Does the Presence of the Cilioretinal Artery Affect the Incidence, Clinical Picture and Progression of Age-Related Macular Degeneration?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ophthalmologic Examination
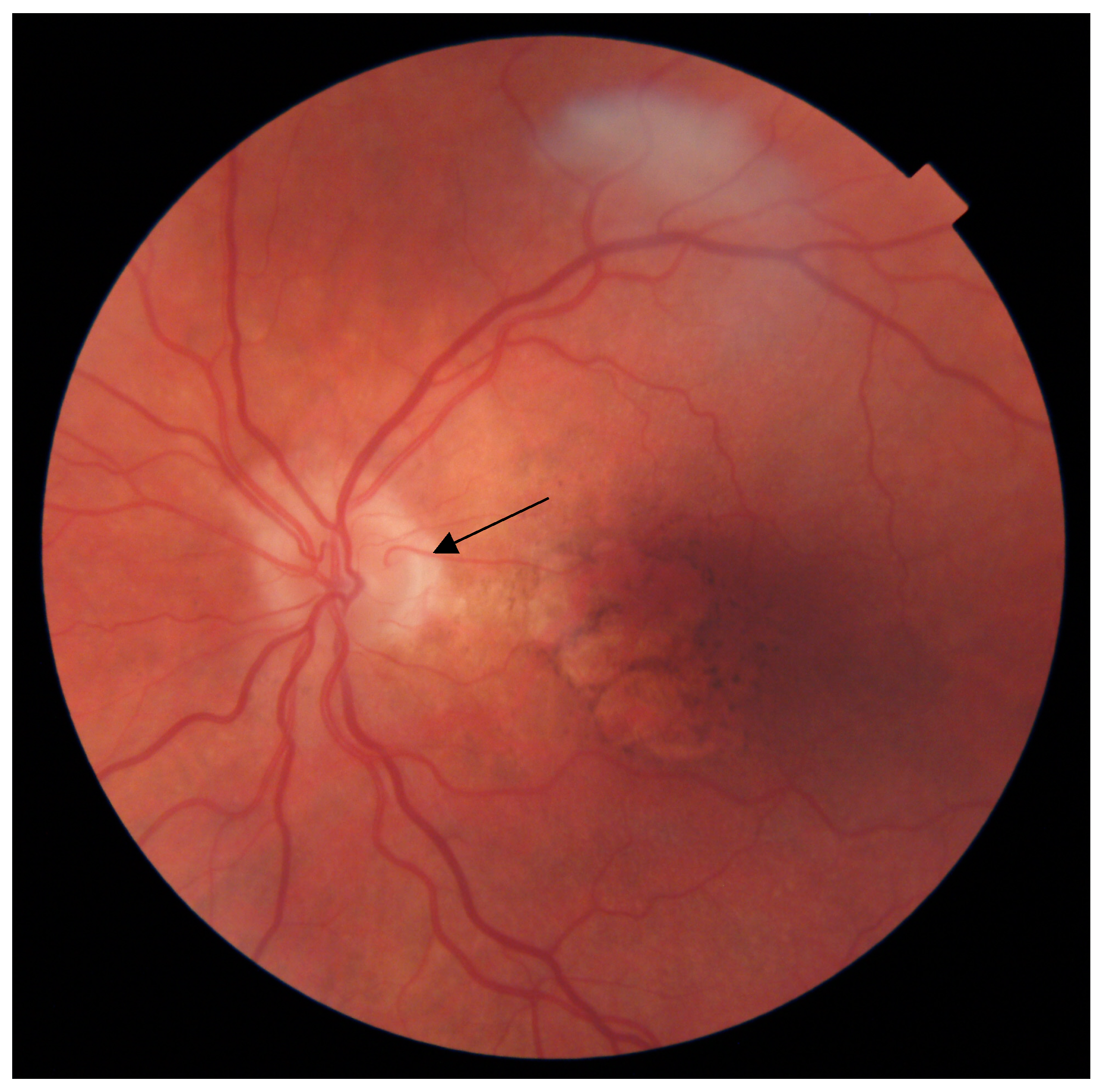
2.3. CRA Identification
2.4. Optical Coherence Tomography
2.5. Phenotypic AMD Description
2.6. Retinal Vessel Analysis
2.7. Genotyping
2.8. Statistical Analysis
3. Results
3.1. Prevalence of the CRA in the AMD and Control Groups
3.2. The Impact of CRA on the Clinical Picture of AMD
3.3. Influence of the CRA on AMD Progression
3.4. Impact of the CRA on Clinical and Morphologic Retinal and Choroidal Parameters
3.5. Associations between CFH Y402H and ARMS2 A69S Polymorphisms and CRA Prevalence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Lutty, G.A. Bruch’s Membrane and the Choroid in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2021, 1256, 89–119. [Google Scholar] [CrossRef]
- Chirco, K.R.; Sohn, E.H.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. Structural and molecular changes in the aging choroid: Implications for age-related macular degeneration. Eye 2017, 31, 10–25. [Google Scholar] [CrossRef]
- Lutty, G.A.; McLeod, D.S.; Bhutto, I.A.; Edwards, M.M.; Seddon, J.M. Choriocapillaris dropout in early age-related macular degeneration. Exp. Eye Res. 2020, 192, 107939. [Google Scholar] [CrossRef] [PubMed]
- Lipecz, A.; Miller, L.; Kovacs, I.; Czakó, C.; Csipo, T.; Baffi, J.; Csiszar, A.; Tarantini, S.; Ungvari, Z.; Yabluchanskiy, A.; et al. Micro-vascular contributions to age-related macular degeneration (AMD): From mechanisms of choriocapillaris aging to novel in-terventions. Geroscience 2019, 41, 813–845. [Google Scholar] [CrossRef]
- Harada, N.; Nagai, N.; Mushiga, Y.; Ozawa, Y. Choriocapillaris Flow Imbalance in Fellow Eyes in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2022, 63, 13. [Google Scholar] [CrossRef]
- Hayreh, S.S. The cilio-retinal arteries. Br. J. Ophthalmol. 1963, 47, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Blood supply of the optic nerve head. Ophthalmologica 1996, 210, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Schwartz, B. Role of the Temporal Cilioretinal Artery in Retaining Central Visual Field in Open-angle Glaucoma. Ophthalmology 1992, 99, 696–699. [Google Scholar] [CrossRef]
- Winther-Tham, C.; Lindblom, B. Presence of cilioretinal arteries in eyes with age-related macular degeneration. Acta Ophthalmol. 1994, 72, 397. [Google Scholar]
- Snyder, K.; Yazdanyar, A.; Mahajan, A.; Yiu, G. Association Between the Cilioretinal Artery and Choroidal Neovascularization in Age-Related Macular Degeneration: A Secondary Analysis From the Age-Related Eye Disease Study. JAMA Ophthalmol. 2018, 136, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Bavinger, J.C.; Ying, G.S.; Daniel, E.; Grunwald, J.E.; Maguire, M.G. Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Association Between Cilioretinal Arteries and Advanced Age-Related Macular Degeneration: Secondary Analysis of the Comparison of Age-Related Macular Degeneration Treatment Trials (CATT). JAMA Ophthalmol. 2019, 137, 1306–1311. [Google Scholar] [PubMed]
- Krytkowska, E.; Ulanczyk, Z.; Grabowicz, A.; Mozolewska-Piotrowska, K.; Safranow, K.; Palucha, A.; Krawczyk, M.; Sikora, P.; Matczynska, E.; Stahl, A.; et al. Retinal Vessel Functionality Is Linked with ARMS2 A69S and CFH Y402H Polymorphisms and Choroidal Status in AMD Patients. Investig. Opthalmol. Vis. Sci. 2021, 62, 30. [Google Scholar] [CrossRef]
- Krytkowska, E.; Masiuk, M.; Kawa, M.P.; Grabowicz, A.; Rynio, P.; Kazimierczak, A.; Safranow, K.; Gutowski, P.; Machalińska, A. Impact of Carotid Endarterectomy on Choroidal Thickness and Volume in Enhanced Depth Optical Coherence Tomography Imaging. J. Ophthalmol. 2020, 2020, 8326207. [Google Scholar] [CrossRef]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Shirasawa, M.; Uchino, E.; Terasaki, H.; Tomita, M. Choroidal Structure in Normal Eyes and after Photodynamic Therapy Determined by Binarization of Optical Coherence Tomographic Images. Investig. Opthalmol. Vis. Sci. 2014, 55, 3893–3899. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Gupta, P.; Tan, K.-A.; Cheung, C.M.G.; Wong, T.-Y.; Cheng, C.-Y. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci. Rep. 2016, 6, 21090. [Google Scholar] [CrossRef]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Spaide, R.F.; Curcio, C.A. Drusen characterization with multimodal imaging. Retina 2010, 30, 1441–1454. [Google Scholar] [CrossRef]
- Spaide, R.F. Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina 2018, 38, 708–716. [Google Scholar] [CrossRef]
- Fukuda, Y.; Sakurada, Y.; Yoneyama, S.; Kikushima, W.; Sugiyama, A.; Matsubara, M.; Tanabe, N.; Iijima, H. Clinical and genetic characteristics of pachydrusen in patients with exudative age-related macular degeneration. Sci. Rep. 2019, 9, 1190. [Google Scholar] [CrossRef] [PubMed]
- Wightman, A.J.; Guymer, R.H. Reticular pseudodrusen: Current understanding. Clin. Exp. Optom. 2019, 102, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Cougnard-Grégoire, A.; Delyfer, M.-N.; Combillet, F.; Rougier, M.-B.; Schweitzer, C.; Dartigues, J.-F.; Korobelnik, J.-F.; Delcourt, C. Multimodal Imaging of Reticular Pseudodrusen in a Population-Based Setting: The Alienor Study. Investig. Opthalmol. Vis. Sci. 2016, 57, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Castro-Navarro, V.; Behar-Cohen, F.; Chang, W.; Joussen, A.M.; Lai, T.Y.Y.; Navarro, R.; Pearce, I.; Yanagi, Y.; Okada, A.A. Pachychoroid: Current concepts on clinical features and pathogenesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Warrow, D.J.; Hoang, Q.V.; Freund, K.B. Pachychoroid pigment epitheliopathy. Retina 2013, 33, 1659–1672. [Google Scholar] [CrossRef]
- Chen, G.; Tzekov, R.; Li, W.; Jiang, F.; Mao, S.; Tong, Y. Subfoveal Choroidal Thickness in Central Serous Chorioretinopathy: A Me-ta-Analysis. PLoS ONE 2017, 12, e0169152. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef]
- Machalińska, A.; Pius-Sadowska, E.; Babiak, K.; Sałacka, A.; Safranow, K.; Kawa, M.P.; Machaliński, B. Correlation between Flick-er-Induced Retinal Vessel Vasodilatation and Plasma Biomarkers of Endothelial Dysfunction in Hypertensive Patients. Curr. Eye Res. 2018, 43, 128–134. [Google Scholar]
- Ulańczyk, Z.; Grabowicz, A.; Mozolewska-Piotrowska, K.; Safranow, K.; Kawa, M.P.; Pałucha, A.; Krawczyk, M.; Sikora, P.; Matczyńska, E.; Machaliński, B.; et al. Genetic factors associated with age-related macular degeneration: Identification of a novel PRPH2 single nucleotide polymorphism associated with increased risk of the disease. Acta Ophthalmol. 2021, 99, 739–749. [Google Scholar] [CrossRef]
- Awan, K.J. Arterial Vascular Anomalies of the Retina. Arch Ophthalmol. 1977, 95, 1197–1202. [Google Scholar] [CrossRef]
- Justice, J., Jr.; Lehmann, R.P. Cilioretinal arteries. A study based on review of stereo fundus photographs and fluorescein angio-graphic findings. Arch Ophthalmol. 1976, 94, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, I.; Karahan, E.; Zengin, M. Comparement of stereo fundus photography with fundus fluorescein angiography for the incidence of cilioretinal arteries. Spektrum Augenheilkd. 2013, 27, 245–248. [Google Scholar] [CrossRef]
- Mehre, K.S. Incidence of cilio-retinal artery in indians. Br. J. Ophthalmol. 1965, 49, 52–53. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.M.; Chen, L. Incidence of cilioretinal arteries in Chinese Han population. Int. J. Ophthalmol. 2011, 4, 323–325. [Google Scholar] [PubMed]
- Lindenmuth, K.A.; Skuta, G.L.; Musch, D.C.; Bueche, M. Significance of cilioretinal arteries in primary open angle glaucoma. Arch Ophthalmol. 1988, 106, 1691–1693. [Google Scholar] [CrossRef]
- Kim, D.I.; Yoon, C.K.; Yu, H.G. Unilateral Cilioretinal Artery and Advanced Age-Related Macular Degeneration: A Retrospective Cross-Sectional Study. Am. J. Ophthalmol. 2021, 237, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Ocular vascular occlusive disorders: Natural history of visual outcome. Prog. Retin. Eye Res. 2014, 41, 1–25. [Google Scholar] [CrossRef]
- Trinh, M.; Kalloniatis, M.; Nivison-Smith, L. Vascular Changes in Intermediate Age-Related Macular Degeneration Quantified Using Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Shihab, Z.M.; Beebe, W.E.; Wentlandt, T. Possible Significance of Cilioretinal Arteries in Open-angle Glaucoma. Ophthalmology 1985, 92, 880–883. [Google Scholar] [CrossRef]
- Mikelberg, F.S.; Drance, S.M.; Schulzer, M.; Wijsman, K. Possible significance of cilioretinal arteries in low-tension glaucoma. Can. J. Ophthalmol. 1990, 25, 298–300. [Google Scholar]
- Toulouie, S.; Chang, S.; Pan, J.; Snyder, K.; Yiu, G. Relationship of Retinal Vessel Caliber with Age-Related Macular Degeneration. J. Ophthalmol. 2022, 2022, 8210599. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Dahlöf, B. Cardiovascular Disease Risk Factors: Epidemiology and Risk Assessment. Am. J. Cardiol. 2010, 105 (Suppl. S1), 3A–9A. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Khan, O.A.; Shearman, C. Is there a fetal origin of peripheral vascular disease? Heart 2005, 91, 869–870. [Google Scholar] [CrossRef]
- Jønsson, L.H.; Larsen, M.; Olsen, E.M.; Skovgaard, A.M.; Munch, I.C. Incidence of cilioretinal arteries in 11- to 12-year-old children and association with maternal smoking during pregnancy: The Copenhagen Child Cohort 2000 Eye Study. Acta Ophthalmol. 2021, 99, e1162–e1167. [Google Scholar] [CrossRef] [PubMed]
- Taarnhøj, N.C.; Munch, I.C.; Kyvik, K.O.; Sander, B.; Kessel, L.; Sørensen, T.I.; Hougaard, J.L.; Larsen, M. Heritability of cilioretinal arteries: A twin study. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3850–3854. [Google Scholar] [CrossRef]
- Baneke, A.J.; Williams, K.M.; Mahroo, O.A.; Mohamed, M.; Hammond, C.J. A twin study of cilioretinal arteries, tilted discs and situs inversus. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Meng, J.; Cheng, K.; He, W.; Qi, J.; Lu, Z.-L.; Lu, Y.; Zhu, X. Contrast Sensitivity Function: A More Sensitive Index for Assessing Protective Effects of the Cilioretinal Artery on Macular Function in High Myopia. Investig. Opthalmol. Vis. Sci. 2022, 63, 25. [Google Scholar] [CrossRef]
- Spaide, R.F.; Ooto, S.; Curcio, C.A. Subretinal drusenoid deposits AKA pseudodrusen. Surv. Ophthalmol. 2018, 63, 782–815. [Google Scholar] [CrossRef]
- Inan, U.U.; Yavaş, G.; Oztürk, F. Können zilioretinale Arterien altersabhängige Makuladegeneration beeinflussen? [Does the cilioretinal artery affect age-related macular degeneration?]. Klin. Monbl. Augenheilkd. 2007, 224, 127–128. (In German) [Google Scholar] [CrossRef] [PubMed]
- Holm, L.M.; Hesgaard, H.B.; Vinding, T.; Nielsen, N.V.; Knudsen, L.L. Cilioretinal Artery and Visual Acuity in the Elderly. Investig. Ophthalmol. Vis. Sci. 2006, 47, 463. [Google Scholar]
| Parameter | AMD Group | Control Group | ||||
|---|---|---|---|---|---|---|
| CRA Present | CRA Absent | p | CRA Present | CRA Absent | p | |
| Number of subjects | 67 | 220 | 0.313 | 27 | 83 | 0.27 |
| Sex (male/female) | 16.8/27.2 | 83.2/72.8 | 0.05 | 28.6/23.2 | 71.4/76.8 | 0.61 |
| Age (years) (mean ± SD) | 72.39 (7.89) | 73.31 (8.11) | 0.31 | 73.96 (6.57) | 75.52 (5.28) | 0.27 |
| Hypertension [%] | 71.88 | 62.38 | 0.18 | 66.67 | 74.24 | 0.6 |
| Duration of hypertension (years) (mean ± SD) | 9.91 (9.48) | 8.15 (9.89) | 0.09 | 9.96 (12.78) | 8.99 (8.57) | 0.82 |
| History of ischemic heart disease (%) | 10.94 | 16.92 | 0.33 | 16.67 | 9.09 | 0.45 |
| Duration of ischemic heart disease (years) (mean ± SD) | 0.75 (2.92) | 1.4 (4.55) | 0.51 | 1.46 (5.0) | 0.58 (2.52) | 0.66 |
| History of myocardial infarction (%) | 1.56 | 7.96 | 0.08 | 8.33 | 6.06 | 0.66 |
| Stroke (%) | 4.69 | 2 | 0.37 | 4.17 | 3.03 | 1.0 |
| Peripheral artery disease [%] | 1.56 | 6.47 | 0.2 | 12.55 | 4.5 | 0.34 |
| Current smokers [%] | 12.5 | 16.34 | 0.55 | 4.17 | 6.15 | 1.0 |
| Former smokers [%] | 42.19 | 55.94 | 0.06 | 20.83 | 34.85 | 0.3 |
| Period without smoking [years] (mean ± SD) | 4.47 (8.79) | 7.01 (15) | 0.1 | 2.33 (5.42) | 6.49 (11.3) | 0.16 |
| Smoking pack-years (mean ± SD) | 12.74 (21.22) | 14.45 (18.76) | 0.13 | 2.08 (4.82) | 7.55 (15.17) | 0.13 |
| BMI [kg/m2] (mean ± SD) | 27.8 (4.66) | 26.68 (3.97) | 0.08 | 26.71 (2.92) | 26.48 (4.02) | 0.63 |
| WHR [arbitrary units] (mean ± SD) | 0.9 (0.09) | 0.89 (0.09) | 0.41 | 0.88 (0.1) | 0.88 (0.09) | 0.94 |
| MAP [mmHg] (mean ± SD) | 97.96 (11.61) | 97.28 (11.0) | 0.95 | 99.93 (9.37) | 97.96 (9.57) | 0.38 |
| Physical activity [MET] (mean ± SD) | 1946.28 (2883.21) | 1513.57 (1871.18) | 0.61 | 1489.19 (1497.22) | 1386.21 (1566.13) | 0.75 |
| Clinical Parameter | CRA Present | CRA Absent | p-Value | |
|---|---|---|---|---|
| Pachychoroid (Y/N) | 6/72 | 28/492 | 0.577 | |
| Pachyvessels (Y/N) | 22/56 | 129/392 | 0.608 | |
| Pachydrusen (Y/N) | 5/73 | 31/492 | 0.93 | |
| Subretinal drusenoid deposits (SDD) (Y/N) | 24/54 | 142/379 | 0.609 | |
| Soft drusen (Y/N) | 32/31 | 249/161 | 0.168 | |
| Drusen size (µm) [mean ± SD] | 2.08 ± 0.96 | 1.88 ± 1.14 | 0.293 | |
| AMD stage | Early (yes [%]) | 47.37 | 21.38 | 0.016 |
| Intermediate (yes [%]) | 47.37 | 56.60 | ||
| Late (yes [%]) | 5.26 | 22.01 | ||
| Geographic atrophy (Y/N) | 1/22 | 35/183 | 0.216 | |
| CNV (Y/N) | 16/6 | 129/55 | 1.000 | |
| Clinical Parameter | AMD Group | Control Group | ||||
|---|---|---|---|---|---|---|
| CRA Present (IQR) | CRA Absent (IQR) | p | CRA Present (IQR) | CRA Absent (IQR) | p | |
| BCDVA (logMAR) | 0.3 (0.6) | 0.4 (0.5) | 0.3 | 0.2 (0.3) | 0.2 (0.3) | 0.72 |
| AV (mm3) | 7.11 (3.42) | 6.71 (3.23) | 0.09 | 7.29 (2.03) | 6.83 (2.93) | 0.63 |
| ATC (μm) | 311 (146) | 273 (142) | 0.14 | 277 (111) | 269.5 (118) | 1.0 |
| AVC (mm3) | 0.24 (0.11) | 0.21 (0.11) | 0.14 | 0.22 (0.08) | 0.21(0.09) | 1.0 |
| CVI | 0.65 (0.03) | 0.65 (0.04) | 0.92 | 0.67 (0.04) | 0.66 (0.03) | 0.04 |
| CRT (μm) | 286.5 (61) | 289 (84) | 0.65 | 265 (28) | 276 (27) | 0.23 |
| AVR | 0.86 (0.1) | 0.85 (0.09) | 0.28 | 0.86 (0.06) | 0.85 (0.09) | 0.91 |
| DAA (%) | 2.85 (3.45) | 2.8 (3.1) | 0.87 | 3.1(2.7) | 3 (3.1) | 0.98 |
| DAV (%) | 3.9 (3) | 4.1 (2.7) | 0.73 | 4.1 (2) | 4 (3.2) | 0.93 |
| Tested SNP | Genotype | % of AMD Patients with CRA | % of AMD Patients without CRA | p-Value | % of Controls with CRA | % of Controls without CRA | p-Value |
|---|---|---|---|---|---|---|---|
| CFH Y402H | TT | 20.75% | 79.25% | 0.38 | 23.68% | 76.32% | 0.46 |
| TC | 25.71% | 74.29% | 32.56% | 67.44% | |||
| CC | 17.44% | 82.56% | 16.67% | 83.33% | |||
| ARMS2 A69S | GG | 24.69% | 75.31% | 0.69 | 27.78% | 72.22% | 0.57 |
| GT | 19.69% | 80.31% | 27.78% | 72.22% | |||
| TT | 21.62% | 78.38% | 0.00% | 100.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krytkowska, E.; Grabowicz, A.; Safranow, K.; Machalińska, A. Does the Presence of the Cilioretinal Artery Affect the Incidence, Clinical Picture and Progression of Age-Related Macular Degeneration? Diagnostics 2023, 13, 1593. https://doi.org/10.3390/diagnostics13091593
Krytkowska E, Grabowicz A, Safranow K, Machalińska A. Does the Presence of the Cilioretinal Artery Affect the Incidence, Clinical Picture and Progression of Age-Related Macular Degeneration? Diagnostics. 2023; 13(9):1593. https://doi.org/10.3390/diagnostics13091593
Chicago/Turabian StyleKrytkowska, Elżbieta, Aleksandra Grabowicz, Krzysztof Safranow, and Anna Machalińska. 2023. "Does the Presence of the Cilioretinal Artery Affect the Incidence, Clinical Picture and Progression of Age-Related Macular Degeneration?" Diagnostics 13, no. 9: 1593. https://doi.org/10.3390/diagnostics13091593
APA StyleKrytkowska, E., Grabowicz, A., Safranow, K., & Machalińska, A. (2023). Does the Presence of the Cilioretinal Artery Affect the Incidence, Clinical Picture and Progression of Age-Related Macular Degeneration? Diagnostics, 13(9), 1593. https://doi.org/10.3390/diagnostics13091593






