaches were categorized as migraine, tension-type headaches (TTHs), secondary types (STHs), and cluster-type headaches (CTHs). The ratio of headache was 30.3% in the rosacea group, which did not show a significant difference compared to the control group (30.3% vs. 25.0%, p = 0.138). In 81.3% of rosacea patients with headaches, headache onset occurred after the diagnosis of rosacea. The rate of patients with headaches was higher in the ETR group compared to the PPR and RhR groups (35.2% vs. 16.2% vs. 23.1%, p = 0.007, respectively). In terms of headache subtypes, the rates of patients with migraine and STHs were higher in the ETR group compared to the PPR and RhR groups, while the rate of patients with TTHs was higher in the RhR group. A positive correlation was found between rosacea severity and migraine severity (r = 0.284, p < 0.05). Among the triggering factors for rosacea, only sunlight was found to be associated with headaches. Lower age, female gender, and moderate to severe rosacea severity were identified as independent factors increasing the likelihood of headaches. A significant portion of rosacea patients experience headaches. Particularly, different subtypes of rosacea may be associated with various types of headaches. This study, highlighting the connection between migraine and ETR, is a pioneering work that demonstrates common pathogenic mechanisms and potential triggers.
1. Introduction
Rosacea is a chronic skin disorder characterized by flushing, erythema, telangiectasia, papulopustular lesions, and ocular manifestations, typically exhibiting a fluctuating course with episodes of exacerbation and remission. The prevalence of rosacea is reported to be between 2–22% [1]. Epidemiological studies have demonstrated an association between rosacea and headaches, with a particular emphasis on migraines [2,3]. This is linked to a common pathophysiology involving neurovascular dysregulation and inflammation [4,5,6].
Both rosacea and migraine are commonly triggered by factors such as physical and mental stress, specific foods and drinks, exposure to ultraviolet light, as well as extreme temperatures [7,8,9]. Additionally, these conditions have been linked to anxiety and depression, significantly impacting the quality of life [9]. On the other hand, headaches include primary types such as tension-type headaches (TTHs), migraines, and cluster-type headaches (CTHs), as well as secondary types (STHs) that arise from pathological conditions [10]. Although recent studies have underscored a possible association between migraines and rosacea, a thorough investigation into the relationship between rosacea and various other types of headaches has not yet been conducted.
We hypothesized that there might be a significant relationship between rosacea and headaches across various subtypes. Therefore, this study aimed to investigate the relationship between rosacea and headaches, focusing on different subtypes, as well as the associated clinical features and triggering factors.
2. Materials and Methods
Following the principles set forth in the Declaration of Helsinki, this prospective study was conducted at the Ankara University Dermatology and Neurology Department from January 2020 to June 2021. The study received approval from the local ethics committee (Approval Date: February 2020, Decision No. I2-77-20). All participants provided their written informed consent.
2.1. Study Population
The study included 300 patients diagnosed with rosacea and 320 control subjects without rosacea or any connected mast cell activation illness. Patients under 18 years of age, those not providing written informed consent, those with any connected mast cell activation illness such as accompanying allergic disease, atopic dermatitis, allergic eczematous dermatitis, or urticaria, those with inflammatory, auto-immune, or photosensitive diseases, or those with diseases causing fibrosis in the skin like scleroderma were excluded from the study.
2.2. Study Protocol
The assessment process involved a comprehensive evaluation conducted by both a dermatologist for skin conditions and a neurologist for neurological aspects. A standardized questionnaire was employed to identify typical triggers in both rosacea and headache subgroups. A detailed medical history, current comorbidities, demographics, and lifestyle factors such as smoking habits, alcohol use, caffeine intake, and sun exposure were collected through a structured questionnaire administered by an interviewer (Supplementary Materials File S1).
Patients with rosacea were assessed by a dermatologist according to the 2019 updated rosacea classification (ROSCO panel). Accordingly, patients were classified based on their predominant rosacea subtype as follows: erythematotelangiectatic (ETR), papulopustular (PPR) (Figure 1), or phymatous (RhR) [11]. They were photographed, and the severity of rosacea in each patient was determined using the National Rosacea Society Rosacea Clinical Scorecard (Supplementary Materials File S1) [12]. All occurrences of burning, stinging, itching, and dryness experienced by patients with rosacea were documented.
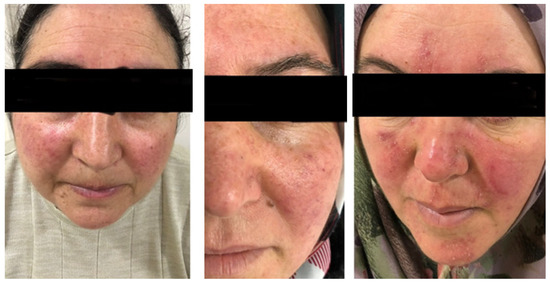
Figure 1.
Patients with the predominant rosacea subtypes of ETR and PPR. On the left, a 55-year-old patient with prominent burning, stinging, and itching symptoms and a moderate severity of ETR for 35 years. In the middle, a 46-year-old patient with moderate severity ETR for 28 years, without burning, stinging, or itching symptoms. On the right, a 42-year-old patient with prominent erythema, burning, stinging, and itching symptoms, and a moderate severity of PPR for 27 years.
Neurological assessments in patients were carried out by a neurologist, and the findings were documented along with the patients’ medical histories. Patients experiencing headaches were assessed using the International Headache Classification [13]. Headaches were categorized as migraine, TTHs, STHs, or CTHs. For measuring headache severity, the Migraine Disability Assessment (MIDAS) Questionnaire was recorded in migraine patients (Supplementary Materials File S1) [14], while the VAS score during attacks was noted in patients with other types of headaches [15].
2.3. Statistical Analysis
Statistical analyses of all data were conducted using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA). The normal distribution of numerical data was determined using the Kolmogorov–Smirnov test. Data meeting normality criteria were expressed as mean ± standard deviation, while those not meeting the criteria were presented as median (min–max). Categorical variables were shown as numbers and percentages. The assessment of differences between two groups in numerical variables was carried out using either the Student’s T-test or the Mann–Whitney U test, contingent upon the normality of the data. For analyses involving three or more groups, the Anova test (post hoc: Bonferroni test) or Kruskal–Wallis H test (post hoc: Dunn’s test) were employed. For the comparison of categorical variables, the Chi-Square test and Fisher’s Exact test were utilized. The relationship between numerical variables was investigated using Pearson and Spearman correlation analyses. Independent factors affecting headaches in rosacea patients were evaluated using multivariable logistic regression analysis with the backward method. A p-value ≤ 0.05 was considered statistically significant.
3. Results
In patients with rosacea, the mean age was 45.8 ± 12.5 years (range = 18–73 years), with a predominant female majority. The median duration of the disease was 16 years (range = 1–50 years), and 40.7% of the patients reported a family history. Among the rosacea patients, 73% (n = 219) had the predominant ETR subtype, followed by 23% (n = 68) with predominant PPR subtype, and 4% (n = 13) with predominant RhR subtype. In 60% of the patients diagnosed with ETR (n = 132), only ETR symptoms were observed, while the remaining patients exhibited concurrent symptoms such as papules, pustules, and rhinophymatous changes. The ratio of headaches was 30.3% in the rosacea group, which did not show a significant difference compared to the control group (30.3% vs. 25.0%, p = 0.138). In rosacea patients who experienced headaches, 25.3% (n = 23) reported mild severity, 37.4% (n = 34) had headaches of moderate severity, and 37.4% (n = 34) faced severe headaches. In these patients, migraines were most frequently observed (Figure 2A). It was also noted that among the rosacea patients with migraines, 61% had migraines without episodic aura, 37% had migraines with episodic aura, and 2% experienced menstrual migraines. The ratio of patients with migraine-type headaches was higher in the rosacea group compared to the control group (18.0% vs. 9.0%, p = 0.011) (Table 1).
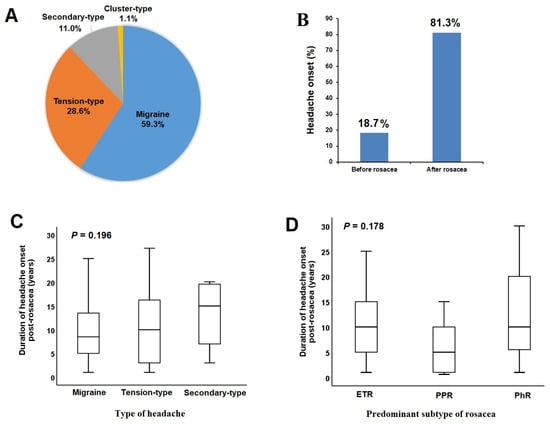
Figure 2.
Distribution of headache types (A) and onset timing (B) in rosacea patients with headaches. Comparison of headache onset duration post-rosacea diagnosis across headache types (C) or rosacea predominant subtypes (D).

Table 1.
Characteristic features of the study population.
In 81.3% of rosacea patients with headaches, headache onset occurred after the diagnosis of rosacea (Figure 2B), with a median headache onset duration of 10 years (range = 1–30 years). The median onset duration of headaches following the diagnosis of rosacea did not show significant differences across headache types (Figure 2C) or rosacea subtypes (Figure 2D). A positive correlation was found between rosacea severity and migraine severity (r = 0.284) (p < 0.05).
Demographic and clinical findings by predominant subtypes of rosacea are presented in Supplementary Table S1. The rate of patients with headaches was higher in the predominant ETR subtype group compared to the predominant PPR subtype and predominant RhR subtype groups (35.2% vs. 16.2% vs. 23.1%, p = 0.007, respectively). In terms of headache subtypes, the rates of patients with migraine and STHs were higher in the predominant ETR subtype group compared to the predominant PPR subtype and predominant RhR subtype groups, while the rate of patients with TTHs was higher in the predominant PhR subtype group. Other headache characteristics, such as severity and triggering factors, did not show significant differences among predominant subtypes of rosacea (Supplementary Table S2).
In rosacea patients with headaches, the mean age was lower (42.6 ± 10.2 vs. 47.2 ± 13.2, p = 0.003), and the ratios of female and patients with peptic ulcer were higher compared to those without headaches. Other demographic characteristics of rosacea patients did not show significant differences between those with and without headaches. The prevalence of burning, stinging, and itching symptoms was higher in rosacea patients with headaches compared to those without (51.6% vs. 30.6%; p = 0.001). Other rosacea symptoms did not show significant differences between the groups. The ratio of patients with moderate and severe rosacea was higher among those with headaches than those without. Among the triggering factors for rosacea, only sunlight was found to be associated with headaches (Table 2).

Table 2.
Demographic and clinical findings in rosacea patients based on the presence of headaches.
The mean age was higher in patients with STHs compared to those with other types of headaches. The distribution of comorbidities did not show significant differences based on the type of headache. The rates of burning, stinging, itching, and facial edematous symptoms were higher in patients with migraine-type headaches compared to those with other types of headaches. The rate of patients with severe rosacea was higher in the migraine group. No significant relationship was found between the triggering factors of rosacea and the types of headaches (Supplementary Table S3).
The ratio of patients reporting stress as a headache trigger was higher in the TTH group, while those reporting coffee, menstruation, and cheese as triggers were more prevalent in the migraine group. In the STH group, a higher ratio of patients identified hypertension as a headache trigger (Supplementary Table S4).
Stress, alcohol, coffee, and menstruation were identified as common triggering factors for both rosacea and headaches (Figure 3) (Supplementary Table S5).
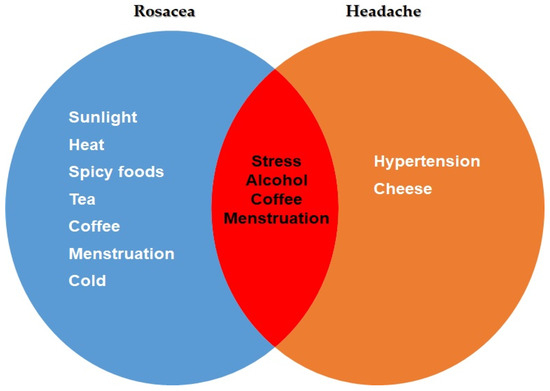
Figure 3.
Common trigger factors for headache and rosacea patients.
In a multivariable regression model incorporating factors associated with headaches in rosacea patients, lower age, female gender, and moderate to severe rosacea severity were identified as independent factors increasing the likelihood of headaches (Table 3).

Table 3.
Independent predictors of increased headache risk in rosacea patients.
4. Discussion
This study stands as a pioneering effort, offering a comprehensive analysis of the relationship between rosacea and headaches based on disease type and triggering factors. Although previous studies have investigated the relationship between rosacea and migraines, this connection has not been explored in the context of predominant subtypes of rosacea and headache subtypes. In rosacea patients, particularly in the predominant ETR subtype, migraine-type headaches were observed more frequently. Furthermore, it was found that headaches frequently emerge subsequent to the onset of rosacea. Lower age, male gender, and rosacea severity had an independent association with headaches.
Consistent with epidemiological studies, which report a headache prevalence of 12% to 54% [3,5,6], patients with rosacea had a higher frequency of headaches compared to the control group. In a meta-analysis study conducted by Stovner et al., which investigated the global prevalence of headaches, it was shown that TTH is the most prevalent type of headache in the general population [16]. Although TTH is the most prevalent primary headache type in the overall population, it is noteworthy that migraine was the most common primary headache in the rosacea group [5,17]. Although these findings indicate a lower likelihood of TTHs in rosacea,, the underlying pathogenesis of TTH is not yet clearly understood [18]. Therefore, this topic deserves further research. On the other hand, the frequency of CTHs were reported to be around 0.5% in both the rosacea and control groups [6]. In a study conducted using national data registers in Denmark, the initial prevalence of migraine was reported as 7.3% in the general population and 12.1% in patients with rosacea. Additionally, it has been shown that the likelihood of developing migraine in patients with rosacea is 1.3-fold higher than in the general population and being over 50 years of age and of female gender are independently associated [3]. In a United Kingdom-based national study that used a reverse approach to investigate the risk of rosacea development in patients with migraine, it was reported that female migraine patients had a 1.2-fold increased risk of rosacea, while this association was not observed in males. Additionally, it was reported that in women, the risk of rosacea increases with age [6]. In the current study, although the duration of rosacea was similar in patients with and without headaches, the mean age was lower. However, in the migraine patients, approximately 96% were female. Moreover, consistent with the studies reported above, female gender had an independent association with the likelihood of developing headaches.
The relationship between rosacea and headaches might be linked to multiple underlying mechanisms. Inflammatory processes, a fundamental aspect of rosacea, could influence neural pathways and contribute to headache development. Previous studies have reported a positive correlation between the duration and severity of rosacea and its inflammatory response [19,20]. In the current study, it was found that a majority of patients with headaches developed their headaches after being diagnosed with rosacea. A shared vascular change between rosacea and certain headache types, along with neurogenic modulation, might play a role [21,22]. The variability in headache types among different rosacea predominant subtypes further suggests a complex interplay, where specific inflammatory and pathophysiological features of each subtype differentially impact neurological outcomes. Neurogenic inflammation and vasodilation are apparent in both the aura and headache phases of a migraine [23,24]. The trigeminal ganglion and its nerve fibers are connected to the meninges and dural vessels, and their stimulation leads to the release of vasoactive neuropeptides, including substance p and calcitonin gene-related peptide. This release culminates in neurogenic inflammation, vasodilation, and migraine pain [25]. Furthermore, the release of neuropeptides can lead to migraine attacks and cause rosacea-like erythema and flushing [26,27]. This potential mechanism may explain the high rates of migraine in rosacea patients with a predominant ETR subtype. Additionally, mast cells, which are part of the innate immune system, connect inflammatory vascular and neurogenic arrangements in rosacea, creating the microvascular unit of the skin. Mast cell counts have been found to be raised in all predominant subtypes of rosacea, especially PPR, even from the beginning [28]. Mast cells contribute to the inflammatory response in rosacea by producing LL-37 and matrix metalloproteinases (MMPs)-9 [7]. In rosacea, it has been reported that the Th17 pathway, which is thought to influence LL-37, is activated and there is an increase in interleukin (IL)-17 [29,30]. In rosacea patients, increased IL-17 levels are associated with inflammation, angiogenesis, and the stimulation of LL-37 and MMP-9 expression [31,32]. On the other hand, factors of both innate and adaptive immunity, including mast cells which are closely linked to dural vessels and nerve pathways that trigger pain sensation, play a significant role in migraines. They stimulate vasodilation, inflammation, and heightened vascular permeability [24,33]. In patients with migraines, an increase in IL-17 and MMP activity has been detected, independent of aura [34,35]. These potential mechanisms, particularly the varying levels of mast cell release in different types of rosacea, may lead to variations in headache types.
The trigger factors of rosacea may play a significant role in the development of headaches following rosacea. Among the physical factors reported to trigger rosacea symptoms, exposure to sunlight, stress, and hot weather are the most frequently mentioned [36]. Bright light, including sunlight, serves both as a trigger and an exacerbating factor for migraine attacks, making individuals with migraines susceptible and vulnerable to the effects of sunlight [37,38]. In this current study, the association of sunlight, a known trigger factor for rosacea, with headaches can be explained. While stress was the most common trigger for headaches in rosacea patients, it was also the second most frequent trigger for rosacea itself. This is consistent with studies suggesting that stress may be a common triggering factor for both rosacea and headaches [39,40]. On the other hand, the low prevalence of TTHs in patients with rosacea does not necessarily mean that these patients experience less stress compared to healthy controls. In the current study, 92.3% of rosacea patients with TTHs reported stress as a triggering factor for their headaches. A meta-analysis study showed that 19.6% of rosacea patients suffer from depression and 15.6% from anxiety. It also revealed that the likelihood of exhibiting symptoms of depression and anxiety is at least twice as high in rosacea patients compared to healthy controls [41]. Transient receptor potential vanilloid (TRPV) receptors, located in various anatomical sites such as dermal nerve endings, the trigeminal ganglion, and scalp arteries, respond to fluctuations in stress and temperature. Rosacea patients exhibit an increased density of receptors and a lower threshold for stimulation, indicating the potential for therapeutic applications in both rosacea and migraines [42,43]. Certain studies propose that the PAR2 receptors, existing alongside the TRPV receptors, may interconnect the neurogenic and inflammatory pathways in both afflictions [43,44]. The precise role of neurogenic inflammation in migraine pathogenesis remains a topic of debate, although it is thought to influence chronicity and pain perception [42,43]. Remarkably, it was observed that the majority of rosacea patients suffering from headaches, particularly migraines, predominantly presented with the ETR subtype, characterized by symptoms such as burning, stinging, and itching. These patients may be more sensitive to migraine-triggering factors such as sunlight [45].
This study had several significant limitations. There were age differences both in the control group and among the rosacea subtypes. The age discrepancy may have negatively influenced the prevalence of migraine in rosacea patients [21]. Inflammatory parameters were not assessed in the study. Additionally, comorbid conditions were not matched between groups. Evaluating these factors could have played a role in shedding light on the inflammation mechanism between headache and rosacea [46].
5. Conclusions
A significant portion of rosacea patients experience headaches. Particularly, different predominant subtypes of rosacea may be associated with various types of headaches. This study, highlighting the connection between migraine and the predominant ETR subtype, is a pioneering work that demonstrates common pathogenic mechanisms and potential triggers. The predominant ETR subtype, categorized as a subtype of rosacea with diverse clinical features and insufficient treatment outcomes, may denote a distinctive patient cohort, which demonstrates classic rosacea symptoms, but stems from different pathogenic mechanisms. These findings could lead to fresh therapeutic strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics14010023/s1, File S1. Rosacea—Headache Patient Evaluation Form; Table S1. Demographic and clinical findings according to predominant subtypes of rosacea; Table S2. Headache findings according to predominant subtypes of rosacea; Table S3. Demographic and clinical findings according to headache type in rosacea patients; Table S4. Other findings according to headache type in rosacea patients; Table S5. Common trigger factors for headache and rosacea patients. References [47,48] are cited in the supplementary materials.
Author Contributions
Conceptualization, M.A. and P.K.; Methodology, M.A., T.S., O.S. and P.K.; Supervision, P.K.; Formal Analysis, M.A., T.S., O.S. and P.K.; Resources, M.A., T.S., O.S. and P.K.; Data Curation, M.A., T.S., O.S. and P.K.; Writing—Original Draft Preparation, M.A.; Writing—Review and Editing, T.S., O.S. and P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ankara University Clinical Research Ethics Committee (Date: February 2020, Decision No: I2-77-20).
Informed Consent Statement
All participants provided their written informed consent.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rainer, B.M.; Kang, S.; Chien, A.L. Rosacea: Epidemiology, pathogenesis, and treatment. Dermatoendocrinology 2017, 9, e1361574. [Google Scholar] [CrossRef] [PubMed]
- Sinikumpu, S.P.; Vahanikkila, H.; Jokelainen, J.; Tasanen, K.; Huilaja, L. Male patients with rosacea have increased risk for migraine: A population-based study. Br. J. Dermatol. 2021, 185, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Ashina, M.; Gaist, D.; Gislason, G.H.; Thyssen, J.P. Prevalence and risk of migraine in patients with rosacea: A population-based cohort study. J. Am. Acad. Dermatol. 2017, 76, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Han, Y.J.; Kim, H.S.; Cho, S.H.; Lee, J.D. Updates on the risk of neuropsychiatric and gastrointestinal comorbidities in rosacea and its possible relationship with the gut-brain-skin axis. Int. J. Mol. Sci. 2020, 21, 8427. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.E.; Andersen, F.S.; Wienholtz, N.; Egeberg, A.; Thyssen, J.P.; Ashina, M. The relationship between migraine and rosacea: Systematic review and meta-analysis. Cephalalgia 2018, 38, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Spoendlin, J.; Voegel, J.J.; Jick, S.S.; Meier, C.R. Migraine, triptans, and the risk of developing rosacea: A population-based study within the United Kingdom. J. Am. Acad. Dermatol. 2013, 69, 399–406. [Google Scholar] [CrossRef]
- Maden, S. Rosacea: An overview of its etiological factors, pathogenesis, classification and therapy options. Dermato 2023, 3, 241–262. [Google Scholar] [CrossRef]
- Hu, X.M.; Li, Z.X.; Zhang, D.Y.; Yang, Y.C.; Zheng, S.Y.; Zhang, Q.; Wan, X.-X.; Li, J.; Yang, R.-H.; Xiong, K. Current research and clinical trends in rosacea pathogenesis. Heliyon 2022, 8, e10874. [Google Scholar] [CrossRef]
- Wienholtz, N.K.F.; Christensen, C.E.; Haugaard, J.H.; Zhang, D.G.; Ashina, M.; Thyssen, J.P.; Egeberg, A. Cohort profile: COpenhagen ROsacea COhort (COROCO) and COpenhagen MIgraine COhort (COMICO). BMJ Open 2020, 10, e039445. [Google Scholar] [CrossRef]
- Ahmed, F. Headache disorders: Differentiating and managing the common subtypes. Br. J. Pain 2012, 6, 124–132. [Google Scholar] [CrossRef]
- Schaller, M.; Almeida, L.M.C.; Bewley, A.; Cribier, B.; Del Rosso, J.; Dlova, N.C.; Gallo, R.L.; Granstein, R.D.; Kautz, G.; Mannis, M.J.; et al. Recommendations for rosacea diagnosis, classification and management: Update from the global ROSacea COnsensus 2019 panel. Br. J. Dermatol. 2020, 182, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, J.; Dahl, M.; Detmar, M.; Drake, L.; Liang, M.H.; Odom, R.; Powell, F. Standard grading system for rosacea: Report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J. Am. Acad. Dermatol. 2004, 50, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J. International classification of headache disorders. Lancet Neurol. 2018, 17, 396–397. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.F.; Lipton, R.B.; Dowson, A.J.; Sawyer, J. Development and testing of the migraine disability assessment (MIDAS) questionnaire to assess headache-related disability. Neurology 2001, 56, S20–S28. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, C.; Benth, J.S.; Grande, R.B.; Aaseth, K.; Russell, M.B. A vertical VAS is a valid instrument for monitoring headache pain intensity. Cephalalgia 2009, 29, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Stovner, L.J.; Hagen, K.; Linde, M.; Steiner, T.J. The global prevalence of headache: An update, with analysis of the influences of methodological factors on prevalence estimates. J. Headache Pain 2022, 23, 34. [Google Scholar] [CrossRef]
- Scripter, C. Headache: Tension-Type Headache. FP Essent. 2018, 473, 17–20. [Google Scholar]
- Steel, S.J.; Robertson, C.E.; Whealy, M.A. Current Understanding of the Pathophysiology and Approach to Tension-Type Headache. Curr. Neurol. Neurosci. Rep. 2021, 21, 56. [Google Scholar] [CrossRef]
- Kulaklı, S.; Oğuz, I.D.; Sarı, I.F.; Sengul, I.; Kulaklı, F.; Akşan, B.; Sengul, D. “Zooming” in the association between rosacea and fibromyalgia syndrome: Is it worth mentioning? Rev. Assoc. Médica Bras. 2023, 69, e20230256. [Google Scholar] [CrossRef]
- Gerber, P.A.; Buhren, B.A.; Steinhoff, M.; Homey, B. Rosacea: The cytokine and chemokine network. J. Investig. Dermatol. Symp. Proc. 2011, 15, 40–47. [Google Scholar] [CrossRef]
- Wienholtz, N.K.F.; Christensen, C.E.; Zhang, D.G.; Rechnagel, A.S.A.; Byrnel, H.V.; Haugaard, J.H.; Ashina, M.; Thyssen, J.P.; Egeberg, A. Clinical characteristics of combined rosacea and migraine. Front Med 2022, 9, 1026447. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cai, M. New insights into the mutual promotion of rosacea, anxiety, and depression from neuroendocrine immune aspects. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R. Neurogenic inflammation and its role in migraine. Semin. Immunopathol. 2018, 40, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Donelan, J.; Kandere-Grzybowska, K.; Konstantinidou, A. The role of mast cells in migraine pathophysiology. Brain Res. Brain Res. Rev. 2005, 49, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Granstein, R.D. Roles of calcitonin gene-related peptide in the skin, and other physiological and pathophysiological functions. Brain Behav. Immun. Health 2021, 18, 100361. [Google Scholar] [CrossRef] [PubMed]
- Karsan, N.; Gosalia, H.; Goadsby, P.J. Molecular mechanisms of migraine: Nitric oxide synthase and neuropeptides. Int. J. Mol. Sci. 2023, 24, 11993. [Google Scholar] [CrossRef]
- Schwab, V.D.; Sulk, M.; Seeliger, S.; Nowak, P.; Aubert, J.; Mess, C.; Rivier, M.; Carlavan, I.; Rossio, P.; Metze, D.; et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J. Investig. Dermatol. Symp. Proc. 2011, 15, 53–62. [Google Scholar] [CrossRef]
- Sakabe, J.; Umayahara, T.; Hiroike, M.; Shimauchi, T.; Ito, T.; Tokura, Y. Calcipotriol increases hCAP18 mRNA expression but inhibits extracellular LL37 peptide production in IL-17/IL-22-stimulated normal human epidermal keratinocytes. Acta Derm. Venereol. 2014, 94, 512–516. [Google Scholar] [CrossRef]
- Woo, Y.R.; Lim, J.H.; Cho, D.H.; Park, H.J. Rosacea: Molecular mechanisms and management of a chronic cutaneous inflammatory condition. Int. J. Mol. Sci. 2016, 17, 1562. [Google Scholar] [CrossRef]
- Hayran, Y.; Sen, O.; Firat Oguz, E.; Yücel, Ç.; Eren, F.; Külcü Çakmak, S.; Yalçın, B. Serum IL-17 levels in patients with rosacea. J. Cosmet. Dermatol. 2022, 21, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Bi, Y.; Li, J.; Zhang, M.; Chen, X.; Zhang, K.; Li, J. IL-17 induces reactive astrocytes and up-regulation of vascular endothelial growth factor (VEGF) through JAK/STAT signaling. Sci. Rep. 2017, 7, 41779. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; D’Ovidio, C.; Conti, C.; Gallenga, C.E.; Lauritano, D.; Caraffa, A.; Kritas, S.K.; Ronconi, G. Progression in migraine: Role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur. J. Pharmacol. 2019, 844, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Avramut, M. Matrix metalloproteinases in neuropathic pain and migraine: Friends, enemies, and therapeutic targets. Pain Res. Treat. 2012, 2012, 952906. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, G.; Hayashi, K.; Morishita, N.; Takeshita, M.; Ishii, C.; Suzuki, S.; Ishimine, R.; Kasuga, A.; Nakazawa, H.; Takamatsu, T.; et al. Experimental and clinical investigation of cytokines in migraine: A narrative review. Int. J. Mol. Sci. 2023, 24, 8343. [Google Scholar] [CrossRef] [PubMed]
- Abram, K.; Silm, H.; Maaroos, H.I.; Oona, M. Risk factors associated with rosacea. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 565–571. [Google Scholar] [CrossRef]
- Maniyar, F.H.; Sprenger, T.; Schankin, C.; Goadsby, P.J. Photic hypersensitivity in the premonitory phase of migraine—A positron emission tomography study. Eur. J. Neurol. 2014, 21, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Marmura, M.J. Triggers, protectors, and predictors in episodic migraine. Curr. Pain Headache Rep. 2018, 22, 81. [Google Scholar] [CrossRef]
- Steinhoff, M.; Schauber, J.; Leyden, J.J. New insights into rosacea pathophysiology: A review of recent findings. J. Am. Acad. Dermatol. 2013, 69, S15–S26. [Google Scholar] [CrossRef]
- Kelman, L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007, 27, 394–402. [Google Scholar] [CrossRef]
- Dai, R.; Lin, B.; Zhang, X.; Lou, Y.; Xu, S. Depression and Anxiety in Rosacea Patients: A Systematic Review and Meta-Analysis. Dermatol. Ther. 2021, 11, 2089–2105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Moore, C.D.; Zhang, J.Y.; Hall, R.P., III; MacLeod, A.S.; Liedtke, W. TRPV4 moves toward center-fold in rosacea pathogenesis. J. Investig. Dermatol. 2017, 137, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Benemei, S.; Dussor, G. TRP channels and migraine: Recent developments and new therapeutic opportunities. Pharmaceuticals 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.J.; Lim, B.J.; Sohn, H.J.; Shin, D.; Oh, S.H. Increased expression of cathelicidin by direct activation of protease-activated receptor 2: Possible implications on the pathogenesis of rosacea. Yonsei Med. J. 2014, 55, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Scharschmidt, T.C.; Yost, J.M.; Truong, S.V.; Steinhoff, M.; Wang, K.C.; Berger, T.G. Neurogenic rosacea: A distinct clinical subtype requiring a modified approach to treatment. Arch. Dermatol. 2011, 147, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, burden, and comorbidity. Neurol. Clin. 2019, 37, 631–649. [Google Scholar] [CrossRef]
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Stewart, W.F.; Lipton, R.B.; Kolodner, K.; Liberman, J.; Sawyer, J. Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia 1999, 19, 107–114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).