Salivary Biomarkers to Differentiate between Streptococcus pneumoniae and Influenza A Virus-Related Pneumonia in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Saliva Collection
2.3. Gel-Free Isobaric Tags for Relative and Absolute Quantitation (iTRAQ) Proteomics
2.4. Salivary Biomarker Determination
2.5. Statistical Analysis
3. Results
3.1. Patients
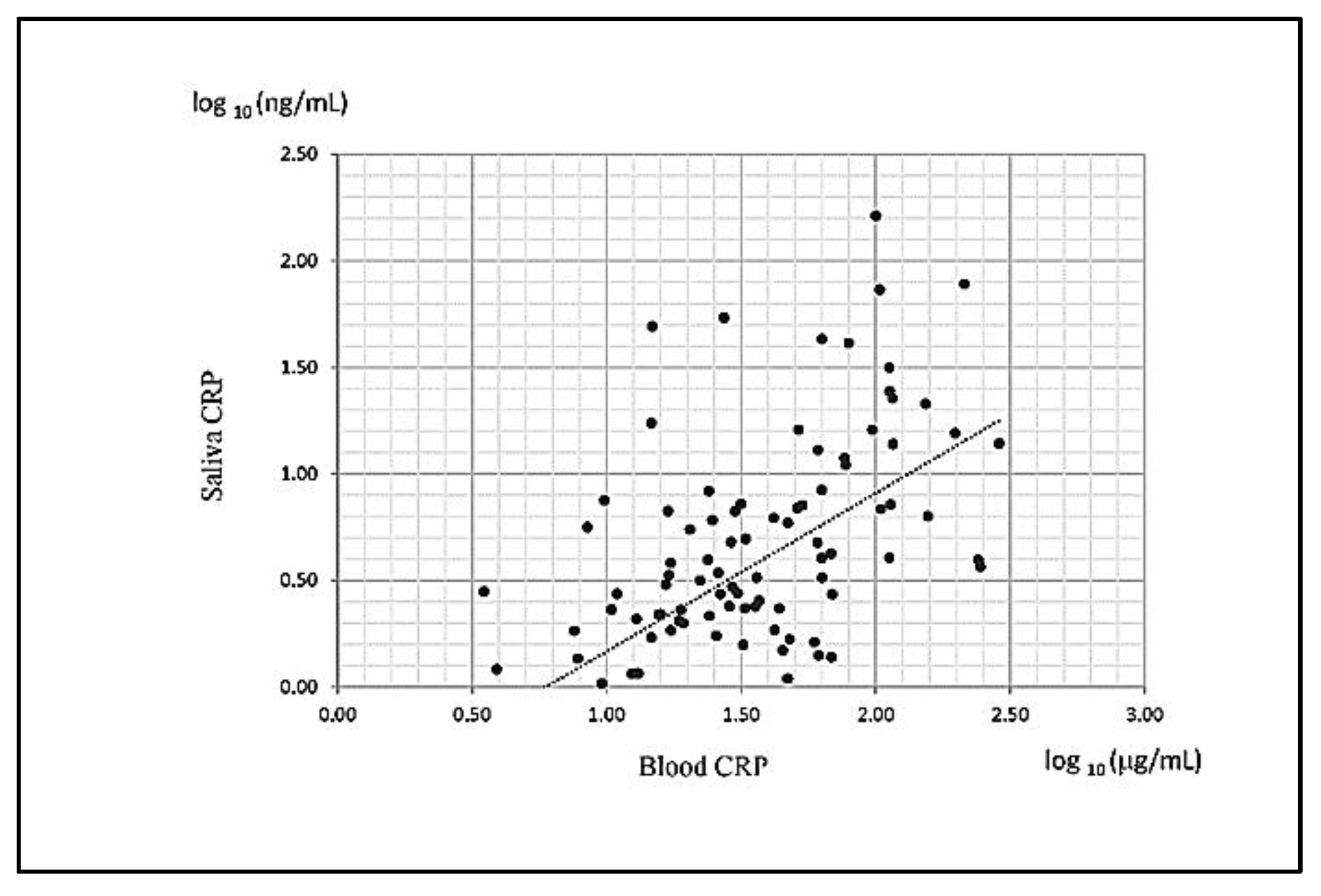
3.2. Salivary and Serum CRP Levels Were Highly Correlated
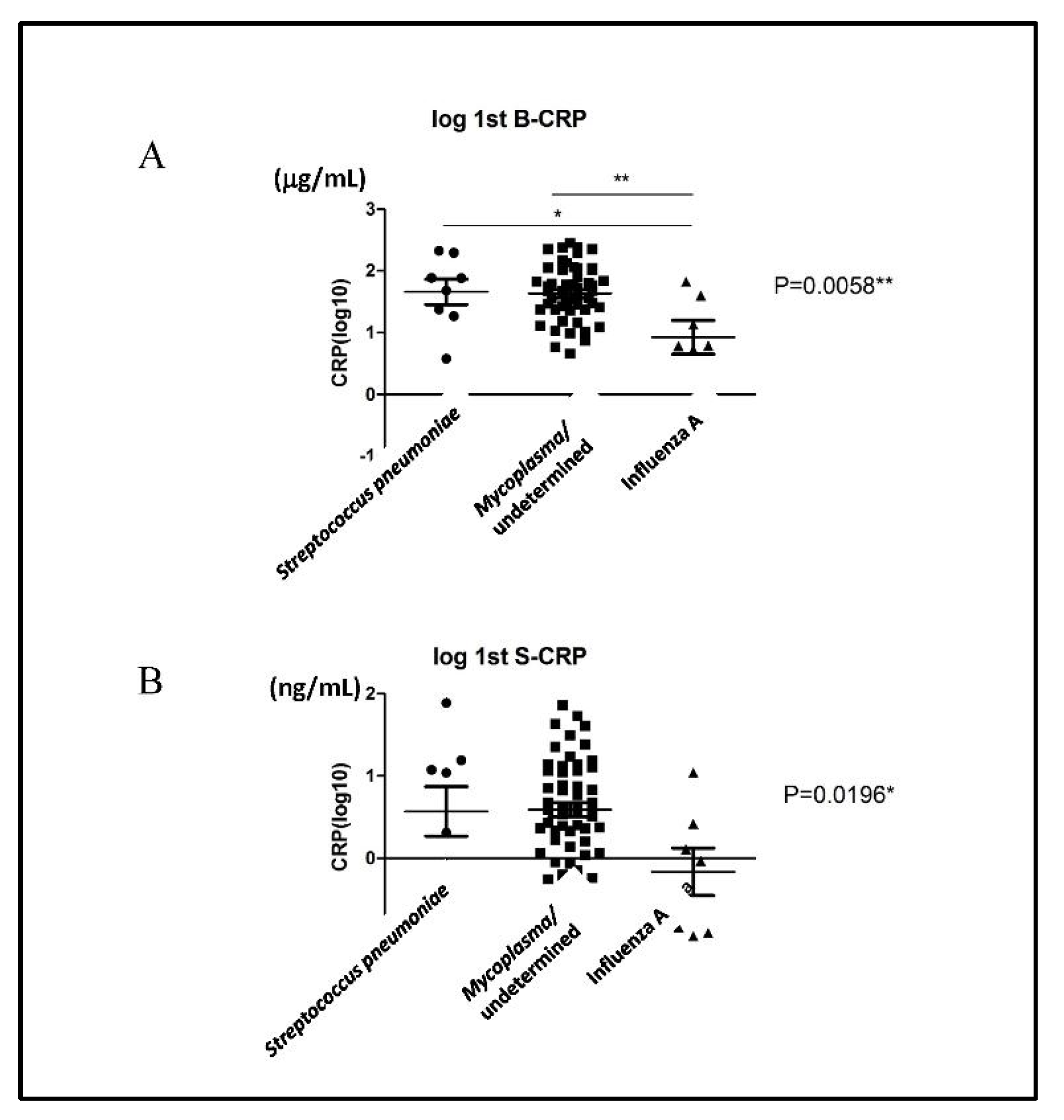
3.3. Salivary CRP Levels Did Not Distinguish Whether the Pathogen Causing Pneumonia in Children was Streptococcus pneumoniae or Influenza A
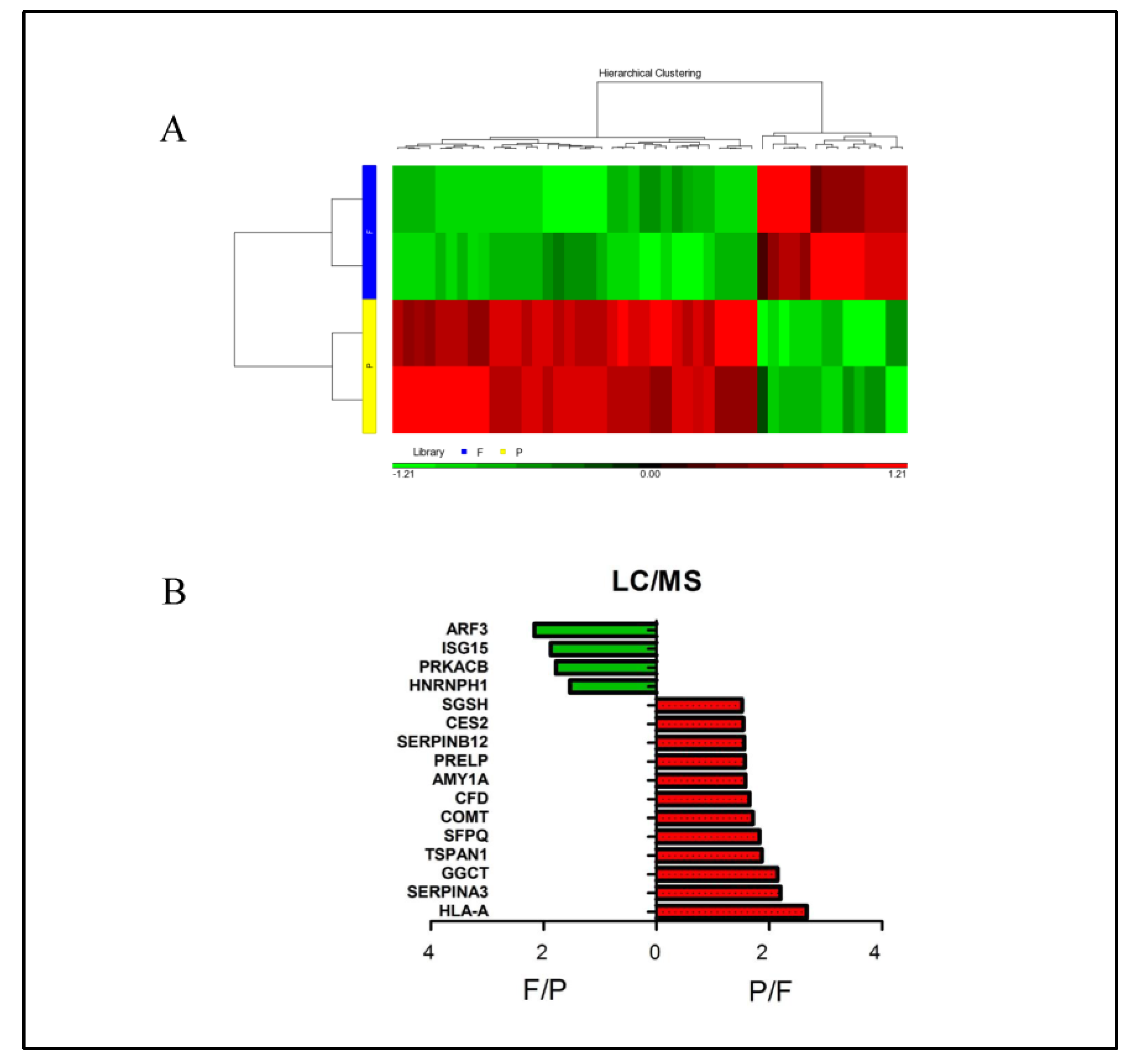
3.4. Identification of the Differential Salivary Biomarkers from Children with Streptococcus pneumoniae and Influenza a Virus Infection by Gel-Free Isobaric Tag for Relative and Absolute Quantitation (iTRAQ) Proteomics
3.5. Different Pathways Were Enriched by Specific Salivary Proteins from Children with Streptococcus pneumoniae and Influenza A Infections
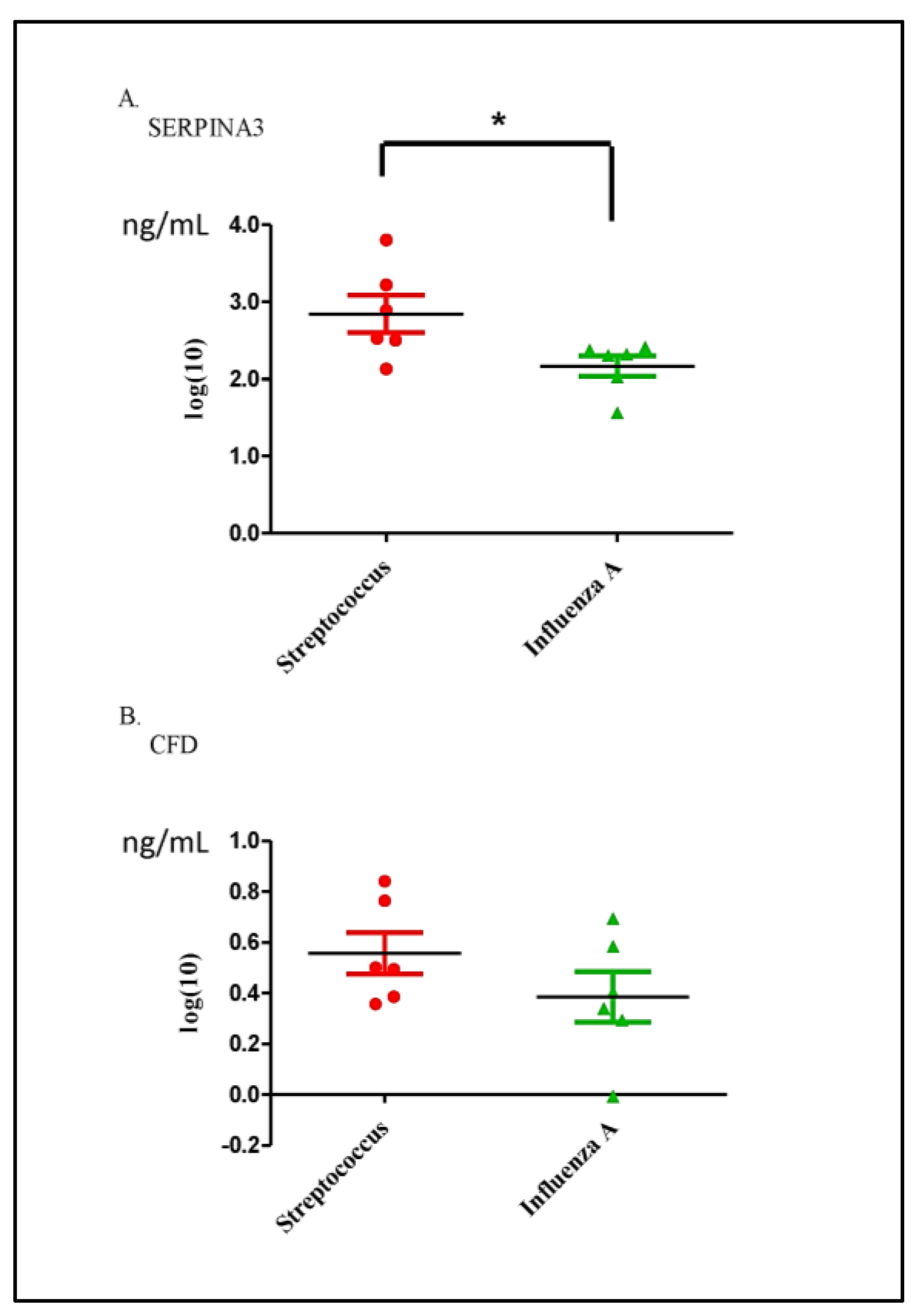
3.6. Validation of Differential Proteins in Saliva Samples between the Streptococcus pneumoniae and Influenza A Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000-19: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.W.; Wallihan, R.; Juergensen, A.; Mejias, A.; Ramilo, O. Community-Acquired Pneumonia in Children: Myths and Facts. Am. J. Perinatol. 2019, 36, S54–S57. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.W.; Wallihan, R.; Desai, A.; Alter, S.; Ambroggio, L.; Cohen, D.M.; El-Assal, O.; Marzec, S.; Florin, T.A.; Keaton, M.; et al. Clinical Characteristics and Etiology of Community-acquired Pneumonia in US Children, 2015–2018. Pediatr. Infect. Dis. J. 2022, 41, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Von Mollendorf, C.; Berger, D.; Gwee, A.; Duke, T.; Graham, S.M.; Russell, F.M.; Mulholland, E.K.; ARI Review Group. Aetiology of childhood pneumonia in low- and middle-income countries in the era of vaccination: A systematic review. J. Glob. Health 2022, 12, 10009. [Google Scholar] [CrossRef]
- Wetzke, M.; Schutz, K.; Kopp, M.V.; Seidenberg, J.; Vogelberg, C.; Ankermann, T.; Happle, C.; Voigt, G.; Koster, H.; Illig, T.; et al. Pathogen spectra in hospitalised and nonhospitalised children with community-acquired pneumonia. ERJ Open Res. 2023, 9, 286. [Google Scholar] [CrossRef]
- Shang, L.; Xu, J.; Cao, B. Viral pneumonia in China: From surveillance to response. Lancet Public Health 2020, 5, e633–e634. [Google Scholar] [CrossRef] [PubMed]
- Sitthikool, K.; Aksilp, C. Accuracy of procalcitonin in detecting severe bacterial infections among critically ill children. Pediatr. Respirol. Crit. Care Med. 2020, 4, 13–17. [Google Scholar] [CrossRef]
- Eschborn, S.; Weitkamp, J.H. Procalcitonin versus C-reactive protein: Review of kinetics and performance for diagnosis of neonatal sepsis. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2019, 39, 893–903. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Babaei, M.; Rezaei, S.; Saghafi Khadem, S.; Shirinbak, I.; Basir Shabestari, S. The Role of Salivary C-Reactive Protein in Systemic and Oral Disorders: A Systematic Review. Med. J. Islam. Repub. Iran 2022, 36, 138. [Google Scholar] [CrossRef]
- Song, M.; Bai, H.; Zhang, P.; Zhou, X.; Ying, B. Promising applications of human-derived saliva biomarker testing in clinical diagnostics. Int. J. Oral Sci. 2023, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Kara, S.S.; Cevik, O. The Potential Use of Saliva as a Biofluid for Systemic Inflammatory Response Monitoring in Children with Pneumonia. Indian J. Pediatr. 2022, 89, 477–483. [Google Scholar] [CrossRef]
- Hua, N.; Corsten, M.; Bello, A.; Bhatt, M.; Milwid, R.; Champredon, D.; Turgeon, P.; Zemek, R.; Dawson, L.; Mitsakakis, N.; et al. Salivary testing for SARS-CoV-2 in the pediatric population: A diagnostic accuracy study. CMAJ Open 2022, 10, E981–E987. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.M.; Tang, K.S.; Cheng, M.C.; Liu, T.Y.; Huang, Y.H.; Chen, C.C.; Yu, H.R. Use of saliva sample to detect C-reactive protein in children with pneumonia. Pediatr. Pulmonol. 2020, 55, 2457–2462. [Google Scholar] [CrossRef]
- Lee, W.J.; Huang, E.Y.; Tsai, C.M.; Kuo, K.C.; Huang, Y.C.; Hsieh, K.S.; Niu, C.K.; Yu, H.R. Role of Serum Mycoplasma pneumoniae IgA, IgM, and IgG in the Diagnosis of Mycoplasma pneumoniae-Related Pneumonia in School-Age Children and Adolescents. Clin. Vaccine Immunol. CVI 2017, 24, e00471-16. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chang, C.H.; Lee, W.J.; Liu, T.Y.; Tsai, C.M.; Tsai, T.A.; Tsai, C.K.; Kuo, K.C.; Chen, C.C.; Niu, C.K.; et al. Altered chemokine profile in Refractory Mycoplasma pneumoniae pneumonia infected children. J. Microbiol. Immunol. Infect. 2021, 54, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-Y.; Yu, H.-R.; Lee, W.-J.; Tsai, C.-M.; Kuo, K.-C.; Chang, C.-H.; Su, Y.-T.; Wang, S.-C.; Niu, C.-K.; Hsieh, K.-S. Role of biocard Mycoplasma immunoglobulin M rapid test in the diagnosis of Mycoplasma pneumoniae infection. Pediatr. Respirol. Crit. Care Med. 2018, 2, 7–10. [Google Scholar] [CrossRef]
- Tsai, T.A.; Tsai, C.K.; Kuo, K.C.; Yu, H.R. Rational stepwise approach for Mycoplasma pneumoniae pneumonia in children. J. Microbiol. Immunol. Infect. 2021, 54, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Yamagata, E.; Murakami, J.; Shirai, R.; Kadota, J. A retrospective study of the patients with positive ImmunoCard Mycoplasma test on an outpatient clinic basis. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2010, 16, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Hernes, S.S.; Hagen, E.; Tofteland, S.; Finsen, N.T.; Christensen, A.; Giske, C.G.; Spindler, C.; Bakke, P.S.; Bjorvatn, B. Transthoracic fine-needle aspiration in the aetiological diagnosis of community-acquired pneumonia. Clin. Microbiol. Infect. 2010, 16, 909–911. [Google Scholar] [CrossRef]
- Levinson, T.; Wasserman, A. C-Reactive Protein Velocity (CRPv) as a New Biomarker for the Early Detection of Acute Infection/Inflammation. Int. J. Mol. Sci. 2022, 23, 8100. [Google Scholar] [CrossRef] [PubMed]
- Benito, J.; Acedo, Y.; Medrano, L.; Barcena, E.; Garay, R.P.; Arri, E.A. Usefulness of new and traditional serum biomarkers in children with suspected appendicitis. Am. J. Emerg. Med. 2016, 34, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Gunaratnam, L.C.; Robinson, J.L.; Hawkes, M.T. Systematic Review and Meta-Analysis of Diagnostic Biomarkers for Pediatric Pneumonia. J. Pediatr. Infect. Dis. Soc. 2021, 10, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Tsalik, E.L.; Henao, R.; Nichols, M.; Burke, T.; Ko, E.R.; McClain, M.T.; Hudson, L.L.; Mazur, A.; Freeman, D.H.; Veldman, T.; et al. Host gene expression classifiers diagnose acute respiratory illness etiology. Sci. Transl. Med. 2016, 8, 322ra311. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Carballa, A.; Barral-Arca, R.; Cebey-Lopez, M.; Bello, X.; Pardo-Seco, J.; Martinon-Torres, F.; Salas, A. Identification of a Minimal 3-Transcript Signature to Differentiate Viral from Bacterial Infection from Best Genome-Wide Host RNA Biomarkers: A Multi-Cohort Analysis. Int. J. Mol. Sci. 2021, 22, 3148. [Google Scholar] [CrossRef] [PubMed]
- Oved, K.; Cohen, A.; Boico, O.; Navon, R.; Friedman, T.; Etshtein, L.; Kriger, O.; Bamberger, E.; Fonar, Y.; Yacobov, R.; et al. A novel host-proteome signature for distinguishing between acute bacterial and viral infections. PLoS ONE 2015, 10, e0120012. [Google Scholar] [CrossRef]
- Stein, M.; Lipman-Arens, S.; Oved, K.; Cohen, A.; Bamberger, E.; Navon, R.; Boico, O.; Friedman, T.; Etshtein, L.; Paz, M.; et al. A novel host-protein assay outperforms routine parameters for distinguishing between bacterial and viral lower respiratory tract infections. Diagn. Microbiol. Infect. Dis. 2018, 90, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Srugo, I.; Klein, A.; Stein, M.; Golan-Shany, O.; Kerem, N.; Chistyakov, I.; Genizi, J.; Glazer, O.; Yaniv, L.; German, A.; et al. Validation of a Novel Assay to Distinguish Bacterial and Viral Infections. Pediatrics 2017, 140, e20163453. [Google Scholar] [CrossRef]
- Ashkenazi-Hoffnung, L.; Oved, K.; Navon, R.; Friedman, T.; Boico, O.; Paz, M.; Kronenfeld, G.; Etshtein, L.; Cohen, A.; Gottlieb, T.M.; et al. A host-protein signature is superior to other biomarkers for differentiating between bacterial and viral disease in patients with respiratory infection and fever without source: A prospective observational study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Gofin, Y.; Fanous, E.; Pasternak, Y.; Prokocimer, Z.; Zagoory-Sharon, O.; Feldman, R.; Codick, G.; Waisbourd-Zinman, O.; Fried, S.; Livni, G. Salivary C-reactive protein-a possible predictor of serum levels in pediatric acute respiratory illness. Eur. J. Pediatr. 2021, 180, 2465–2472. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Lin, C.C.; Abemayor, E.; Wang, M.B.; Wong, D.T. The emerging landscape of salivary diagnostics. Periodontology 2000 2016, 70, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva diagnostics—Current views and directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef]
- Nguyen-Kim, H.; Beckmann, C.; Redondo, M.; Ziliox, J.; Vallet, V.; Berger-Sturm, K.; Overbeck, J.V.; Alberi Auber, L. COVID salivary diagnostics: A comparative technical study. J. Med. Virol. 2022, 94, 4277–4286. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C.; Wong, D.T.W. Role of Saliva and Salivary Diagnostics in the Advancement of Oral Health. J. Dent. Res. 2019, 98, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Desai, G.S.; Mathews, S.T. Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World J. Diabetes 2014, 5, 730–738. [Google Scholar] [CrossRef]
- Datla, S.; Kitchanan, S.; Sethuraman, G. Diagnostic Reliability of Salivary C-Reactive Protein as an Alternative Noninvasive Biomarker of Neonatal Sepsis. Indian Pediatr. 2021, 58, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, J.; Jin, Q. Class I ADP-ribosylation factors are involved in enterovirus 71 replication. PLoS ONE 2014, 9, e99768. [Google Scholar] [CrossRef] [PubMed]
- Belov, G.A.; Fogg, M.H.; Ehrenfeld, E. Poliovirus proteins induce membrane association of GTPase ADP-ribosylation factor. J. Virol. 2005, 79, 7207–7216. [Google Scholar] [CrossRef] [PubMed]
- Perng, Y.C.; Lenschow, D.J. ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef]
- Hinay, A.A., Jr.; Kakee, S.; Kageyama, S.; Tsuneki-Tokunaga, A.; Perdana, W.Y.; Akena, Y.; Nishiyama, S.; Kanai, K. Pro-Inflammatory Cytokines and Interferon-Stimulated Gene Responses Induced by Seasonal Influenza A Virus with Varying Growth Capabilities in Human Lung Epithelial Cell Lines. Vaccines 2022, 10, 1507. [Google Scholar] [CrossRef] [PubMed]
- Han, S.P.; Tang, Y.H.; Smith, R. Functional diversity of the hnRNPs: Past, present and perspectives. Biochem. J. 2010, 430, 379–392. [Google Scholar] [CrossRef]
- Kutluay, S.B.; Emery, A.; Penumutchu, S.R.; Townsend, D.; Tenneti, K.; Madison, M.K.; Stukenbroeker, A.M.; Powell, C.; Jannain, D.; Tolbert, B.S.; et al. Genome-Wide Analysis of Heterogeneous Nuclear Ribonucleoprotein (hnRNP) Binding to HIV-1 RNA Reveals a Key Role for hnRNP H1 in Alternative Viral mRNA Splicing. J. Virol. 2019, 93, e01048-19. [Google Scholar] [CrossRef]
- Tong, L.; Chu, Z.; Gao, X.; Yang, M.; Adam, F.E.A.; Theodore, D.W.P.; Liu, H.; Wang, X.; Xiao, S.; Yang, Z. Newcastle disease virus V protein interacts with hnRNP H1 to promote viral replication. Vet. Microbiol. 2021, 260, 109093. [Google Scholar] [CrossRef] [PubMed]
- Van’t Wout, E.F.; van Schadewijk, A.; Savage, N.D.; Stolk, J.; Hiemstra, P.S. alpha1-antitrypsin production by proinflammatory and antiinflammatory macrophages and dendritic cells. Am. J. Respir. Cell Mol. Biol. 2012, 46, 607–613. [Google Scholar] [CrossRef]
- Pini, L.; Tiberio, L.; Venkatesan, N.; Bezzi, M.; Corda, L.; Luisetti, M.; Ferrarotti, I.; Malerba, M.; Lomas, D.A.; Janciauskiene, S.; et al. The role of bronchial epithelial cells in the pathogenesis of COPD in Z-alpha-1 antitrypsin deficiency. Respir. Res. 2014, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Cheadle, C.E.; Rice, L.V.; Pfeffer, P.E.; Dimeloe, S.; Gupta, A.; Bush, A.; Gooptu, B.; Hawrylowicz, C.M. The Induction of Alpha-1 Antitrypsin by Vitamin D in Human T Cells Is TGF-beta Dependent: A Proposed Anti-inflammatory Role in Airway Disease. Front. Nutr. 2021, 8, 667203. [Google Scholar] [CrossRef] [PubMed]
- Serban, K.A.; Petrache, I. Alpha-1 Antitrypsin and Lung Cell Apoptosis. Ann. Am. Thorac. Soc. 2016, 13 (Suppl S2), S146–S149. [Google Scholar] [CrossRef]
| Pathway Name | Enrichment p-Value |
|---|---|
| Valine, leucine and isoleucine biosynthesis | 0.008463 |
| Salivary secretion | 0.016398 |
| Complement and coagulation cascades | 0.01818 |
| Amoebiasis | 0.023701 |
| Lysosome | 0.030797 |
| 2-Oxocarboxylic acid metabolism | 0.038579 |
| Pantothenate and CoA biosynthesis | 0.039091 |
| Glycosaminoglycan degradation | 0.039091 |
| Pathway Name | Enrichment p-Value | Pathway Name | Enrichment p-Value |
|---|---|---|---|
| Vasopressin-regulated water reabsorption | 0.000496 | cAMP signaling pathway | 0.013366 |
| Amphetamine addiction | 0.001445 | Proteoglycans in cancer | 0.015301 |
| GABAergic synapse | 0.001546 | Nicotinate and nicotinamide metabolism | 0.023197 |
| Long-term potentiation | 0.002042 | Prion diseases | 0.03016 |
| Inflammatory mediator regulation of TRP channels | 0.003325 | Human papillomavirus infection | 0.030801 |
| Oocyte meiosis | 0.003867 | Hedgehog signaling pathway | 0.032532 |
| Platelet activation | 0.004953 | Endocrine and other factor-regulated calcium reabsorption | 0.035263 |
| Dopaminergic synapse | 0.005182 | Cocaine addiction | 0.039439 |
| Insulin signaling pathway | 0.005399 | Ovarian steroidogenesis | 0.039982 |
| Vascular smooth muscle contraction | 0.005638 | Vibrio cholerae infection | 0.047745 |
| Adrenergic signaling in cardiomyocytes | 0.007017 | Pyrimidine metabolism | 0.047745 |
| Oxytocin signaling pathway | 0.007075 | Regulation of lipolysis in adipocytes | 0.049543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, K.-S.; Tsai, C.-M.; Cheng, M.-C.; Huang, Y.-H.; Chang, C.-H.; Yu, H.-R. Salivary Biomarkers to Differentiate between Streptococcus pneumoniae and Influenza A Virus-Related Pneumonia in Children. Diagnostics 2023, 13, 1468. https://doi.org/10.3390/diagnostics13081468
Tang K-S, Tsai C-M, Cheng M-C, Huang Y-H, Chang C-H, Yu H-R. Salivary Biomarkers to Differentiate between Streptococcus pneumoniae and Influenza A Virus-Related Pneumonia in Children. Diagnostics. 2023; 13(8):1468. https://doi.org/10.3390/diagnostics13081468
Chicago/Turabian StyleTang, Kuo-Shu, Chih-Min Tsai, Ming-Chou Cheng, Ying-Hsien Huang, Chih-Hao Chang, and Hong-Ren Yu. 2023. "Salivary Biomarkers to Differentiate between Streptococcus pneumoniae and Influenza A Virus-Related Pneumonia in Children" Diagnostics 13, no. 8: 1468. https://doi.org/10.3390/diagnostics13081468
APA StyleTang, K.-S., Tsai, C.-M., Cheng, M.-C., Huang, Y.-H., Chang, C.-H., & Yu, H.-R. (2023). Salivary Biomarkers to Differentiate between Streptococcus pneumoniae and Influenza A Virus-Related Pneumonia in Children. Diagnostics, 13(8), 1468. https://doi.org/10.3390/diagnostics13081468








