Modeling Based on Ensemble Learning Methods for Detection of Diagnostic Biomarkers from LncRNA Data in Rats Treated with Cis-Platinum-Induced Hepatotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
- Control group (C): The group in which cisplatin vehicle solvent was given intraperitoneally on the first day of the experiment.
- Hepatotoxicity group (CK): The group in which 7 mg/kg cisplatin was given intraperitoneally on the first day of the experiment.
2.2. Performing Histopathological and Immunohistochemical Analysis in Liver Tissue
2.2.1. Histopathological Analysis
- 0 (no change);
- 1 (light);
- 2 (medium);
- 3 (severe) [21].
2.2.2. Immunohistochemical Analysis
2.3. Transcriptomic Analysis
2.3.1. Isolation and Quality Control of Total RNAs from Tissue Samples
2.3.2. NGS Library Preparation and Sequencing for lncRNA Sequences
- Ribosomal RNAs (rRNAs) were eliminated from total RNA, and the remaining RNAs were purified and fragmented.
- rRNA elimination was validated with the Bioanalyzer.
- Then, RNA fragments were reverse transcribed (first strand cDNA synthesis) using random hexamer sequences [23].
- Afterward, the RNA template was eliminated, and the second strand of cDNA (blunt ds cDNA) was synthesized [23].
- A single ‘A’ nucleotide was added to their 3’ ends to prevent the blunt ds cDNA fragments from binding together during the adapter ligation reaction.
- Indexing adapters were then added to hybridize the ds cDNA fragments to the flow cell surface.
- DNA fragments have been enriched.
- The libraries of the samples were normalized and pooled.
- Samples of 50M readings were made with the Illumina NovaSeq 6000 platform as a paired-end (PE) 2 × 150 base [24].
2.4. Data Analysis and Modeling Tasks
3. Results
3.1. Histopathological Results
3.2. Immunohistochemical Results
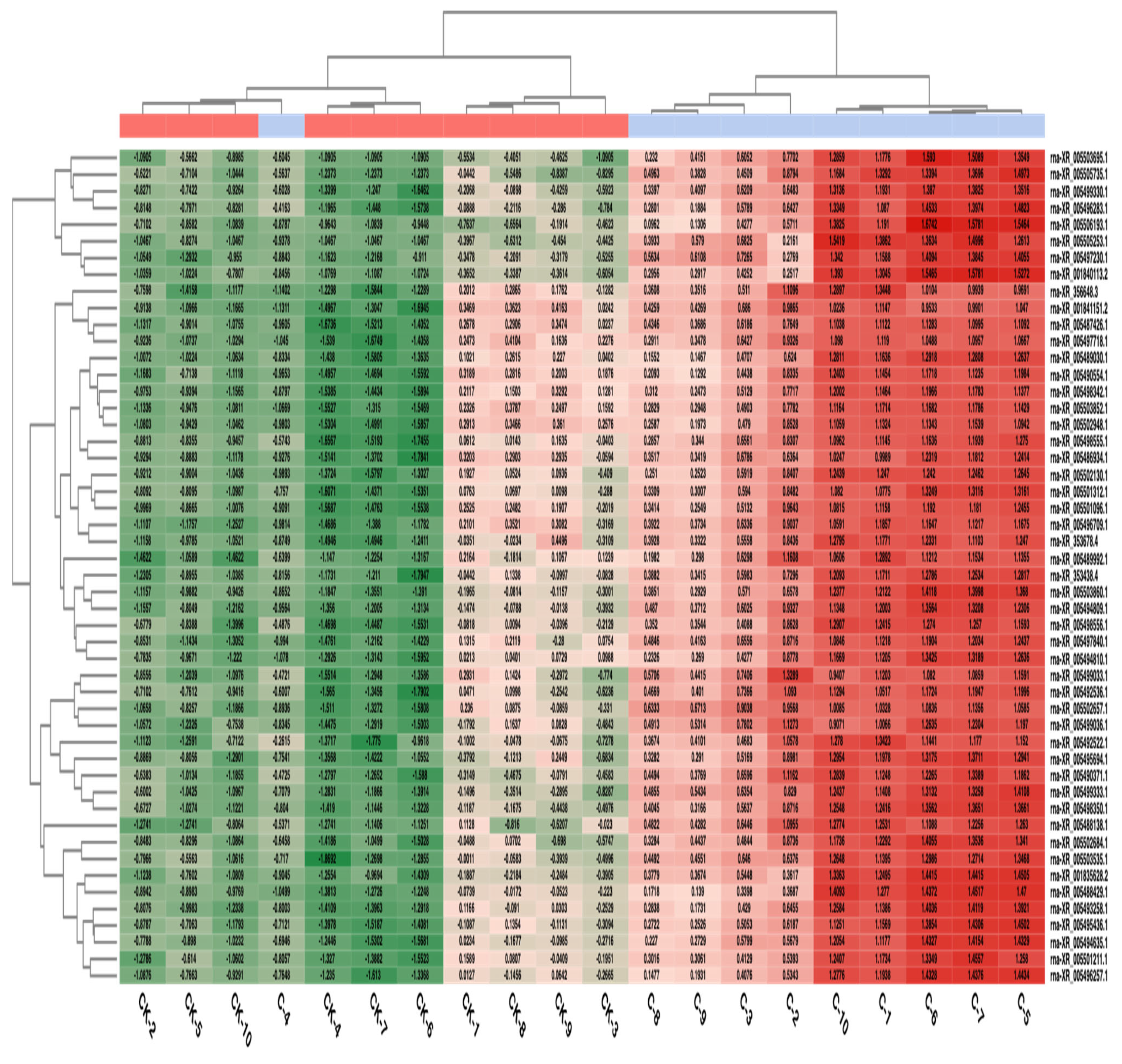
3.3. Transcriptomic Analysis Results
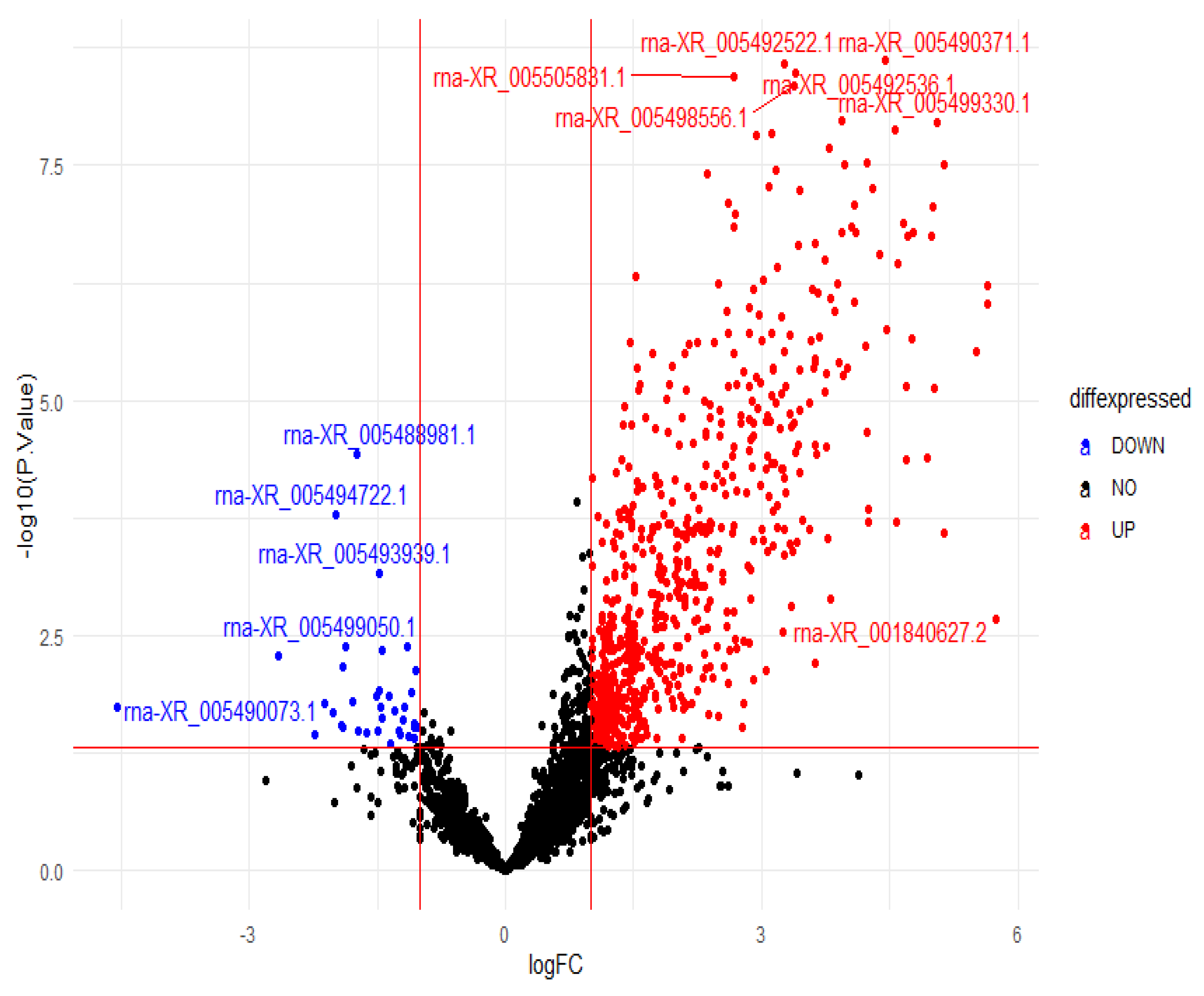
3.4. Differential Expression Results
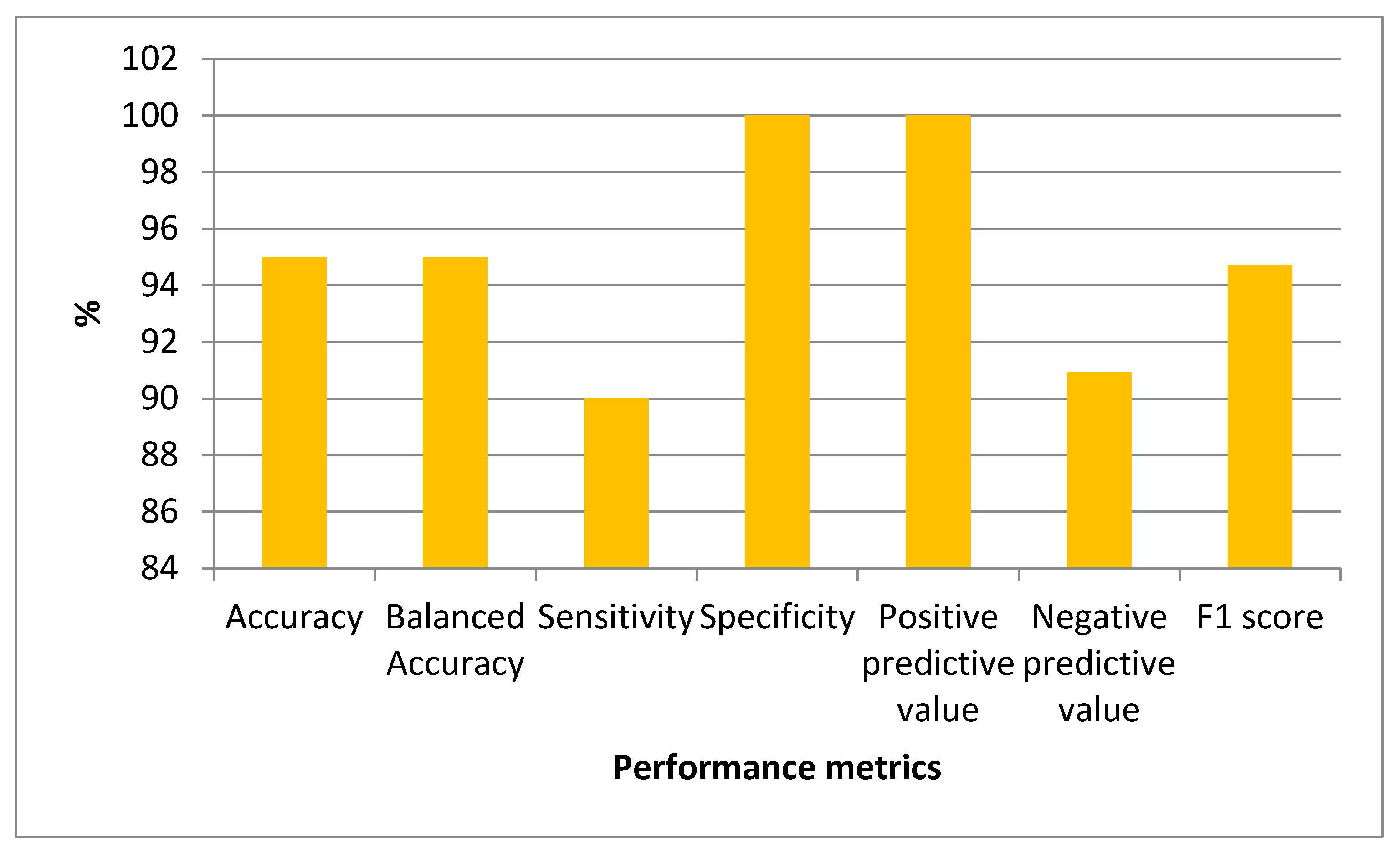
3.5. Biostatistics Analysis and Modeling Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tortora, G.J.; Derrickson, B.H. Introduction to the Human Body; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Zimmerman, H.J. Drug-induced liver disease. Clin. Liver Dis. 2000, 4, 73–96. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Gores, G.J.; Cederbaum, A.I.; Hinson, J.A.; Pessayre, D.; Lemasters, J.J. Mechanisms of hepatotoxicity. Toxicol. Sci. 2002, 65, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Ghabril, M.; Chalasani, N.; Björnsson, E. Drug-induced liver injury: A clinical update. Curr. Opin. Gastroenterol. 2010, 26, 222–226. [Google Scholar] [CrossRef]
- Ramachandran, R.; Kakar, S. Histological patterns in drug-induced liver disease. J. Clin. Pathol. 2009, 62, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Antoine, D.J.; Harrill, A.H.; Watkins, P.B.; Park, B.K. Safety biomarkers for drug-induced liver injury–current status and future perspectives. Toxicol. Res. 2013, 3, 75–85. [Google Scholar] [CrossRef]
- Kayaalp, S. Medical Pharmacology, in Terms of Rational Treatment (Rasyonel Tedavi Yonunden Tibbi Farmakoloji); Hacettepe-Tas Ltd.: Ankara, Turkey, 1998. [Google Scholar]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Aksoy, A.; Karaoglu, A.; Akpolat, N.; Naziroglu, M.; Ozturk, T.; Karagoz, Z.K. Protective role of selenium and high dose vitamin E against cisplatin-induced nephrotoxicty in rats. Asian Pac. J. Cancer Prev. 2015, 16, 6877–6882. [Google Scholar] [CrossRef]
- Naziroglu, M.; Karaoğlu, A.; Aksoy, A.O. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 2004, 195, 221–230. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef]
- Hunt, C.M.; Papay, J.I.; Edwards, R.I.; Theodore, D.; Alpers, D.H.; Dollery, C.; Debruin, T.W.; Adkison, K.K.; Stirnadel, H.A.; Gibbs, T.G. Monitoring liver safety in drug development: The GSK experience. Regul. Toxicol. Pharmacol. 2007, 49, 90–100. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Ağırbaşlı, D.; Ülman, Y.I. Coronary artery disease from a perspective of genomic risk score, ethical approaches and suggestions. Anadolu Kardiyol. Derg. AKD Anatol. J. Cardiol. 2012, 12, 171–177. [Google Scholar] [CrossRef]
- Yang, G.; Lu, X.; Yuan, L. LncRNA: A link between RNA and cancer. Biochim. Biophys. Acta 2014, 1839, 1097–1109. [Google Scholar] [CrossRef]
- Bai, H.; Wu, T.; Jiao, L.; Wu, Q.; Zhao, Z.; Song, J.; Liu, T.; Lv, Y.; Lu, X.; Ying, B. Association of ABCC Gene Polymorphism with Susceptibility to Antituberculosis Drug–Induced Hepatotoxicity in Western Han Patients with Tuberculosis. J. Clin. Pharmacol. 2020, 60, 361–368. [Google Scholar] [CrossRef]
- Aubrecht, J.; Schomaker, S.J.; Amacher, D.E. Emerging hepatotoxicity biomarkers and their potential to improve understanding and management of drug-induced liver injury. Genome Med. 2013, 5, 85. [Google Scholar] [CrossRef]
- Akman, M.; Genç, Y.; Ankarali, H. Random Forests Yöntemi ve Saglik Alaninda Bir Uygulama/Random Forests Methods and an Application in Health Science. Türkiye Klin. Biyoistatistik 2011, 3, 36. [Google Scholar]
- Witten, I.H.; Frank, E. Data mining: Practical machine learning tools and techniques with Java implementations. Acm Sigmod Rec. 2002, 31, 76–77. [Google Scholar] [CrossRef]
- Rokach, L.; Maimon, O. Clustering Methods. In Data Mining and Knowledge Discovery Handbook; Springer: Berlin/Heidelberg, Germany, 2005; pp. 321–352. [Google Scholar]
- Taslidere, E.; Dogan, Z.; Elbe, H.; Vardi, N.; Cetin, A.; Turkoz, Y. Quercetin protection against ciprofloxacin induced liver damage in rats. Biotech. Histochem. Off. Publ. Biol. Stain. Comm. 2016, 91, 116–121. [Google Scholar] [CrossRef]
- Parlakpinar, H.; Ozhan, O.; Ermis, N.; Vardi, N.; Cigremis, Y.; Tanriverdi, L.H.; Colak, C.; Acet, A. Acute and Subacute Effects of Low Versus High Doses of Standardized Panax ginseng Extract on the Heart: An Experimental Study. Cardiovasc. Toxicol. 2019, 19, 306–320. [Google Scholar] [CrossRef]
- Napolitano, M.; Comegna, M.; Succoio, M.; Leggiero, E.; Pastore, L.; Faraonio, R.; Cimino, F.; Passaro, F. Comparative analysis of gene expression data reveals novel targets of senescence-associated microRNAs. PLoS ONE 2014, 9, e98669. [Google Scholar] [CrossRef]
- Arrigoni, A.; Ranzani, V.; Rossetti, G.; Panzeri, I.; Abrignani, S.; Bonnal, R.J.; Pagani, M. Analysis RNA-seq and Noncoding RNA. Methods Mol. Biol. 2016, 1480, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Bioulac-Sage, P.; Balabaud, C. Toxic and drug-induced disorders of the liver. In Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1059–1086. [Google Scholar]
- Teoh, N.C.; Farrell, G.C. Liver Disease Caused by Drugs. U: Sleisenger and Fordtran’s, ur. Gastrointestinal and Liver Disease 9. izd. 2006. Available online: https://clinicalgate.com/liver-disease-caused-by-drugs/ (accessed on 23 April 2023).
- Lee, W.M. Acute liver failure. Semin. Respir. Crit. Care Med. 2012, 33, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Holt, M.P.; Ju, C. Mechanisms of drug-induced liver injury. AAPS J. 2006, 8, E48–E54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef]
- Bilgic, Y.; Akbulut, S.; Aksungur, Z.; Erdemli, M.E.; Ozhan, O.; Parlakpinar, H.; Vardi, N.; Turkoz, Y. Protective effect of dexpanthenol against cisplatin-induced hepatotoxicity. Exp. Ther. Med. 2018, 16, 4049–4057. [Google Scholar] [CrossRef]
- El-Sayyad, H.I.; Ismail, M.F.; Shalaby, F.M.; Abou-El-Magd, R.F.; Gaur, R.L.; Fernando, A.; Raj, M.H.; Ouhtit, A. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int. J. Biol. Sci. 2009, 5, 466–473. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Jäättelä, M. Heat shock proteins as cellular lifeguards. Ann. Med. 1999, 31, 261–271. [Google Scholar] [CrossRef]
- Gupta, R.S.; Ramachandra, N.B.; Bowes, T.; Singh, B. Unusual cellular disposition of the mitochondrial molecular chaperones Hsp60, Hsp70 and Hsp10. Novartis Found. Symp. 2008, 291, 59–140. [Google Scholar] [CrossRef]
- Merendino, A.M.; Bucchieri, F.; Campanella, C.; Marcianò, V.; Ribbene, A.; David, S.; Zummo, G.; Burgio, G.; Corona, D.F.; Conway de Macario, E.; et al. Hsp60 is actively secreted by human tumor cells. PLoS ONE 2010, 5, e9247. [Google Scholar] [CrossRef]
- Henderson, B.; Fares, M.A.; Lund, P.A. Chaperonin 60: A paradoxical, evolutionarily conserved protein family with multiple moonlighting functions. Biol. Rev. Camb. Philos. Soc. 2013, 88, 955–987. [Google Scholar] [CrossRef]
- Hoter, A.; Rizk, S.; Naim, H.Y. Heat Shock Protein 60 in Hepatocellular Carcinoma: Insights and Perspectives. Front. Mol. Biosci. 2020, 7, 60. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, H.; Zheng, C.; Gao, J.; Fu, Q.; Hu, N.; Shao, X.; Zhou, Y.; Xiong, J.; Nie, K.; et al. Oncogenic HSP60 regulates mitochondrial oxidative phosphorylation to support Erk1/2 activation during pancreatic cancer cell growth. Cell Death Dis. 2018, 9, 161. [Google Scholar] [CrossRef]
- Meng, Q.; Li, B.X.; Xiao, X. Toward Developing Chemical Modulators of Hsp60 as Potential Therapeutics. Front. Mol. Biosci. 2018, 5, 35. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, Q.; Fu, X.Y.; Luo, W.S. Heat shock protein 60 overexpression is associated with the progression and prognosis in gastric cancer. PLoS ONE 2014, 9, e107507. [Google Scholar] [CrossRef]
- Theocharis, S.E.; Kanelli, H.; Margeli, A.P.; Spiliopoulou, C.A.; Koutselinis, A.S. Metallothionein and heat shock protein expression during acute liver injury and regeneration in rats. Clin. Chem. Lab. Med. 2000, 38, 1137–1140. [Google Scholar] [CrossRef]
- Theocharis, S.E.; Margeli, A.P.; Koutselinis, A. Metallothionein: A multifunctional protein from toxicity to cancer. Int. J. Biol. Markers 2003, 18, 162–169. [Google Scholar] [CrossRef]
- Wen, C.; Yang, S.; Zheng, S.; Feng, X.; Chen, J.; Yang, F. Analysis of long non-coding RNA profiled following MC-LR-induced hepatotoxicity using high-throughput sequencing. J. Toxicol. Environ. Health A 2018, 81, 1165–1172. [Google Scholar] [CrossRef]
- Zeng, N.; Zhang, Z.; Jiang, H.; Li, R.; Chang, C.; Wang, F.; Xu, D.; Fan, Q.; Wang, T.; Xiao, Y.; et al. LncRNA-241 inhibits 1,2-Dichloroethane-induced hepatic apoptosis. Toxicol. Vitr. 2019, 61, 104650. [Google Scholar] [CrossRef] [PubMed]
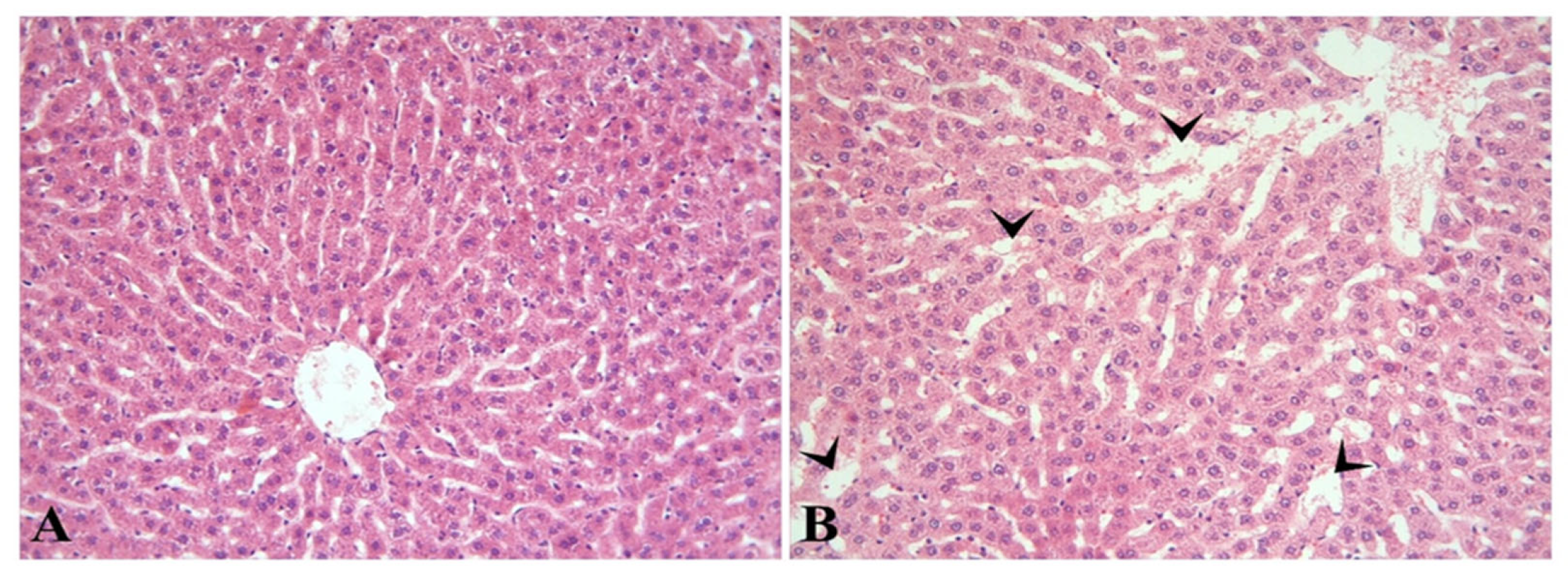



| Groups * | Hepatocyte Degeneration | Sinusoidal Dilation | Hsp60 Immunoreactivity |
|---|---|---|---|
| Control | 0 (0–2) | 0 (0–2) | 2 (0–9) |
| Hepatotoxicity | 0 (0–2) | 1 (0–2) a | 4 (0–12) a |
| Variables | Mean ± SD |
|---|---|
| Rat weight starting (g) | 230.2 ± 15.44 |
| Rat weight end (g) | 228.7 ± 14.974 |
| Liver weight (g) | 8.019 ± 0.844 |
| Variables | Control | Hepatotoxicity |
|---|---|---|
| Rat weight starting (g) | 227.2 ± 19.96 | 233.2 ± 9.211 |
| Rat weight end (g) | 234.2 ± 16.705 | 223.2 ± 11.272 |
| Liver weight (g) | 7.92 ± 1.103 | 8.117 ± 0.515 |
| Gene Name | Chromosome | ID | Group | LogFC | p | |||
|---|---|---|---|---|---|---|---|---|
| CK | C | |||||||
| Mean ± SD | Median (Min-Max) | Mean ± SD | Median (Min-Max) | |||||
| LOC120094596 | NC_051344.1 | rna_XR_005488981.1 | 43.6 ± 15.665 | 39 (20–74) | 12.9 ± 9.291 | 11.5 (0–32) | −2.58 | <0.001 * |
| LOC120097437 | NC_051351.1 | rna_XR_005494824.1 | 0.6 ± 0.843 | 0 (0–2) | 3.8 ± 1.033 | 4 (2–6) | 2.04 | <0.001 ** |
| LOC120096352 | NC_051348.1 | rna_XR_005492760.1 | 1.8 ± 2.044 | 1 (0–5) | 12.5 ± 5.126 | 13.5 (4–18) | 2.38 | <0.001 ** |
| LOC120102815 | NC_051340.1 | rna_XR_005504579.1 | 1.5 ± 1.269 | 1.5 (0–3) | 8 ± 3.712 | 8 (2–14) | 1.80 | <0.001 * |
| LOC120101756 | NC_051338.1 | rna_XR_005502657.1 | 31.8 ± 31.091 | 18.5 (7–108) | 402.7 ± 151.646 | 424 (43–548) | 3.34 | <0.001 ** |
| LOC120099881 | NC_051336.1 | rna_XR_005499304.1 | 4.7 ± 4.572 | 4 (0–14) | 49.8 ± 18.66 | 55.5 (7–75) | 2.93 | <0.001 * |
| LOC102557053 | NC_051341.1 | rna_XR_005505831.1 | 54.3 ± 19.402 | 50 (28–89) | 351.9 ± 115.959 | 364.5 (107–484) | 2 | <0.001 * |
| LOC103693406 | NC_051345.1 | rna_XR_005490393.1 | 8.9 ± 7.534 | 7 (1–23) | 57.8 ± 17.536 | 63 (16–77) | 2.29 | <0.001 ** |
| LOC120098296 | NC_051353.1 | rna_XR_005496472.1 | 3.9 ± 4.332 | 3 (0–14) | 13.4 ± 5.358 | 13 (7–23) | 1.38 | 0.001 ** |
| LOC120094640 | NC_051344.1 | rna_XR_005489054.1 | 5 ± 5.85 | 2.5 (0–17) | 28.6 ± 8.708 | 29 (9–38) | 2.19 | <0.001 ** |
| LOC102552566 | NC_051336.1 | rna_XR_001836079.2 | 1.2 ± 0.919 | 1 (0–3) | 9.6 ± 3.565 | 10 (3–16) | 2.40 | 0.001 * |
| LOC120096990 | NC_051350.1 | rna_XR_005493970.1 | 2.1 ± 1.37 | 2.5 (0–4) | 20.6 ± 8.669 | 21 (3–31) | 2.69 | <0.001 * |
| LOC120096276 | NC_051348.1 | rna_XR_005492536.1 | 213.2 ± 123.706 | 194.5 (64–482) | 2101.1 ± 720.824 | 2139 (586–2927) | 2.63 | <0.001 * |
| LOC120099800 | NC_051336.1 | rna_XR_005499033.1 | 14.5 ± 15.58 | 8 (4–54) | 168.9 ± 67.258 | 162.5 (49–310) | 3.10 | <0.001 ** |
| LOC102553540 | NC_051351.1 | rna_XR_360532.3 | 1.4 ± 1.713 | 1 (0–5) | 17.7 ± 7.273 | 19 (5–30) | 2.99 | <0.001 ** |
| LOC120096269 | NC_051348.1 | rna_XR_005492522.1 | 5.4 ± 3.627 | 5 (0–11) | 50.7 ± 11.47 | 55 (30–64) | 2.67 | <0.001 * |
| LOC120094133 | NC_051343.1 | rna_XR_005488138.1 | 2.7 ± 3.889 | 1 (0–12) | 56.7 ± 21.386 | 60.5 (11–81) | 3.98 | <0.001 ** |
| Model | Metric | Value (%) (95% CI) |
|---|---|---|
| Bagging | Acc | 85 (69.4–100) |
| B-Acc | 85 (69.4–100) | |
| Se | 70 (34.75–93.33) | |
| Sp | 100 (69.15–100) | |
| Ppv | 100 (59–100) | |
| Npv | 76.92 (56.39–89.57) | |
| F1-score | 82.4 (65.6–99.1) | |
| Boosting | Acc | 85 (69.4–100) |
| B-Acc | 85 (69.4–100) | |
| Se | 70 (34.75–93.33) | |
| Sp | 100 (69.15–100) | |
| Ppv | 100 (59–100) | |
| Npv | 76.92 (56.39–89.57) | |
| F1-score | 82.4 (65.6–99.1) | |
| Stacking | Acc | 90 (68.3–98.77) |
| B-Acc | 90 (68.3–98.77) | |
| Se | 80 (44.39–97.48) | |
| Sp | 100 (69.15–100) | |
| Ppv | 100 | |
| Npv | 83.3 (59.14–94.53) | |
| F1-score | 88.9 (75.1–100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucukakcali, Z.; Colak, C.; Gozukara Bag, H.G.; Balikci Cicek, I.; Ozhan, O.; Yildiz, A.; Danis, N.; Koc, A.; Parlakpinar, H.; Akbulut, S. Modeling Based on Ensemble Learning Methods for Detection of Diagnostic Biomarkers from LncRNA Data in Rats Treated with Cis-Platinum-Induced Hepatotoxicity. Diagnostics 2023, 13, 1583. https://doi.org/10.3390/diagnostics13091583
Kucukakcali Z, Colak C, Gozukara Bag HG, Balikci Cicek I, Ozhan O, Yildiz A, Danis N, Koc A, Parlakpinar H, Akbulut S. Modeling Based on Ensemble Learning Methods for Detection of Diagnostic Biomarkers from LncRNA Data in Rats Treated with Cis-Platinum-Induced Hepatotoxicity. Diagnostics. 2023; 13(9):1583. https://doi.org/10.3390/diagnostics13091583
Chicago/Turabian StyleKucukakcali, Zeynep, Cemil Colak, Harika Gozde Gozukara Bag, Ipek Balikci Cicek, Onural Ozhan, Azibe Yildiz, Nefsun Danis, Ahmet Koc, Hakan Parlakpinar, and Sami Akbulut. 2023. "Modeling Based on Ensemble Learning Methods for Detection of Diagnostic Biomarkers from LncRNA Data in Rats Treated with Cis-Platinum-Induced Hepatotoxicity" Diagnostics 13, no. 9: 1583. https://doi.org/10.3390/diagnostics13091583
APA StyleKucukakcali, Z., Colak, C., Gozukara Bag, H. G., Balikci Cicek, I., Ozhan, O., Yildiz, A., Danis, N., Koc, A., Parlakpinar, H., & Akbulut, S. (2023). Modeling Based on Ensemble Learning Methods for Detection of Diagnostic Biomarkers from LncRNA Data in Rats Treated with Cis-Platinum-Induced Hepatotoxicity. Diagnostics, 13(9), 1583. https://doi.org/10.3390/diagnostics13091583








