Application of Novel Technologies in Cardiac Electrotherapy to Prevent Complications
Abstract
1. Introduction
2. History of Presentation
2.1. Previous History
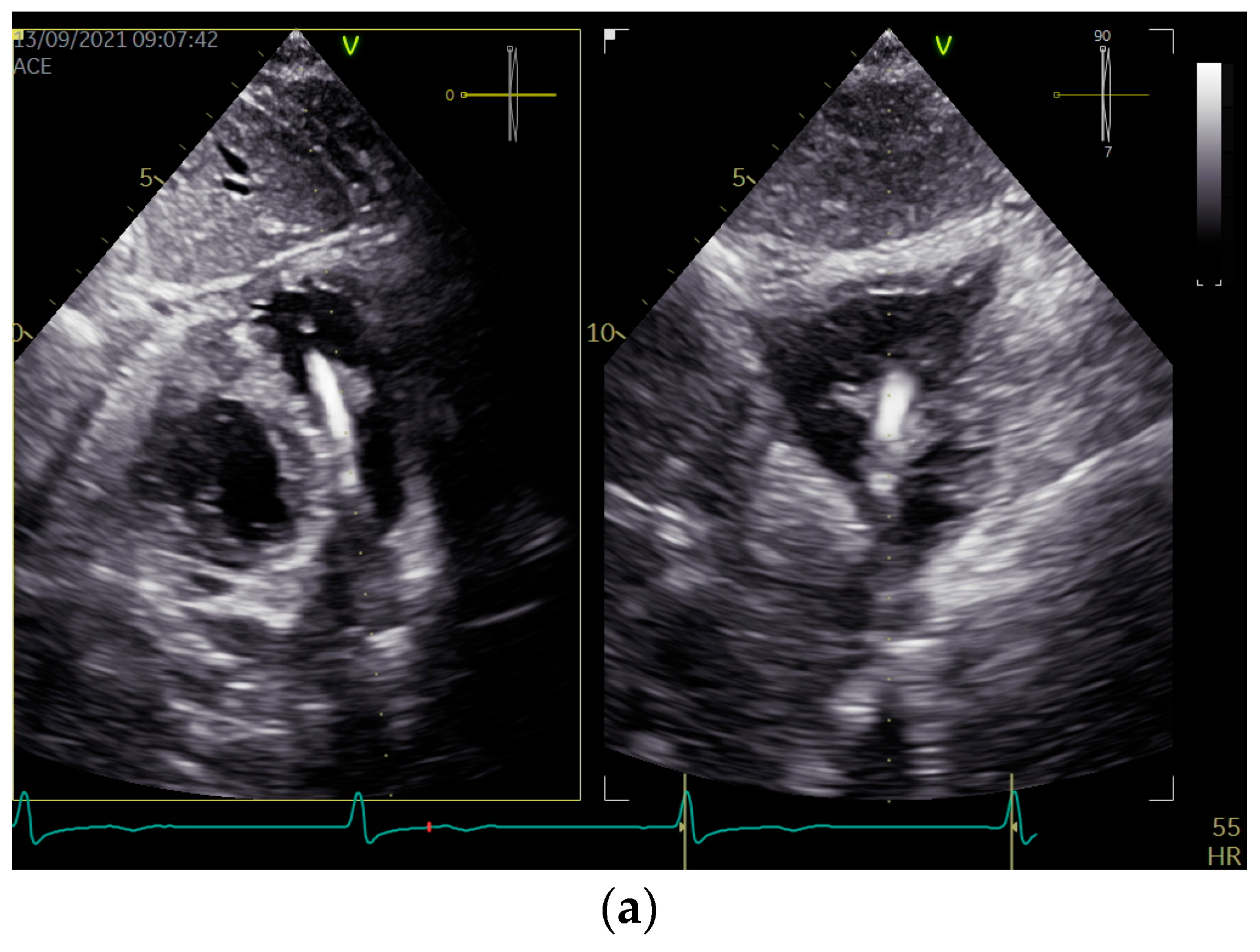
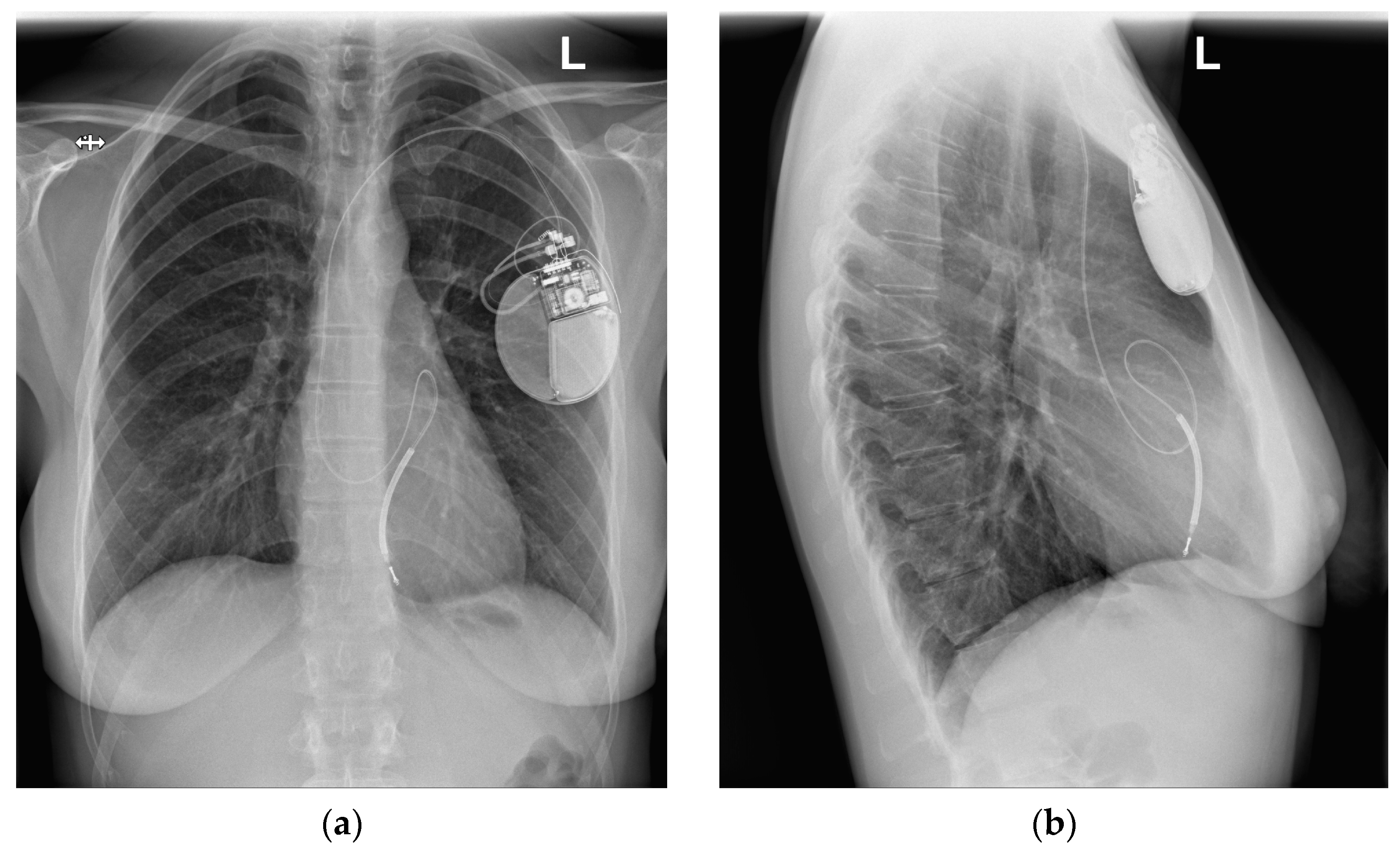
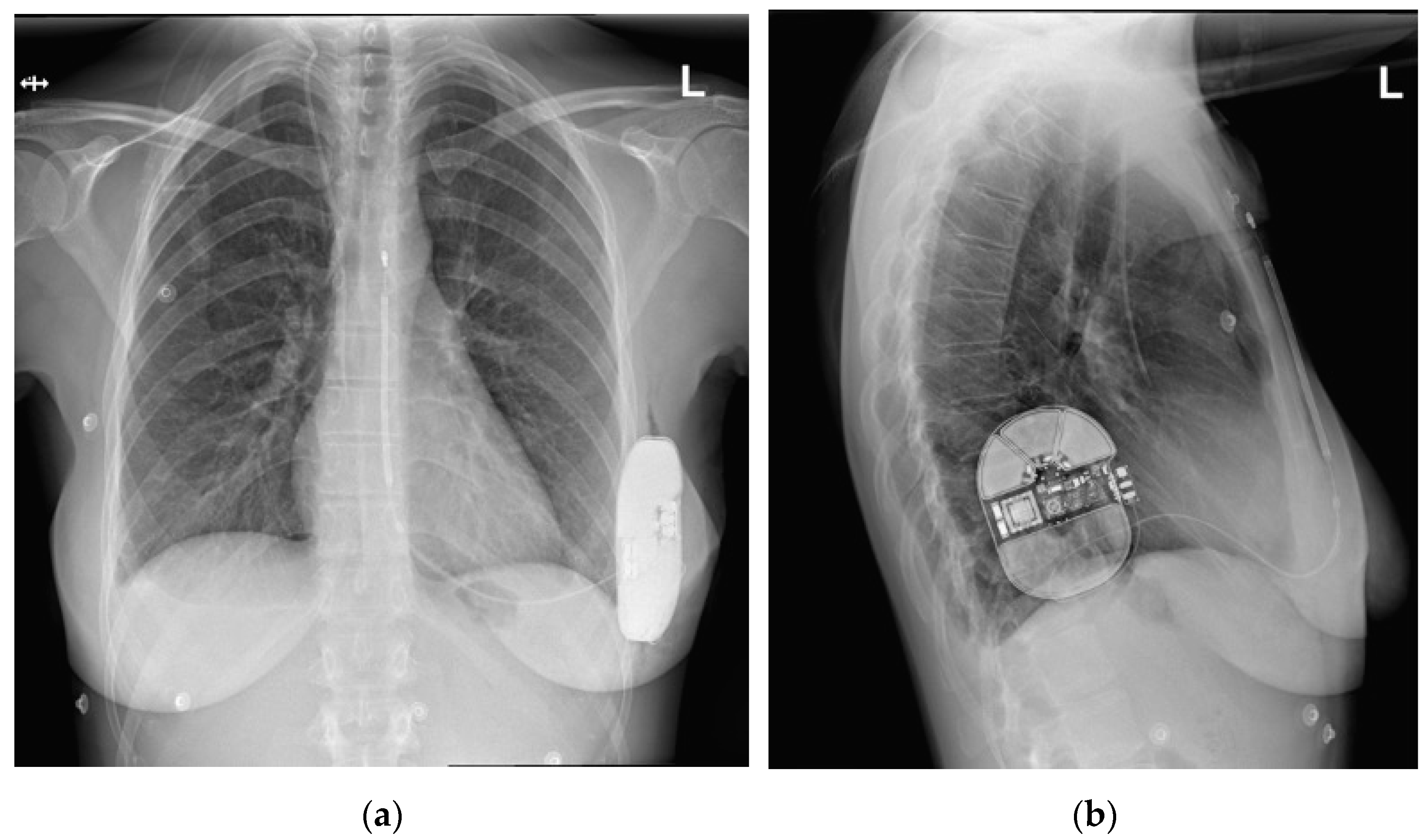
2.2. Presented Hospitalization
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elmqvist, R. Review of Early Pacemaker Development. Pacing Clin. Electrophysiol. 1978, 1, 535–536. [Google Scholar] [CrossRef]
- Mirowski, M.; Mower, M.M.; Reid, P.R. The Automatic Implantable Defibrillator. Am. Heart J. 1980, 100, 1089–1092. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomstrom-Lundqvist, C.; Mazzanti, A.; Bloma, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the Europea. Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Arribas, F.; Auricchio, A.; Wolpert, C.; Merkely, B.; Merino, J.L.; Boriani, G.; Van Der Velde, E.; Camm, J.; Vardas, P. The EHRA White Book. Europace 2012, 14 (Suppl. S3), iii1–iii55. [Google Scholar] [CrossRef]
- Prutkin, J.M.; Reynolds, M.R.; Bao, H.; Curtis, J.P.; Al-Khatib, S.M.; Aggarwal, S.; Uslan, D.Z. Rates of and Factors Associated with Infection in 200,909 Medicare Implantable Cardioverter-Defibrillator Implants Results from the National Cardiovascular Data Registry. Circulation 2014, 130, 1037–1043. [Google Scholar] [CrossRef]
- Kirkfeldt, R.E.; Johansen, J.B.; Nohr, E.A.; Jorgensen, O.D.; Nielsen, J.C. Complications after Cardiac Implantable Electronic Device Implantations: An Analysis of a Complete, Nationwide Cohort in Denmark. Eur. Heart J. 2014, 35, 1186–1194. [Google Scholar] [CrossRef]
- Han, H.C.; Hawkins, N.M.; Pearman, C.M.; Birnie, D.H.; Krahn, A.D. Epidemiology of Cardiac Implantable Electronic Device Infections: Incidence and Risk Factors. Europace 2021, 23, iv3–iv10. [Google Scholar] [CrossRef]
- Frausing, M.H.J.P.; Kronborg, M.B.; Johansen, J.B.; Nielsen, J.C. Avoiding Implant Complications in Cardiac Implantable Electronic Devices: What Works? Europace 2021, 23, 163–173. [Google Scholar] [CrossRef]
- Domagała, S.J.; Domagała, M.; Chyła, J.; Wojciechowska, C.; Janion, M.; Polewczyk, A. Ten-Year Study of Late Electrotherapy Complications. Single-Centre Analysis of Indications and Safety of Transvenous Leads Extraction. Kardiol. Pol. 2018, 76, 1350–1359. [Google Scholar] [CrossRef]
- Polewczyk, A.; Jacheć, W.; Polewczyk, A.M.; Tomasik, A.; Janion, M.; Kutarski, A. Infectious Complications in Patients with Cardiac Implantable Electronic Devices: Risk Factors, Prevention, and Prognosis. Polish Arch. Intern. Med. 2017, 127, 597–607. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Grazia Bongiorni, M.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Polewczyk, M.; Jacheć, W.; Polewczyk, A.M.; Polewczyk, A.; Czajkowski, M.; Kutarski, A. Leads dislodged into the pulmonary vascular bed in patients with cardiac implantable electronic devices. Postep. Kardiol. Interwencyjnej 2016, 12, 348–354. [Google Scholar] [CrossRef]
- Paskudzka, D.; Kołodzińska, A.; Stolarz, P.; Łyżwiński, Ł.; Grabowski, M.; Kotarski, A.; Opolski, G. Management of the late endocardial lead dislocation into the pulmonary trunk. Heart Beat J. 2018, 3, 72–76. [Google Scholar] [CrossRef]
- Michalak, M.; Kutarski, A.; Zawadzka-Byśko, M.; Opolski, G.; Grabowski, M. Transvenous Extraction of a Broken Atrial Lead Embolised into the Pulmonary Artery Using a Pigtail Catheter. Kardiol. Pol. 2015, 73, 464. [Google Scholar] [CrossRef]
- Polewczyk, M.; Polewczyk, A.M.; Kutarski, A.; Polewczyk, A. Proximal end of 15-year-old ventricular electrode penetrating pulmonary tissue—A source of infection and a challenge for transvenous lead extraction. Postep. Kardiol. Interwencyjnej 2015, 11, 248–249. [Google Scholar] [CrossRef]
- Tay, J.K.; Dweck, M.R.; Francis, C.M.; Grubb, N.R. Unusual complication of a migrant pacemaker lead. Europace 2009, 11, 1122–1124. [Google Scholar] [CrossRef]
- Erkan, H.; Varol, O.; Karadeniz, A.; Erkan, M. Embolisation of Permanent Pacemaker Lead to Pulmonary Artery: A 15-Year Follow Up. Kardiol. Pol. 2014, 72, 759. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen, K.A.; et al. 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, W.; Zhou, C.; Yin, Y.; Lu, S.; Liu, G.; Duan, C.; Cao, M.; Li, M.; Steen, E.; et al. Meta-analysis of the incidence of lead dislodgement with conventional and leadless pacemaker systems. Pacing Clin. Electrophysiol. 2018, 41, 1365–1371. [Google Scholar] [CrossRef]
- Ghani, A.; Delnoy, P.P.; Ramdat Misier, A.R.; Smit, J.J.; Adiyaman, A.; Ottervanger, J.P.; Elvan, A. Incidence of lead dislodgement, malfunction and perforation during the first year following device implantation. Neth. Heart J. 2014, 22, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Udyavar, A.R.; Pandurangi, U.M.; Latchumanadhas, K.; Mullasari, A.S. Repeated fracture of pacemaker leads with migration into the pulmonary circulation and temporary pacemaker wire insertion via the azygous vein. J. Postgrad. Med. 2008, 54, 28–31. [Google Scholar] [CrossRef]
- Drögemüller, A.; Schultz, H.; Senges, J.; Seidl, K. Catheter-based rescue of a split pacemaker lead in the pulmonary artery. Z. Kardiol. 2003, 92, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Halder, V.; Shrimanth, Y.S.; Gawalkar, A.A.; Chauhan, R.; Prasad, K.; Rohit, M.K.; Karri, S.; Walia, S.; Singh, A. Acquired cyanosis secondary to RVOT obstruction due to pacemaker lead prolapse and vegetation in a child with ASD. J. Cardiol. Cases. 2021, 25, 207–209. [Google Scholar] [CrossRef]
- Enab, H.; Cunnington, C.; Zaidi, A. An Unusual Cause of Pacemaker Lead Displacement: ‘Reverse Ratchet’ Syndrome. Heart Int. 2021, 15, 103–105. [Google Scholar] [CrossRef]
- Dalvi, B.V.; Rajani, R.M.; Lokhandwala, Y.Y.; Sathe, S.V.; Kulkarni, H.L.; Kale, P.A. Unusual case of pacemaker lead migration. Cathet. Cardiovasc. Diagn. 1990, 21, 95–96. [Google Scholar] [CrossRef]
- Mayer, E.D.; Saggau, W.; Welsch, M.; Tanzeem, A.; Späth, J.; Schmitz, W.; Schwarz, F.; Jauernig, R.; Kaden, F. Late pulmonary embolization of a retained pacemaker electrode fragment after attempted transatrial extraction. Thorac. Cardiovasc. Surg. 1985, 33, 128–130. [Google Scholar] [CrossRef]
- Stein, A.; Mazzitelli, D.; Kolb, C. Very-late proarrhythmia of a migrant pacemaker lead. J. Electrocardiol. 2011, 44, 232–234. [Google Scholar] [CrossRef]
- Golzio, P.G.; Bongiorni, M.G.; Chiribiri, A.; Franco, E. Extraction of the inner coil of a pacemaker lead slid into the pulmonary artery. Pacing Clin. Electrophysiol. 2007, 30, 273–275. [Google Scholar] [CrossRef]
- Galea, N.; Bandera, F.; Lauri, C.; Autore, C.; Laghi, A.; Erba, P.A. Multimodality Imaging in the Diagnostic Work-Up of Endocarditis and Cardiac Implantable Electronic Device (CIED) Infection. J. Clin. Med. 2020, 9, 2237. [Google Scholar] [CrossRef]
- Polewczyk, A.; Kutarski, A.; Tomaszewski, A.; Brzozowski, W.; Czajkowski, M.; Polewczyk, M.; Janion, M. Lead Dependent Tricuspid Dysfunction: Analysis of the Mechanism and Management in Patients Referred for Transvenous Lead Extraction. Cardiol. J. 2013, 20, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Małecka, B.; Kutarski, A.; Zabek, A.; Maziarz, A.; Pytkowski, M. Percutaneous Removal of Endocardial Implantable Cardioverter-Defibrillator Lead Displaced to the Right Pulmonary Artery. Cardiol. J. 2010, 17, 293–298. [Google Scholar] [PubMed]
- Suga, C.; Hayes, D.L.; Hyberger, L.K.; Lloyd, M.A. Is there an adverse outcome from abandoned pacing leads? J. Interv. Card. Electrophysiol. 2000, 4, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, K.; Kempa, M.; Grabowski, M.; Tajstra, M.; Sokal, A.; Cygankiewicz, I.; Zwoliński, R.; Michalak, M.; Kowara, M.; Budrejko, S.; et al. Multicentre early experience with totally subcutaneous cardioverter-defibrillators in Poland. Arch. Med. Sci. 2019, 16, 764–771. [Google Scholar] [CrossRef]
- Kempa, M.; Przybylski, A.; Budrejko, S.; Krupa, W.; Kaczmarek, K.; Kurek, A.; Syska, P.; Sokal, A.; Grabowski, M.; Jagielski, D.; et al. Multicenter Registry of Subcutaneous Cardioverter—Defibrillator Implantations: A preliminary report. Kardiol. Pol. 2021, 79, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Kempa, M.; Przybylski, A.; Budrejko, S.; Fabiszak, T.; Lewandowski, M.; Kaczmarek, K.; Tajstra, M.; Grabowski, M.; Mitkowski, P.; Tubek, S.; et al. Utilization of Subcutaneous Cardioverter-Defibrillator in Poland and Europe-Comparison of the Results of Multi-Center Registries. Int. J. Environ. Res. Public Health 2021, 8, 7178. [Google Scholar] [CrossRef]


| Device | Lead Impedance | Sensing (R Wave) | Pacing Threshold | Ventricular Pacing | Follow-Up |
|---|---|---|---|---|---|
| Teligen 100 (Boston Scientific) | 409 Ohm | 20 mV | 0.9 V/0.4 ms | 0% | No episodes of ventricular arrhythmias, no interventions |
| Parameter | Result |
|---|---|
| CRP (mg/L; normal 0–5) | <0.4 |
| PCT (ng/mL; normal 0–0.5) | 0.01 |
| D-dimer (ug/L; normal < 500) | 302 |
| Hemoglobin (g/L; normal 12–15) | 13.1 |
| RBC (T/L; normal 3.8–4.8) | 4.57 |
| PLT (G/L; normal 150–410) | 292 |
| WBC (G/L; normal 4–10) | 9.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budrejko, S.; Kempa, M.; Rohun, J.; Daniłowicz-Szymanowicz, L.; Zienciuk-Krajka, A.; Faran, A.; Raczak, G. Application of Novel Technologies in Cardiac Electrotherapy to Prevent Complications. Diagnostics 2023, 13, 1584. https://doi.org/10.3390/diagnostics13091584
Budrejko S, Kempa M, Rohun J, Daniłowicz-Szymanowicz L, Zienciuk-Krajka A, Faran A, Raczak G. Application of Novel Technologies in Cardiac Electrotherapy to Prevent Complications. Diagnostics. 2023; 13(9):1584. https://doi.org/10.3390/diagnostics13091584
Chicago/Turabian StyleBudrejko, Szymon, Maciej Kempa, Justyna Rohun, Ludmiła Daniłowicz-Szymanowicz, Agnieszka Zienciuk-Krajka, Anna Faran, and Grzegorz Raczak. 2023. "Application of Novel Technologies in Cardiac Electrotherapy to Prevent Complications" Diagnostics 13, no. 9: 1584. https://doi.org/10.3390/diagnostics13091584
APA StyleBudrejko, S., Kempa, M., Rohun, J., Daniłowicz-Szymanowicz, L., Zienciuk-Krajka, A., Faran, A., & Raczak, G. (2023). Application of Novel Technologies in Cardiac Electrotherapy to Prevent Complications. Diagnostics, 13(9), 1584. https://doi.org/10.3390/diagnostics13091584