Alterations in Macular Microvasculature in Pterygium Patients Measured by OCT Angiography
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design and Subjects
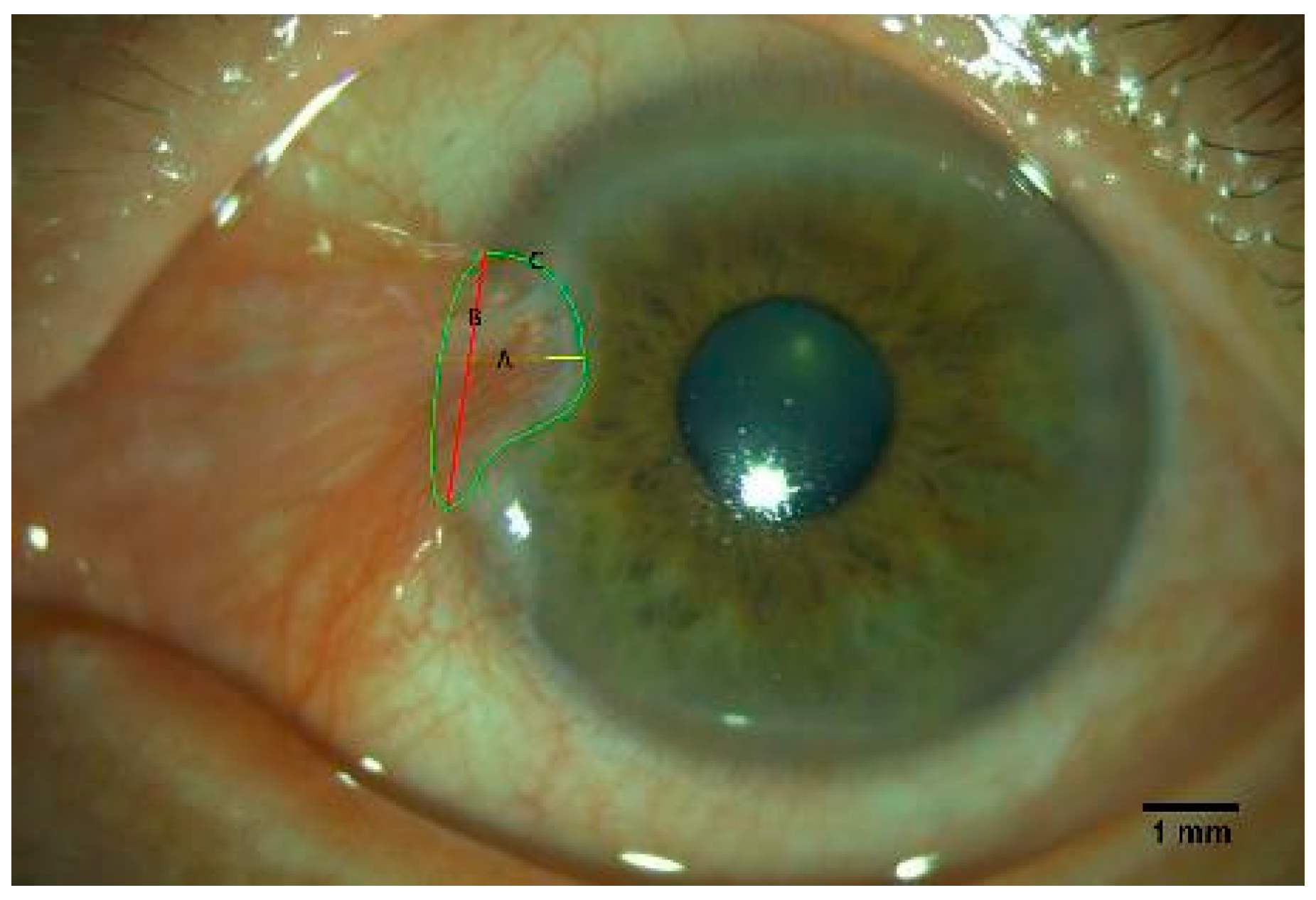
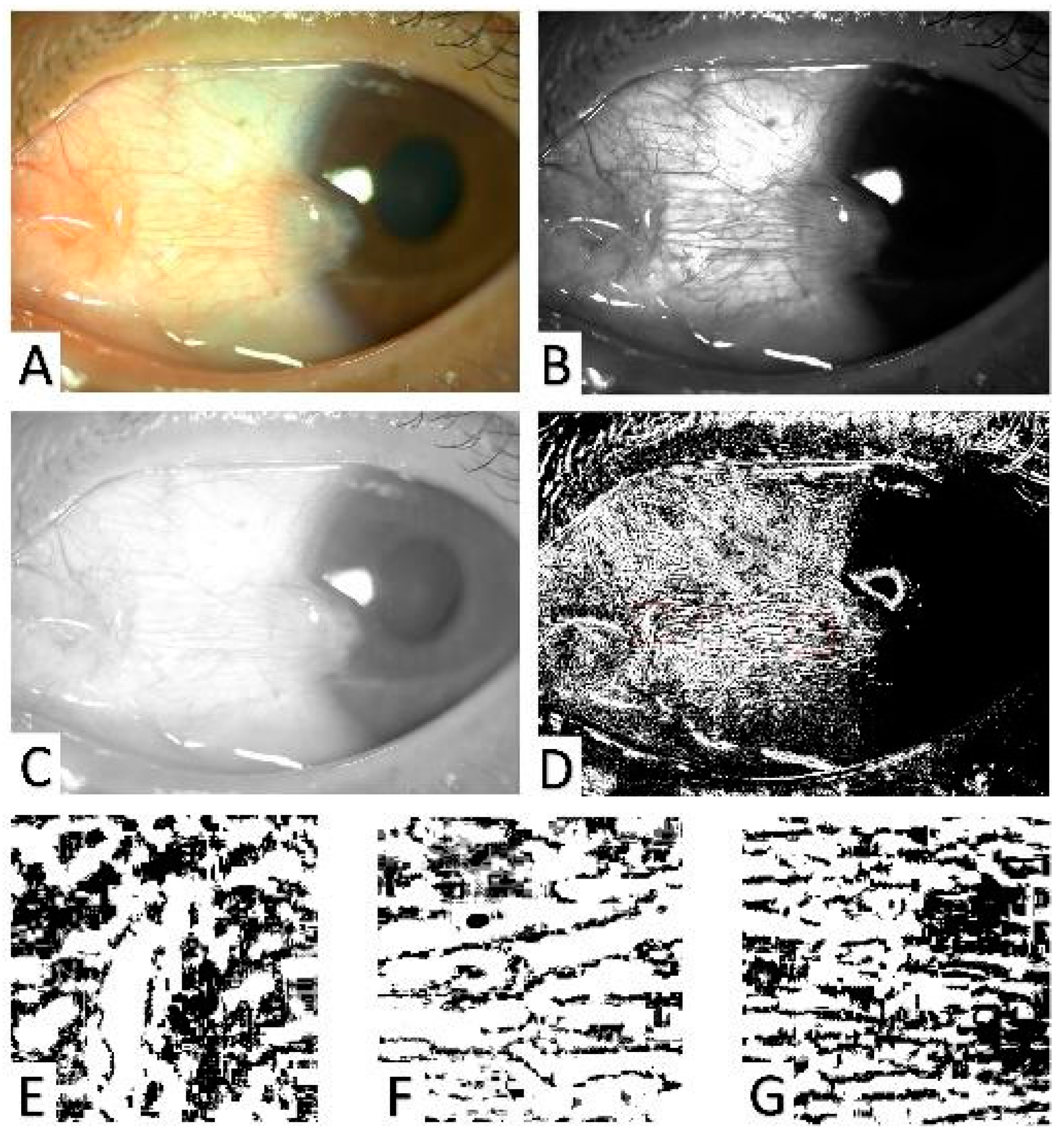
2.3. Anterior Segment Image Acquisition and Evaluation of the Pterygium
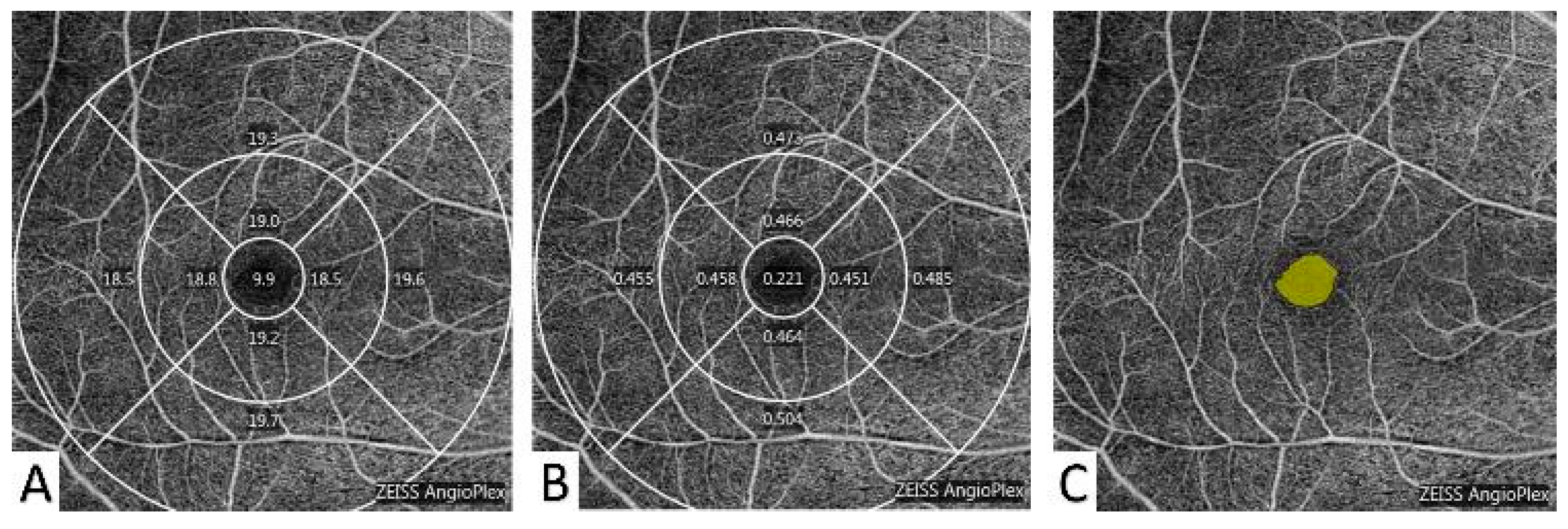
2.4. Optical Coherence Tomography Angiography (OCTA) Measurements
2.5. Statistical Analysis
3. Results
3.1. The Clinical Features of the Participants
3.2. The OCTA Parameters of the Participants
3.3. Correlation Analysis between the OCTA Parameters and the Clinical Features of Pterygia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suarez, M.F.; Echenique, J.; López, J.M.; Medina, E.; Irós, M.; Serra, H.M.; Fini, M.E. Transcriptome Analysis of Pterygium and Pinguecula Reveals Evidence of Genomic Instability Associated with Chronic Inflammation. Int. J. Mol. Sci. 2021, 22, 12090. [Google Scholar] [CrossRef]
- Azzolini, C.; Pagani, I.S.; Pirrone, C.; Borroni, D.; Donati, S.; Al Oum, M.; Pigni, D.; Chiaravalli, A.M.; Vinciguerra, R.; Simonelli, F.; et al. Expression of VEGF-A, Otx homeobox and p53 family genes in proliferative vitreoretinopathy. Mediat. Inflamm. 2013, 2013, 857380. [Google Scholar] [CrossRef]
- Chui, J.; Di Girolamo, N.; Wakefield, D.; Coroneo, M.T. The pathogenesis of pterygium: Current concepts and their therapeutic implications. Ocul. Surf. 2008, 6, 24–43. [Google Scholar] [CrossRef]
- Urbinati, F.; Borroni, D.; Rodríguez-Calvo-de-Mora, M.; Sánchez-González, J.-M.; García-Lorente, M.; Zamorano-Martín, F.; Rachwani-Anil, R.; Ortiz-Pérez, S.; Romano, V.; Rocha-de-Lossada, C. Pseudopterygium: An Algorithm Approach Based on the Current Evidence. Diagnostics 2022, 12, 1843. [Google Scholar] [CrossRef]
- Klein, R.; Peto, T.; Bird, A.; Vannewkirk, M.R. The epidemiology of age-related macular degeneration. Am. J. Ophthalmol. 2004, 137, 486–495. [Google Scholar] [CrossRef]
- Modenese, A.; Korpinen, L.; Gobba, F. Solar Radiation Exposure and Outdoor Work: An Underestimated Occupational Risk. Int. J. Environ. Res. Public Health 2018, 15, 2063. [Google Scholar] [CrossRef]
- Wang, F.; Ge, Q.M.; Shu, H.Y.; Liao, X.L.; Liang, R.B.; Li, Q.Y.; Zhang, L.J.; Gao, G.P.; Shao, Y. Decreased retinal microvasculature densities in pterygium. Int. J. Ophthalmol. 2021, 14, 1858–1867. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.Q.; Liang, R.B.; Zhang, L.J.; Shu, H.Y.; Liao, X.L.; Pan, Y.C.; Wu, J.L.; Su, T.; Shao, Y. Decreased Macular Retinal Thickness in Patients With Pterygium. Front. Neurol. 2022, 13, 881190. [Google Scholar] [CrossRef]
- Venugopal, J.P.; Rao, H.L.; Weinreb, R.N.; Pradhan, Z.S.; Dasari, S.; Riyazuddin, M.; Puttiah, N.K.; Rao, D.A.S.; Devi, S.; Mansouri, K.; et al. Repeatability of vessel density measurements of optical coherence tomography angiography in normal and glaucoma eyes. Br. J. Ophthalmol. 2018, 102, 352–357. [Google Scholar] [CrossRef]
- Lei, J.; Durbin, M.K.; Shi, Y.; Uji, A.; Balasubramanian, S.; Baghdasaryan, E.; Al-Sheikh, M.; Sadda, S.R. Repeatability and Reproducibility of Superficial Macular Retinal Vessel Density Measurements Using Optical Coherence Tomography Angiography En Face Images. JAMA Ophthalmol. 2017, 135, 1092–1098. [Google Scholar] [CrossRef]
- Kulikov, A.N.; Maltsev, D.S.; Burnasheva, M.A. Improved analysis of foveal avascular zone area with optical coherence tomography angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 2293–2299. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, H.; Jiang, H.; Wang, H.; Wang, P.; Xu, Y.; Li, F.; Xu, B.; Yao, X.; Zou, J. Macular vessel density and foveal avascular zone parameters in patients after acute primary angle closure determined by OCT angiography. Sci. Rep. 2020, 10, 18717. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, D.; Tang, Z.; Ng, D.S.; Cheung, C.Y. Optical coherence tomography angiography in diabetic retinopathy: An updated review. Eye 2021, 35, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Ooi, A.Z.H.; Embong, Z.; Abd Hamid, A.I.; Zainon, R.; Wang, S.L.; Ng, T.F.; Hamzah, R.A.; Teoh, S.S.; Ibrahim, H. Interactive Blood Vessel Segmentation from Retinal Fundus Image Based on Canny Edge Detector. Sensors 2021, 21, 6380. [Google Scholar] [CrossRef]
- Fukushima, A.; Tomita, T. Image analyses of the kinetic changes of conjunctival hyperemia in histamine-induced conjunctivitis in Guinea pigs. Cornea 2009, 28, 694–698. [Google Scholar] [CrossRef]
- Sumi, T.; Yoneda, T.; Fukuda, K.; Hoshikawa, Y.; Kobayashi, M.; Yanagi, M.; Kiuchi, Y.; Yasumitsu-Lovell, K.; Fukushima, A. Development of automated conjunctival hyperemia analysis software. Cornea 2013, 32 (Suppl. S1), S52–S59. [Google Scholar] [CrossRef]
- Schuerch, K.; Frech, H.; Zinkernagel, M. Conjunctival Microangiopathy in Diabetes Mellitus Assessed with Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2020, 9, 10. [Google Scholar] [CrossRef]
- Zou, J.; Liu, K.; Li, F.; Xu, Y.; Shen, L.; Xu, H. Combination of optical coherence tomography (OCT) and OCT angiography increases diagnostic efficacy of Parkinson’s disease. Quant. Imaging Med. Surg. 2020, 10, 1930–1939. [Google Scholar] [CrossRef]
- Kwon, J.; Choi, J.; Shin, J.W.; Lee, J.; Kook, M.S. Alterations of the Foveal Avascular Zone Measured by Optical Coherence Tomography Angiography in Glaucoma Patients With Central Visual Field Defects. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1637–1645. [Google Scholar] [CrossRef]
- Shiroma, H.; Higa, A.; Sawaguchi, S.; Iwase, A.; Tomidokoro, A.; Amano, S.; Araie, M. Prevalence and risk factors of pterygium in a southwestern island of Japan: The Kumejima Study. Am. J. Ophthalmol. 2009, 148, 766–771.e1. [Google Scholar] [CrossRef]
- Coroneo, M.T. Pterygium as an early indicator of ultraviolet insolation: A hypothesis. Br. J. Ophthalmol. 1993, 77, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.G.; Cox, C.; West, S. The differential effect of ultraviolet light exposure on cataract rate across regions of the lens. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3919–3923. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, C.K.; Tan, L.T.H.; Pusparajah, P.; Htar, T.T.; Chuah, L.-H.; Lee, V.S.; Low, L.E.; Tang, S.Y.; Chan, K.-G.; Goh, B.H. Detrimental Effects of UVB on Retinal Pigment Epithelial Cells and Its Role in Age-Related Macular Degeneration. Oxidative Med. Cell. Longev. 2020, 2020, 1904178. [Google Scholar] [CrossRef] [PubMed]
- Chalam, K.V.; Khetpal, V.; Rusovici, R.; Balaiya, S. A review: Role of ultraviolet radiation in age-related macular degeneration. Eye Contact Lenses 2011, 37, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Chanwimol, K.; Hirano, T.; Bedolla, A.; Tepelus, T.; Taweebanjongsin, W.; Marion, K.M.; Sadda, S. Evaluation of retinal vessel quantity within individual retinal structural layers using optical coherence tomography angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2111–2116. [Google Scholar] [CrossRef]
- Hill, J.C.; Maske, R. Pathogenesis of pterygium. Eye 1989, 3 Pt 2, 218–226. [Google Scholar] [CrossRef]
- Soliman, W.; Mohamed, T.A. Spectral domain anterior segment optical coherence tomography assessment of pterygium and pinguecula. Acta Ophthalmol. 2012, 90, 461–465. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoo, S.H.; Chung, J.K. Reconstruction of the limbal vasculature after limbal-conjunctival autograft transplantation in pterygium surgery: An angiography study. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7925–7933. [Google Scholar] [CrossRef]
- Gupta, N.; Motlagh, M.; Singh, G. Anatomy, Head and Neck, Eye Arteries, BTI—StatPearls, January 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537063/ (accessed on 1 January 2023).
- Shi, W.-Q.; Han, T.; Liu, R.; Xia, Q.; Xu, T.; Wang, Y.; Cai, S.; Luo, S.-L.; Shao, Y.; Wu, R. Retinal Microvasculature and Conjunctival Vessel Alterations in Patients With Systemic Lupus Erythematosus-An Optical Coherence Tomography Angiography Study. Front. Med. 2021, 8, 724283. [Google Scholar] [CrossRef]
- Jo, Y.H.; Sung, K.R.; Shin, J.W. Effects of Age on Peripapillary and Macular Vessel Density Determined Using Optical Coherence Tomography Angiography in Healthy Eyes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3492–3498. [Google Scholar] [CrossRef]
- Joachim, N.; Mitchell, P.; Rochtchina, E.; Tan, A.G.; Wang, J.J. Incidence and progression of reticular drusen in age-related macular degeneration: Findings from an older Australian cohort. Ophthalmology 2014, 121, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Clemons, T.E.; Milton, R.C.; Klein, R.; Seddon, J.M.; Ferris, F.L. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology 2005, 112, 533–539. [Google Scholar] [PubMed]
- Merle, B.M.J.; Silver, R.E.; Rosner, B.; Seddon, J.M. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: A prospective cohort study. Am. J. Clin. Nutr. 2015, 102, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Xie, B.; Barba, H.; Nadeem, U.; Movahedan, A.; Deng, N.; Spedale, M.; D’Souza, M.; Luo, W.; Leone, V.; et al. Absence of Gut Microbiota Is Associated with RPE/Choroid Transcriptomic Changes Related to Age-Related Macular Degeneration Pathobiology and Decreased Choroidal Neovascularization. Int. J. Mol. Sci. 2022, 23, 9676. [Google Scholar] [CrossRef] [PubMed]
- Borroni, D.; Romano, V.; Kaye, S.B.; Somerville, T.; Napoli, L.; Fasolo, A.; Gallon, P.; Ponzin, D.; Esposito, A.; Ferrari, S. Metagenomics in ophthalmology: Current findings and future prospectives. BMJ Open Ophthalmol. 2019, 4, e000248. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Unilateral Pterygium (n = 25) | Bilateral Pterygium (n = 21) | Control (n = 25) | p-Value |
|---|---|---|---|---|
| Age, years | 56.00 (50.5–59.5) | 55.6 ± 7.4 | 51.3 ± 10.7 | 0.215 |
| Gender no. (%) | 1.000 | |||
| Female | 12 (48.0) | 10 (47.6) | 12 (48.0) | |
| Male | 13 (52.0) | 11 (52.4) | 13 (52.0) | |
| BCVA, logMAR | 5.0 (5.0–5.1) | 5.0 (5.0–5.1) | 5.1 (5.1–5.1) | 0.006 a |
| IOP, mmHg | 14.6 ± 2.0 | 15.5 ± 2.3 | 14.4 ± 2.2 | 0.235 |
| Eye laterality no. (%) | 0.781 | |||
| Right | 13 (52.0) | 9 (42.9) | 13 (52.0) | |
| Left | 12 (48.0) | 12 (57.1) | 12 (48.0) | |
| Duration time, years | 3.0 (1.0–8.5) | 3.0 (0.4–8.0) | / | 0.646 |
| Pterygium width, mm | 5.5 ± 1.6 | 4.7 ± 1.2 | / | 0.066 |
| Pterygium length, mm | 3.5 ± 1.3 | 2.8 ± 0.9 | / | 0.053 |
| Pterygium area, mm2 | 11.0 (5.4–16.8) | 7.0 ± 3.8 | / | 0.066 |
| PPC, % | 33.1 ± 10.3 | 30.8 ± 14.0 | / | 0.848 |
| SSI | 10.0 (8.0–10.0) | 10.0 (8.5–10.0) | 9.0 (9.0–10.0) | 0.975 |
| OCTA Parameters | Unilateral Pterygium | Bilateral Pterygium | Control | p-Value |
|---|---|---|---|---|
| VLD | ||||
| Central | 6.82 ± 3.54 | 7.18 ± 3.38 | 7.76 ± 2.69 | 0.359 |
| Inner | 17.10 (14.70–18.55) | 17.00 (14.78–18.48) | 17.56 ± 1.65 | 0.171 |
| IS | 16.50 (14.05–18.75) | 17.10 (15.30–18.68) | 17.57 ± 1.92 | 0.173 |
| IN | 16.80 (13.80–18.80) | 17.85 (16.40–18.30) | 18.45 (17.10–19.23) | 0.050 |
| II | 17.20 (14.65–18.75) | 16.45 (14.03–18.65) | 17.63 ± 1.45 | 0.097 |
| IT | 17.30 (15.15–18.65) | 17.00 (14.43–18.25) | 17.95 (16.23–18.88) | 0.350 |
| Outer | 17.31 ± 1.81 | 17.55 (15.90–18.85) | 17.76 ± 1.51 | 0.497 |
| OS | 18.00 (16.70–19.00) | 17.95 (15.45–18.80) | 17.70 ± 1.46 | 0.615 |
| ON | 19.20 (18.75–20.00) | 19.24 ± 0.73 | 19.95 (19.53–20.48) | 0.013 a |
| OI | 17.34 ± 2.01 | 16.50 (15.3–17.7) | 17.95 ± 1.40 | 0.108 |
| OT | 17.10 (13.55–17.80) | 15.51 ± 3.17 | 16.25 (13.93–18.45) | 0.672 |
| Full | 16.80 ± 2.05 | 17.10 (15.50–18.60) | 17.44 ± 1.48 | 0.423 |
| FAZ | ||||
| Area | 0.27 (0.15–0.38) | 0.30 ± 0.13 | 0.29 ± 0.10 | 0.789 |
| Perimeter | 2.13 ± 0.62 | 2.23 ± 0.51 | 2.33 ± 0.46 | 0.405 |
| Circularity | 0.73 (0.64–0.79) | 0.75 (0.63–0.79) | 0.67 ± 0.13 | 0.514 |
| VPD | ||||
| Central | 0.15 ± 0.08 | 0.16 ± 0.08 | 0.17 ± 0.06 | 0.266 |
| Inner | 0.40 (0.34–0.45) | 0.41 (0.36–0.44) | 0.42 ± 0.04 | 0.215 |
| IS | 0.38 (0.33–0.45) | 0.42 (0.36–0.45) | 0.42 ± 0.05 | 0.267 |
| IN | 0.40 (0.33–0.44) | 0.43 (0.39–0.43) | 0.44 (0.40–0.45) | 0.052 |
| II | 0.40 (0.34–0.45) | 0.39 (0.33–0.44) | 0.42 ± 0.04 | 0.089 |
| IT | 0.41 (0.35–0.44) | 0.40 (0.35–0.44) | 0.41 ± 0.06 | 0.516 |
| Outer | 0.43 ± 0.05 | 0.43 (0.39–0.46) | 0.43 (0.42–0.47) | 0.492 |
| OS | 0.44 (0.41–0.47) | 0.45 (0.39–0.46) | 0.44 ± 0.04 | 0.787 |
| ON | 0.48 (0.46–0.49) | 0.47 ± 0.02 | 0.49 (0.48–0.50) | 0.027 b |
| OI | 0.44 ± 0.05 | 0.42 (0.39–0.47) | 0.44 ± 0.04 | 0.392 |
| OT | 0.41 (0.30–0.44) | 0.41 (0.31–0.44) | 0.39 (0.34–0.46) | 0.714 |
| Full | 0.41 ± 0.05 | 0.42 (0.38–0.45) | 0.42 (0.41–0.46) | 0.400 |
| Group | Parameter | Regression Formula | r | Adjusted R2 | Durbin–Watson | p-Value |
|---|---|---|---|---|---|---|
| Unilateral pterygium | VLD in ON | 20.691–0.541 PPC | 0.541 | 0.261 | 2.122 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Wu, Z.; Yuan, R.; Wang, P.; Xu, H.; Xu, Y.; Yao, X.; Wang, H.; Zou, J. Alterations in Macular Microvasculature in Pterygium Patients Measured by OCT Angiography. Diagnostics 2023, 13, 1603. https://doi.org/10.3390/diagnostics13091603
Cai Y, Wu Z, Yuan R, Wang P, Xu H, Xu Y, Yao X, Wang H, Zou J. Alterations in Macular Microvasculature in Pterygium Patients Measured by OCT Angiography. Diagnostics. 2023; 13(9):1603. https://doi.org/10.3390/diagnostics13091603
Chicago/Turabian StyleCai, Yingjun, Zhenkai Wu, Ruolan Yuan, Pingbao Wang, Huizhuo Xu, Yi Xu, Xueyan Yao, Hua Wang, and Jing Zou. 2023. "Alterations in Macular Microvasculature in Pterygium Patients Measured by OCT Angiography" Diagnostics 13, no. 9: 1603. https://doi.org/10.3390/diagnostics13091603
APA StyleCai, Y., Wu, Z., Yuan, R., Wang, P., Xu, H., Xu, Y., Yao, X., Wang, H., & Zou, J. (2023). Alterations in Macular Microvasculature in Pterygium Patients Measured by OCT Angiography. Diagnostics, 13(9), 1603. https://doi.org/10.3390/diagnostics13091603






