Simultaneous, Multi-Channel, Near-Infrared Fluorescence Visualization of Mesenteric Lymph Nodes Using Indocyanine Green and Methylene Blue: A Demonstration in a Porcine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Fluorescence Imaging System
2.2. Preparation of the Dyes
2.3. Surgical Procedure
2.4. Image Acquisition and Statistical Analysis
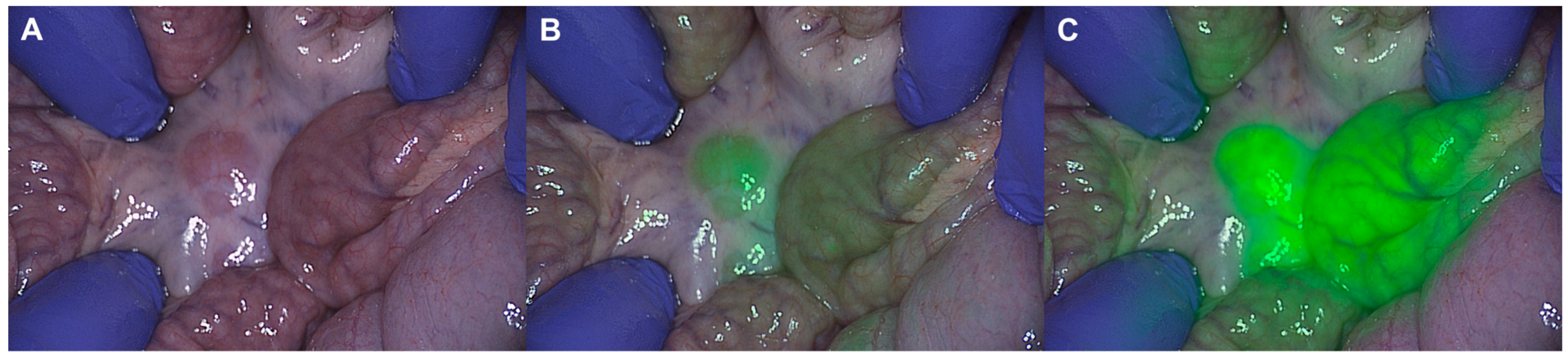
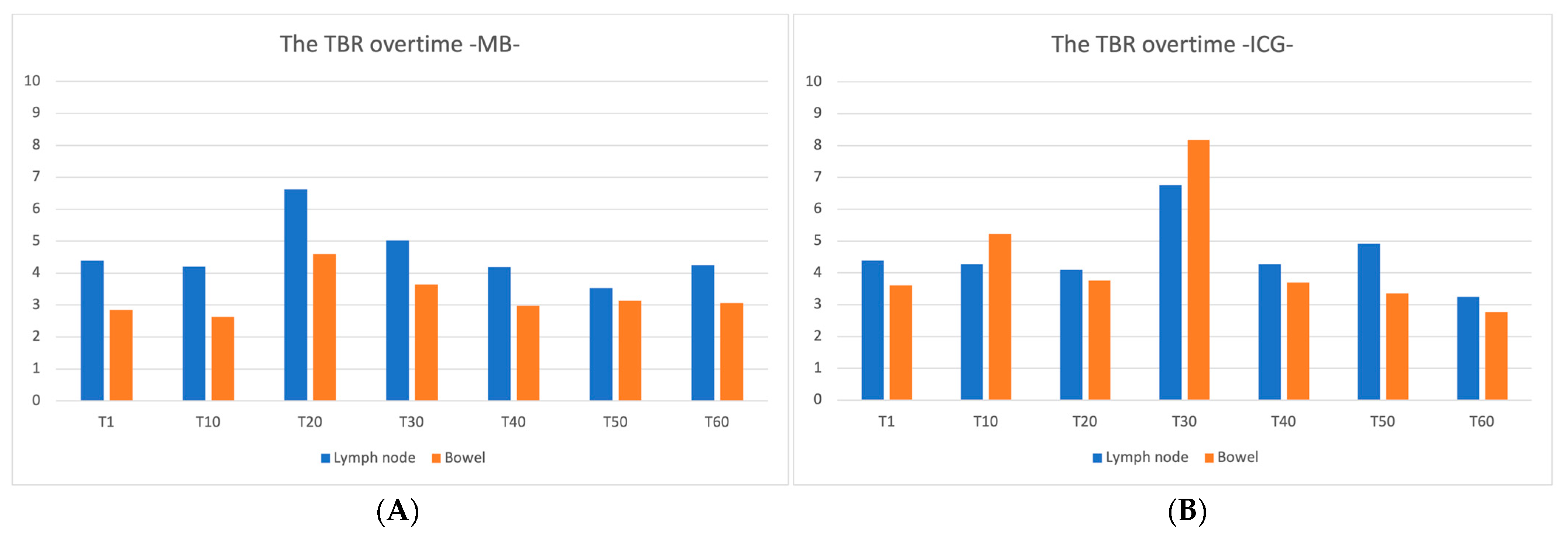
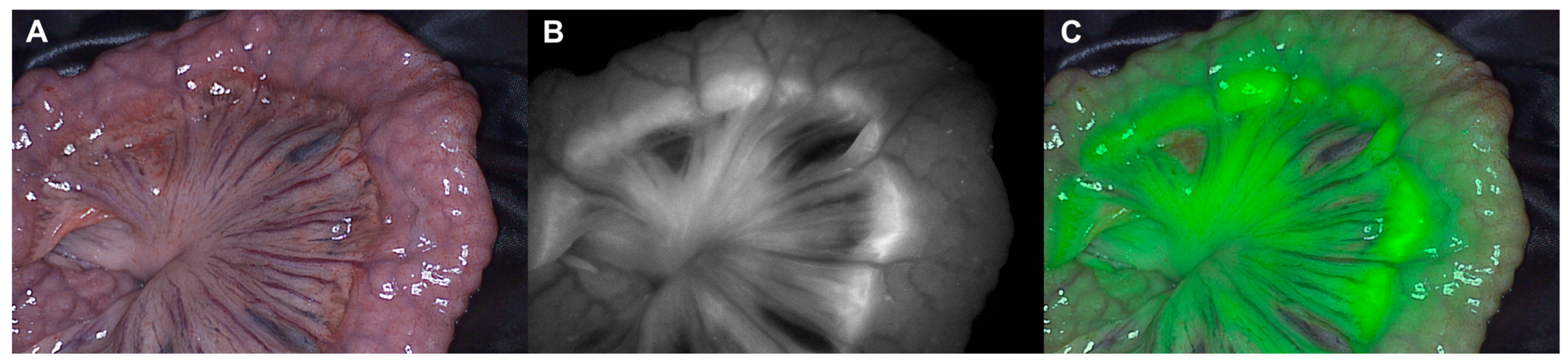
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 3 September 2020).
- Heald, R.J.; Husband, E.M.; Ryall, R.D. The mesorectum in rectal cancer surgery—The clue to pelvic recurrence? Br. J. Surg. 1982, 69, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Enker, W.E.; Havenga, K.; Thaler, H.T.; Cohen, A.M.; Polyak, T.; Cranor, M.; McDermott, K. Total Mesorectal Excision with Pelvic Autonomic Nerve Preservation in the Operative Treatment of Rectal Carcinoma. Rectal Cancer Surg. 1997, 220–243. [Google Scholar] [CrossRef]
- Emile, S.H.; de Lacy, F.B.; Keller, D.S.; Martin-Perez, B.; Alrawi, S.; Lacy, A.M.; Chand, M. Evolution of transanal total mesorectal excision for rectal cancer: From top to bottom. World, J. Gastrointest. Surg. 2018, 10, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Steele, R.J. Anterior resection with total mesorectal excision. J. R. Coll. Surg. Edinb. 1999, 44, 40–45. [Google Scholar] [PubMed]
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation—Technical notes and outcome. Color. Dis. 2009, 11, 354–364. [Google Scholar] [CrossRef]
- West, N.P.; Hohenberger, W.; Weber, K.; Perrakis, A.; Finan, P.J.; Quirke, P. Complete Mesocolic Excision With Central Vascular Ligation Produces an Oncologically Superior Specimen Compared With Standard Surgery for Carcinoma of the Colon. J. Clin. Oncol. 2010, 28, 272–278. [Google Scholar] [CrossRef]
- Zheng, M.-H.; Zhang, S.; Feng, B. Complete mesocolic excision: Lessons from anatomy translating to better oncologic outcome. World J. Gastrointest. Oncol. 2016, 8, 235. [Google Scholar] [CrossRef]
- Simoni, O.D.; De Simoni, O.; Barina, A.; Sommariva, A.; Tonello, M.; Mario, G.; Mattara, G.; Toniato, A.; Pilati, P.; Franzato, B. Complete mesocolic excision versus conventional hemicolectomy in patients with right colon cancer: A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 881–892. [Google Scholar] [CrossRef]
- Olofsson, F.; Buchwald, P.; Elmståhl, S.; Syk, I. Wide excision in right-sided colon cancer is associated with decreased survival. Scand. J. Surg. 2013, 102, 241–245. [Google Scholar] [CrossRef]
- Olofsson, F.; Buchwald, P.; Elmståhl, S.; Syk, I. No benefit of extended mesenteric resection with central vascular ligation in right-sided colon cancer. Color. Dis. 2016, 18, 773–778. [Google Scholar] [CrossRef]
- Bertelsen, C.A.; Kirkegaard-Klitbo, A.; Nielsen, M.; Leotta, S.M.G.; Daisuke, F.; Gögenur, I. Pattern of Colon Cancer Lymph Node Metastases in Patients Undergoing Central Mesocolic Lymph Node Excision: A Systematic Review. Dis. Colon Rectum 2016, 59, 1209–1221. [Google Scholar] [CrossRef]
- Alhassan, N.; Yang, M.; Wong-Chong, N.; Liberman, A.S.; Charlebois, P.; Stein, B.; Fried, G.M.; Lee, L. Comparison between conventional colectomy and complete mesocolic excision for colon cancer: A systematic review and pooled analysis: A review of CME versus conventional colectomies. Surg. Endosc. 2019, 33, 8–18. [Google Scholar] [CrossRef]
- Díaz-Vico, T.; Fernández-Hevia, M.; Suárez-Sánchez, A.; García-Gutiérrez, C.; Mihic-Góngora, L.; Fernández-Martínez, D.; Álvarez-Pérez, J.A.; Otero-Díez, J.L.; Granero-Trancón, J.E.; García-Flórez, L.J. Complete Mesocolic Excision and D3 Lymphadenectomy versus Conventional Colectomy for Colon Cancer: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2021, 28, 8823–8837. [Google Scholar] [CrossRef]
- Nishigori, N.; Koyama, F.; Nakagawa, T.; Nakamura, S.; Ueda, T.; Inoue, T.; Kawasaki, K.; Obara, S.; Nakamoto, T.; Fujii, H.; et al. Visualization of Lymph/Blood Flow in Laparoscopic Colorectal Cancer Surgery by ICG Fluorescence Imaging (Lap-IGFI). Ann. Surg. Oncol. 2016, 23, 266–274. [Google Scholar] [CrossRef]
- Agnus, V.; Pesce, A.; Boni, L.; Van Den Bos, J.; Morales-Conde, S.; Paganini, A.M.; Quaresima, S.; Balla, A.; La Greca, G.; Plaudis, H.; et al. Fluorescence-based cholangiography: Preliminary results from the IHU-IRCAD-EAES EURO-FIGS registry. Surg. Endosc. 2020, 34, 3888–3896. [Google Scholar] [CrossRef]
- Keller, D.S.; Joshi, H.M.; Rodriguez-Justo, M.; Walsh, D.; Coffey, J.C.; Chand, M. Using fluorescence lymphangiography to define the ileocolic mesentery: Proof of concept for the watershed area using real-time imaging. Tech. Coloproctology 2017, 21, 757–760. [Google Scholar] [CrossRef]
- Okuno, K. Surgical treatment for digestive cancer. Current issues—Colon cancer. Dig. Surg. 2007, 24, 108–114. [Google Scholar] [CrossRef]
- Enker, W.E.; Laffer, U.T.; Block, G.E. Enhanced survival of patients with colon and rectal cancer is based upon wide anatomic resection. Ann. Surg. 1979, 190, 350–360. [Google Scholar] [CrossRef]
- West, N.P.; Kobayashi, H.; Takahashi, K.; Perrakis, A.; Weber, K.; Hohenberger, W.; Sugihara, K.; Quirke, P. Understanding optimal colonic cancer surgery: Comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J. Clin. Oncol. 2012, 30, 1763–1769. [Google Scholar] [CrossRef]
- Dip, F.; Boni, L.; Bouvet, M.; Carus, T.; Diana, M.; Falco, J.; Gurtner, G.C.; Ishizawa, T.; Kokudo, N.; Menzo, E.L.; et al. Consensus Conference Statement on the General Use of Near-infrared Fluorescence Imaging and Indocyanine Green Guided Surgery: Results of a Modified Delphi Study. Ann. Surg. 2022, 275, 685–691. [Google Scholar] [CrossRef]
- Nguyen, J.Q.M.; McWade, M.; Thomas, G.; Beddard, B.T.; Herington, J.L.; Paria, B.C.; Schwartz, H.S.; Halpern, J.L.; Holt, G.E.; Mahadevan-Jansen, A. Development of a modular fluorescence overlay tissue imaging system for wide-field intraoperative surgical guidance. J. Med. Imaging 2018, 5, 021220. [Google Scholar] [CrossRef] [PubMed]
- Dzurinko, V.L.; Gurwood, A.S.; Price, J.R. Intravenous and indocyanine green angiography. Optometry 2004, 75, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Novotny, H.R.; Alvis, D.L. A Method of Photographing Fluorescence in Circulating Blood in the Human Retina. Circulation 1961, 24, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Dornseifer, U.; Sturtz, G.; Ninkovic, M. Sensitivity and specificity of ICG angiography in free flap reexploration. J. Reconstr. Microsurg. 2010, 26, 311–316. [Google Scholar] [CrossRef]
- Perry, D.; Bharara, M.; Armstrong, D.G.; Mills, J. Intraoperative fluorescence vascular angiography: During tibial bypass. J. Diabetes Sci. Technol. 2012, 6, 204–208. [Google Scholar] [CrossRef]
- Benya, R.; Quintana, J.; Brundage, B. Adverse reactions to indocyanine green: A case report and a review of the literature. Catheter. Cardiovasc. Diagn. 1989, 17, 231–233. [Google Scholar] [CrossRef]
- Nagata, J.; Fukunaga, Y.; Akiyoshi, T.; Konishi, T.; Fujimoto, Y.; Nagayama, S.; Yamamoto, N.; Ueno, M. Colonic Marking With Near-Infrared, Light-Emitting, Diode-Activated Indocyanine Green for Laparoscopic Colorectal Surgery. Dis. Colon Rectum 2016, 59, e14–e18. [Google Scholar] [CrossRef]
- Jafari, M.D.; Lee, K.H.; Halabi, W.J.; Mills, S.D.; Carmichael, J.C.; Stamos, M.J.; Pigazzi, A. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg. Endosc. 2013, 27, 3003–3008. [Google Scholar] [CrossRef]
- Jafari, M.D.; Wexner, S.D.; Martz, J.E.; McLemore, E.C.; Margolin, D.A.; Sherwinter, D.A.; Lee, S.W.; Senagore, A.J.; Phelan, M.J.; Stamos, M.J. Perfusion Assessment in Laparoscopic Left-Sided/Anterior Resection (PILLAR II): A Multi-Institutional Study. J. Am. Coll. Surg. 2015, 220, 82–92.e1. [Google Scholar] [CrossRef]
- Kudszus, S.; Roesel, C.; Schachtrupp, A.; Höer, J.J. Intraoperative laser fluorescence angiography in colorectal surgery: A noninvasive analysis to reduce the rate of anastomotic leakage. Langenbeck’s Arch. Surg. 2010, 395, 1025–1030. [Google Scholar] [CrossRef]
- Sherwinter, D.A.; Gallagher, J.; Donkar, T. Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: A feasibility study. Color. Dis. 2013, 15, 91–96. [Google Scholar] [CrossRef]
- Spota, A.; Al-Taher, M.; Felli, E.; Morales Conde, S.; Dal Dosso, I.; Moretto, G.; Spinoglio, G.; Baiocchi, G.; Vilallonga, R.; Impellizzeri, H.; et al. Fluorescence-based bowel anastomosis perfusion evaluation: Results from the IHU-IRCAD-EAES EURO-FIGS registry. Surg. Endosc. 2021, 35, 7142–7153. [Google Scholar] [CrossRef]
- van Manen, L.; Handgraaf, H.J.M.; Diana, M.; Dijkstra, J.; Ishizawa, T.; Vahrmeijer, A.L.; Mieog, J.S.D. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J. Surg. Oncol. 2018, 118, 283–300. [Google Scholar] [CrossRef]
- Cahill, R.A.; Anderson, M.; Wang, L.M.; Lindsey, I.; Cunningham, C.; Mortensen, N.J. Near-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasia. Surg. Endosc. 2012, 26, 197–204. [Google Scholar] [CrossRef]
- Kusano, M.; Tajima, Y.; Yamazaki, K.; Kato, M.; Watanabe, M.; Miwa, M. Sentinel Node Mapping Guided by Indocyanine Green Fluorescence Imaging: A New Method for Sentinel Node Navigation Surgery in Gastrointestinal Cancer. Dig. Surg. 2008, 25, 103–108. [Google Scholar] [CrossRef]
- Verbeek, F.P.R.; Tummers, Q.R.J.; Rietbergen, D.D.D.; Peters, A.A.W.; Schaafsma, B.E.; van de Velde, C.J.H.; Frangioni, J.V.; van Leeuwen, F.W.B.; Gaarenstroom, K.N.; Vahrmeijer, A.L. Sentinel Lymph Node Biopsy in Vulvar Cancer Using Combined Radioactive and Fluorescence Guidance. Int. J. Gynecol. Cancer 2015, 25, 1086–1093. [Google Scholar] [CrossRef]
- Watanabe, J.; Ota, M.; Suwa, Y.; Ishibe, A.; Masui, H.; Nagahori, K. Evaluation of lymph flow patterns in splenic flexural colon cancers using laparoscopic real-time indocyanine green fluorescence imaging. Int. J. Colorectal Dis. 2017, 32, 201–207. [Google Scholar] [CrossRef]
- Raabe, A.; Nakaji, P.; Beck, J.; Kim, L.J.; Hsu, F.P.K.; Kamerman, J.D.; Seifert, V.; Spetzler, R.F. Prospective evaluation of surgical microscope—Integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J. Neurosurg. 2005, 103, 982–989. [Google Scholar] [CrossRef]
- Cwalinski, T.; Polom, W.; Marano, L.; Roviello, G.; D’Angelo, A.; Cwalina, N.; Matuszewski, M.; Roviello, F.; Jaskiewicz, J.; Polom, K. Methylene Blue—Current Knowledge, Fluorescent Properties, and Its Future Use. J. Clin. Med. 2020, 9, 3538. [Google Scholar] [CrossRef]
- Barnes, T.G.; Hompes, R.; Birks, J.; Mortensen, N.J.; Jones, O.; Lindsey, I.; Guy, R.; George, B.; Cunningham, C.; Yeung, T.M. Methylene blue fluorescence of the ureter during colorectal surgery. Surg. Endosc. 2018, 32, 4036–4043. [Google Scholar] [CrossRef]
- Al-Taher, M.; van den Bos, J.; Schols, R.M.; Bouvy, N.D.; Stassen, L.P.S. Fluorescence Ureteral Visualization in Human Laparoscopic Colorectal Surgery Using Methylene Blue. J. Laparoendosc. Adv. Surg. Tech. 2016, 26, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, F.P.R.; van der Vorst, J.R.; Schaafsma, B.E.; Swijnenburg, R.-J.; Gaarenstroom, K.N.; Elzevier, H.W.; van de Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L. Intraoperative Near Infrared Fluorescence Guided Identification of the Ureters Using Low Dose Methylene Blue: A First in Human Experience. J. Urol. 2013, 190, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Staniloaie, D.; Budin, C.; Vasile, D.; Iancu, G.; Ilco, A.; Voiculescu, D.I.; Trandafir, A.F.; Ammar, T.; Suliman, E.; Suliman, E.; et al. Role of methylene blue in detecting the sentinel lymph node in colorectal cancer: In vivo vs. ex vivo technique. Exp. Ther. Med. 2021, 23, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Märkl, B.; Kerwel, T.G.; Wagner, T.; Anthuber, M.; Arnholdt, H.M. Methylene blue injection into the rectal artery as a simple method to improve lymph node harvest in rectal cancer. Mod. Pathol. 2007, 20, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, P.; Zheng, Z.; Chen, T.; Wei, H. Modified methylene blue injection improves lymph node harvest in rectal cancer. ANZ J. Surg. 2017, 87, 247–251. [Google Scholar] [CrossRef]
- Polom, W.; Migaczewski, M.; Skokowski, J.; Swierblewski, M.; Cwalinski, T.; Kalinowski, L.; Pedziwiatr, M.; Matuszewski, M.; Polom, K. Multispectral Imaging Using Fluorescent Properties of Indocyanine Green and Methylene Blue in Colorectal Surgery-Initial Experience. J. Clin. Med. 2022, 11, 368. [Google Scholar] [CrossRef]
- Le Voyer, T.E.; Sigurdson, E.R.; Hanlon, A.L.; Mayer, R.J.; Macdonald, J.S.; Catalano, P.J.; Haller, D.G. Colon cancer survival is associated with increasing number of lymph nodes analyzed: A secondary survey of intergroup trial INT-0089. J. Clin. Oncol. 2003, 21, 2912–2919. [Google Scholar] [CrossRef]
- Swanson, R.S.; Compton, C.C.; Stewart, A.K.; Bland, K.I. The Prognosis of T3N0 Colon Cancer Is Dependent on the Number of Lymph Nodes Examined. Ann. Surg. Oncol. 2003, 10, 65–71. [Google Scholar] [CrossRef]
- National Cancer Institute. 2020 AJCC Cancer Staging Manual, 8th ed.; Definitions; National Cancer Institute: Bethesda, MD, USA, 2020. [CrossRef]
- Compton, C.C. Key issues in reporting common cancer specimens: Problems in pathologic staging of colon cancer. Arch. Pathol. Lab. Med. 2006, 130, 318–324. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Wong, S.L.; Ji, H.; Hollenbeck, B.K.; Morris, A.M.; Baser, O.; Birkmeyer, J.D. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA 2007, 298, 2149–2154. [Google Scholar] [CrossRef]
- Goldstein, N.S. Lymph Node Recoveries From 2427 pT3 Colorectal Resection Specimens Spanning 45 Years. Am. J. Surg. Pathol. 2002, 26, 179–189. [Google Scholar] [CrossRef]
- Emile, S.H.; Elfeki, H.; Shalaby, M.; Sakr, A.; Sileri, P.; Laurberg, S.; Wexner, S.D. Sensitivity and specificity of indocyanine green near-infrared fluorescence imaging in detection of metastatic lymph nodes in colorectal cancer: Systematic review and meta-analysis. J. Surg. Oncol. 2017, 116, 730–740. [Google Scholar] [CrossRef]
- van der Pas, M.H.; Ankersmit, M.; Stockmann, H.B.; Silvis, R.; van Grieken, N.C.; Bril, H.; Meijerink, W.J. Laparoscopic Sentinel Lymph Node Identification in Patients with Colon Carcinoma Using a Near-Infrared Dye: Description of a New Technique and Feasibility Study. J. Laparoendosc. Adv. Surg. Tech. 2013, 367–371. [Google Scholar] [CrossRef]
- Chand, M.; Keller, D.S.; Joshi, H.M.; Devoto, L.; Rodriguez-Justo, M.; Cohen, R. Feasibility of fluorescence lymph node imaging in colon cancer: FLICC. Tech. Coloproctology 2018, 22, 271–277. [Google Scholar] [CrossRef]
- Liberale, G.; Vankerckhove, S.; Galdon, M.G.; Donckier, V.; Larsimont, D.; Bourgeois, P. Fluorescence imaging after intraoperative intravenous injection of indocyanine green for detection of lymph node metastases in colorectal cancer. Eur. J. Surg. Oncol. 2015, 41, 1256–1260. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, T.; Wang, X.; Li, J.; Zhao, H. Real-time near-infrared fluorescence imaging mediated by blue dye in breast cancer patients. J. Surg. Oncol. 2020, 121, 964–966. [Google Scholar] [CrossRef]
- Sarli, L.; Bader, G.; Iusco, D.; Salvemini, C.; Mauro, D.D.; Mazzeo, A.; Regina, G.; Roncoroni, L. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur. J. Cancer 2005, 41, 272–279. [Google Scholar] [CrossRef]
- Erlichman, C.; O’Connell, M.; Kahn, M.; Marsoni, S.; Torri, V.; Tardio, B.; Zaniboni, A.; Pancera, G.; Martignoni, G.; Labianca, R.; et al. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J. Clin. Oncol. 1999, 17, 1356–1363. [Google Scholar]
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8507 patients from 22 randomised trials. Lancet 2001, 358, 1291–1304. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Hermanek, P.; Hohenberger, W.; Klimpfinger, M.; Köckerling, F.; Papadopoulos, T. The pathological assessment of mesorectal excision: Implications for further treatment and quality management. Int. J. Colorectal. Dis. 2003, 18, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Yellinek, S.; Krizzuk, D.; Nogueras, J.; Wexner, S. Ureteral Injury During Colorectal Surgery: Two Case Reports and a Literature Review. J. Anus. Rectum. Colon. 2018, 2, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Speicher, P.J.; Goldsmith, Z.G.; Nussbaum, D.P.; Turley, R.S.; Peterson, A.C.; Mantyh, C.R. Ureteral stenting in laparoscopic colorectal surgery. J. Surg. Res. 2014, 190, 98–103. [Google Scholar] [CrossRef]
- Andersen, P.; Andersen, L.M.; Iversen, L.H. Iatrogenic ureteral injury in colorectal cancer surgery: A nationwide study comparing laparoscopic and open approaches. Surg. Endosc. 2015, 29, 1406–1412. [Google Scholar] [CrossRef]
- Chahin, F.; Dwivedi, A.J.; Paramesh, A.; Chau, W.; Agrawal, S.; Chahin, C.; Kumar, A.; Tootla, A.; Tootla, F.; Silva, Y.J. The implications of lighted ureteral stenting in laparoscopic colectomy. JSLS 2002, 6, 49–52. [Google Scholar]
- van den Bos, J.; Al-Taher, M.; Bouvy, N.D.; Stassen, L.P.S. Near-infrared fluorescence laparoscopy of the ureter with three preclinical dyes in a pig model. Surg. Endosc. 2019, 33, 986–991. [Google Scholar] [CrossRef]
- Farnam, R.W.; Arms, R.G.; Klaassen, A.H.; Sorger, J.M. Intraoperative ureter visualization using a near-infrared imaging agent. J. Biomed. Opt. 2019, 24, 1–8. [Google Scholar] [CrossRef]
- Ginimuge, P.R.; Jyothi, S.D. Methylene blue: Revisited. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 517–520. [Google Scholar] [CrossRef]
- Matsui, A.; Tanaka, E.; Choi, H.S.; Kianzad, V.; Gioux, S.; Lomnes, S.J.; Frangioni, J.V. Real-time, near-infrared, fluorescence-guided identification of the ureters using methylene blue. Surgery 2010, 148, 78–86. [Google Scholar] [CrossRef]
- Sinex, J.E. Pulse oximetry: Principles and limitations. Am. J. Emerg. Med. 1999, 17, 59–67. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Leikin, J.B.; Murray, P.; Corbridge, T.C. Adult toxicology in critical care: Part II: Specific poisonings. Chest 2003, 123, 897–922. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, N.; Al-Difaie, Z.; Scheepers, M.H.M.C.; Heuvelings, D.J.I.; Rodríguez-Luna, M.R.; Marescaux, J.; Diana, M.; Stassen, L.P.S.; Bouvy, N.D.; Al-Taher, M. Simultaneous, Multi-Channel, Near-Infrared Fluorescence Visualization of Mesenteric Lymph Nodes Using Indocyanine Green and Methylene Blue: A Demonstration in a Porcine Model. Diagnostics 2023, 13, 1469. https://doi.org/10.3390/diagnostics13081469
Okamoto N, Al-Difaie Z, Scheepers MHMC, Heuvelings DJI, Rodríguez-Luna MR, Marescaux J, Diana M, Stassen LPS, Bouvy ND, Al-Taher M. Simultaneous, Multi-Channel, Near-Infrared Fluorescence Visualization of Mesenteric Lymph Nodes Using Indocyanine Green and Methylene Blue: A Demonstration in a Porcine Model. Diagnostics. 2023; 13(8):1469. https://doi.org/10.3390/diagnostics13081469
Chicago/Turabian StyleOkamoto, Nariaki, Zaid Al-Difaie, Max H. M. C. Scheepers, Danique J. I. Heuvelings, María Rita Rodríguez-Luna, Jacques Marescaux, Michele Diana, Laurents P. S. Stassen, Nicole D. Bouvy, and Mahdi Al-Taher. 2023. "Simultaneous, Multi-Channel, Near-Infrared Fluorescence Visualization of Mesenteric Lymph Nodes Using Indocyanine Green and Methylene Blue: A Demonstration in a Porcine Model" Diagnostics 13, no. 8: 1469. https://doi.org/10.3390/diagnostics13081469
APA StyleOkamoto, N., Al-Difaie, Z., Scheepers, M. H. M. C., Heuvelings, D. J. I., Rodríguez-Luna, M. R., Marescaux, J., Diana, M., Stassen, L. P. S., Bouvy, N. D., & Al-Taher, M. (2023). Simultaneous, Multi-Channel, Near-Infrared Fluorescence Visualization of Mesenteric Lymph Nodes Using Indocyanine Green and Methylene Blue: A Demonstration in a Porcine Model. Diagnostics, 13(8), 1469. https://doi.org/10.3390/diagnostics13081469







