Assessing Passengers’ Motion Sickness Levels Based on Cerebral Blood Oxygen Signals and Simulation of Actual Ride Sensation
Abstract
1. Introduction
2. Background
2.1. Definition and Causes of Motion Sickness
2.2. Recognition and Classification of Motion Sickness
2.3. Motion Sickness Recognition Based on Cerebral Blood Oxygen Signals
2.4. Motivation and Structure of This Study
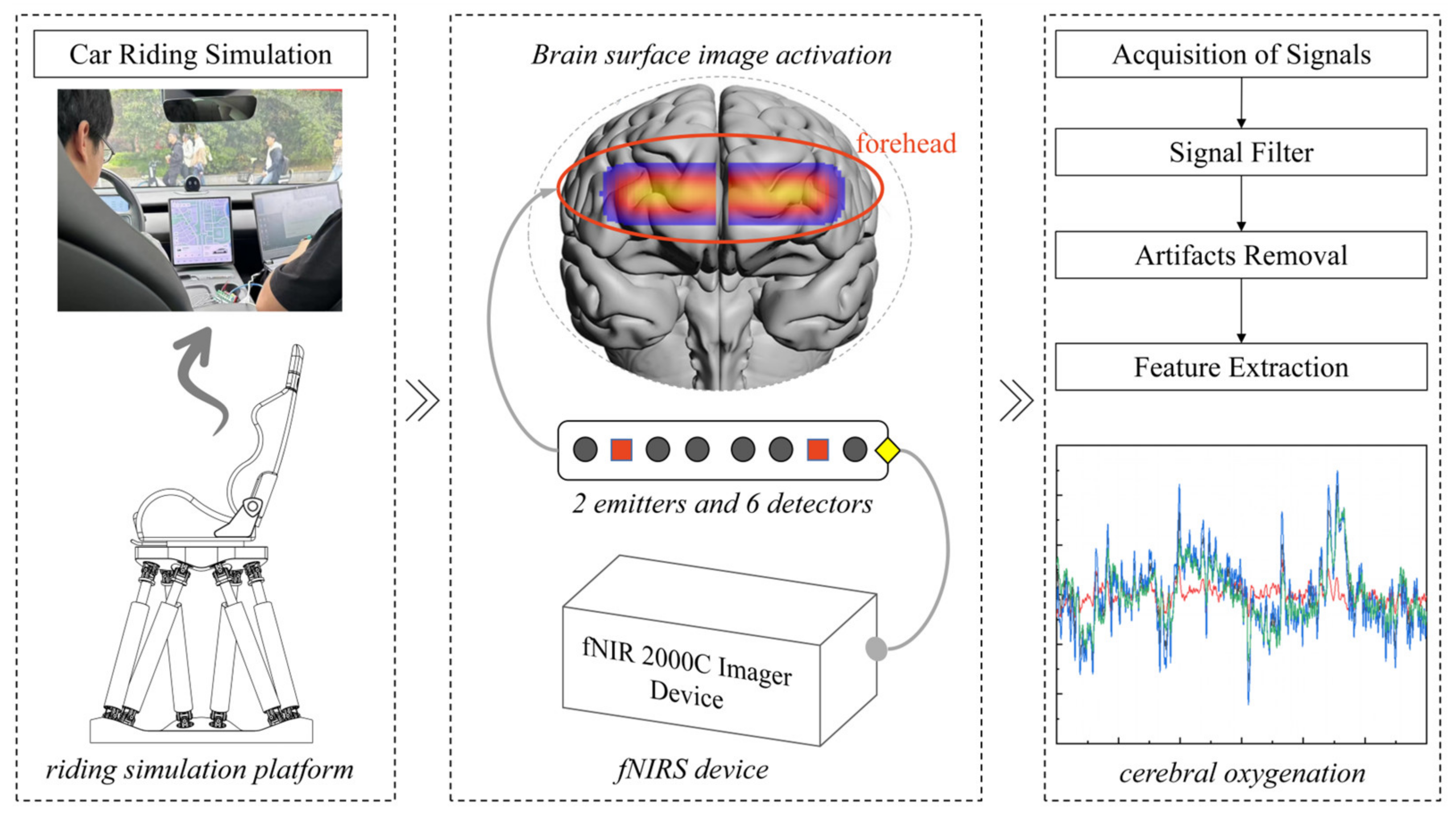
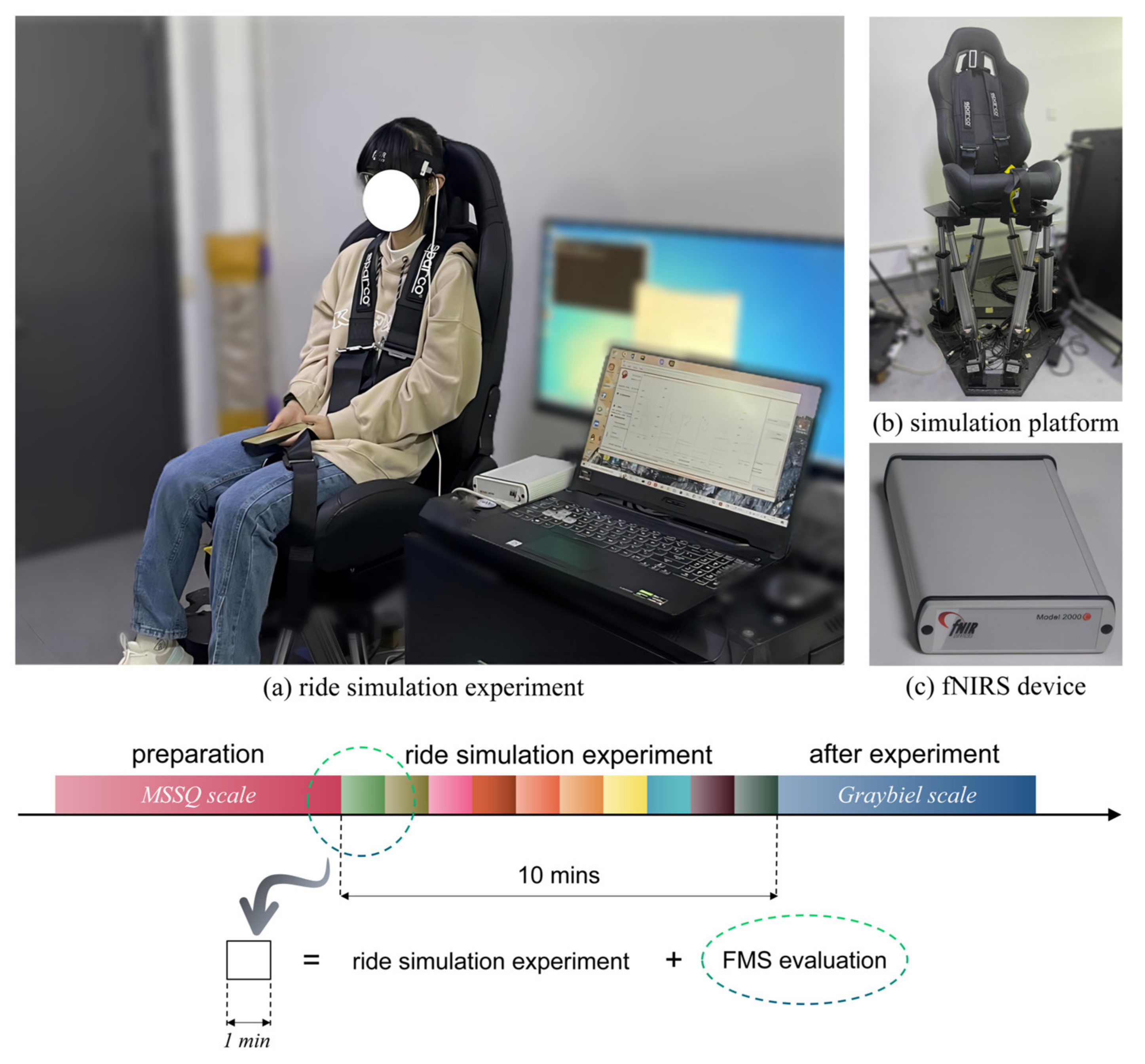
3. Design of the Ride Simulation Experiment of Passengers
4. Calculation and Preprocessing of Cerebral Blood Oxygen Signals
5. Establishment of Motion Sickness Evaluation Model for Passengers
5.1. Extraction of Multiple Characteristic Parameters from Cerebral Blood Oxygen Signals
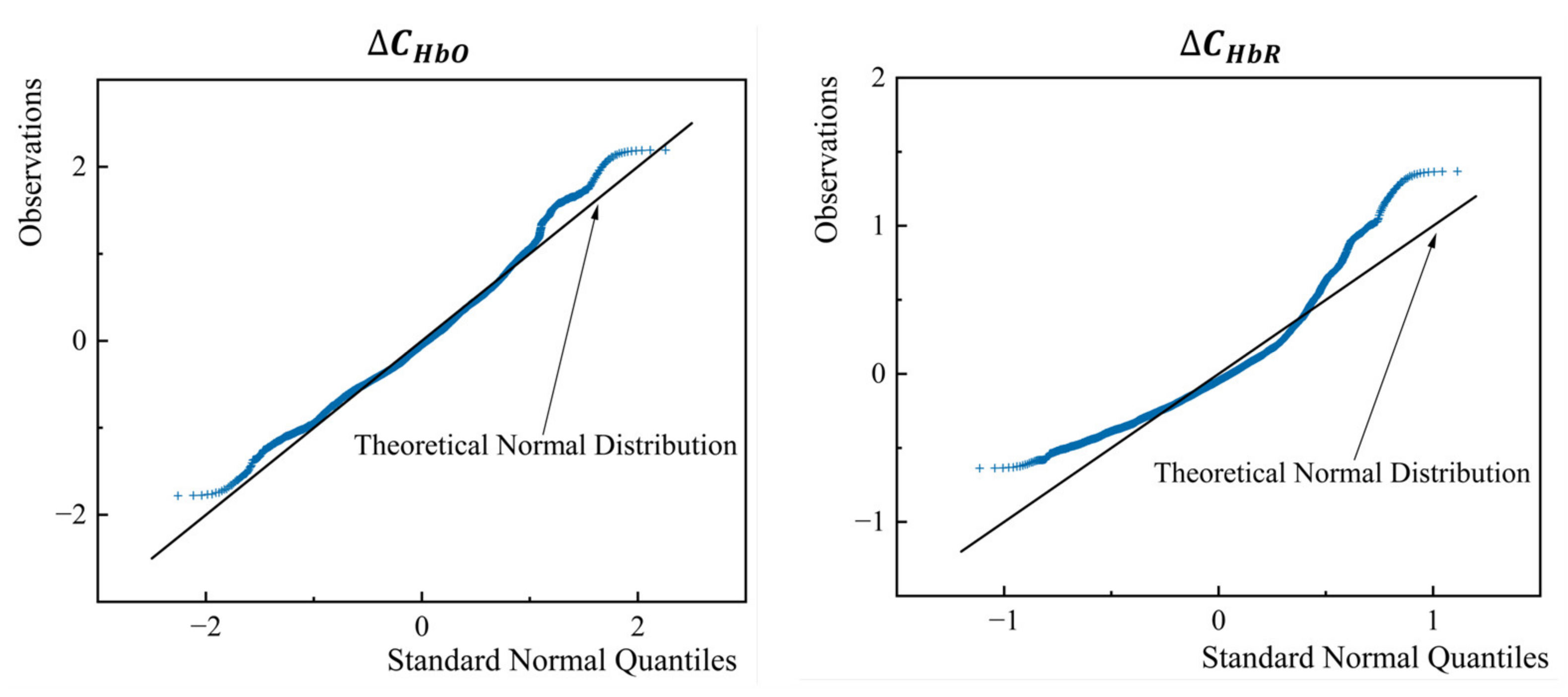
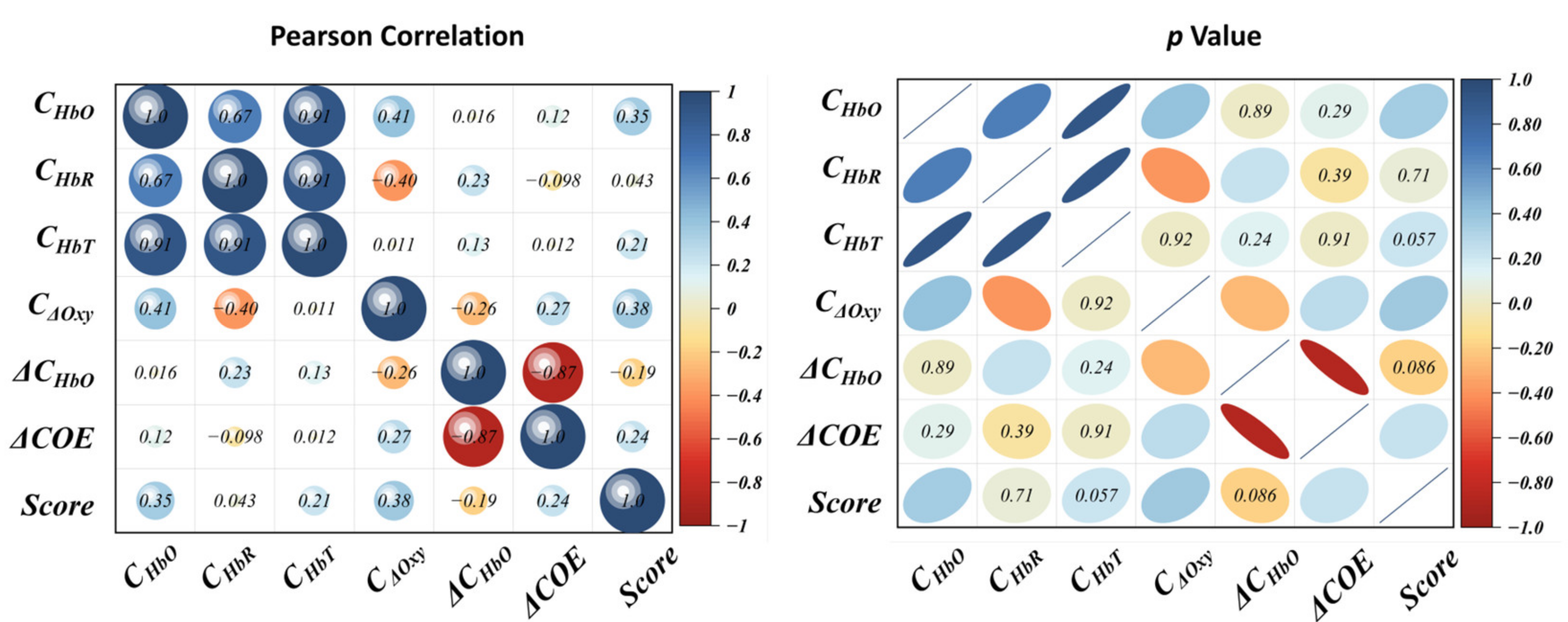
5.2. Normality Test and Correlation Test of the Parameters
5.3. Bayesian Ridge Regression Algorithm
5.4. Evaluation Model Establishment of MSL
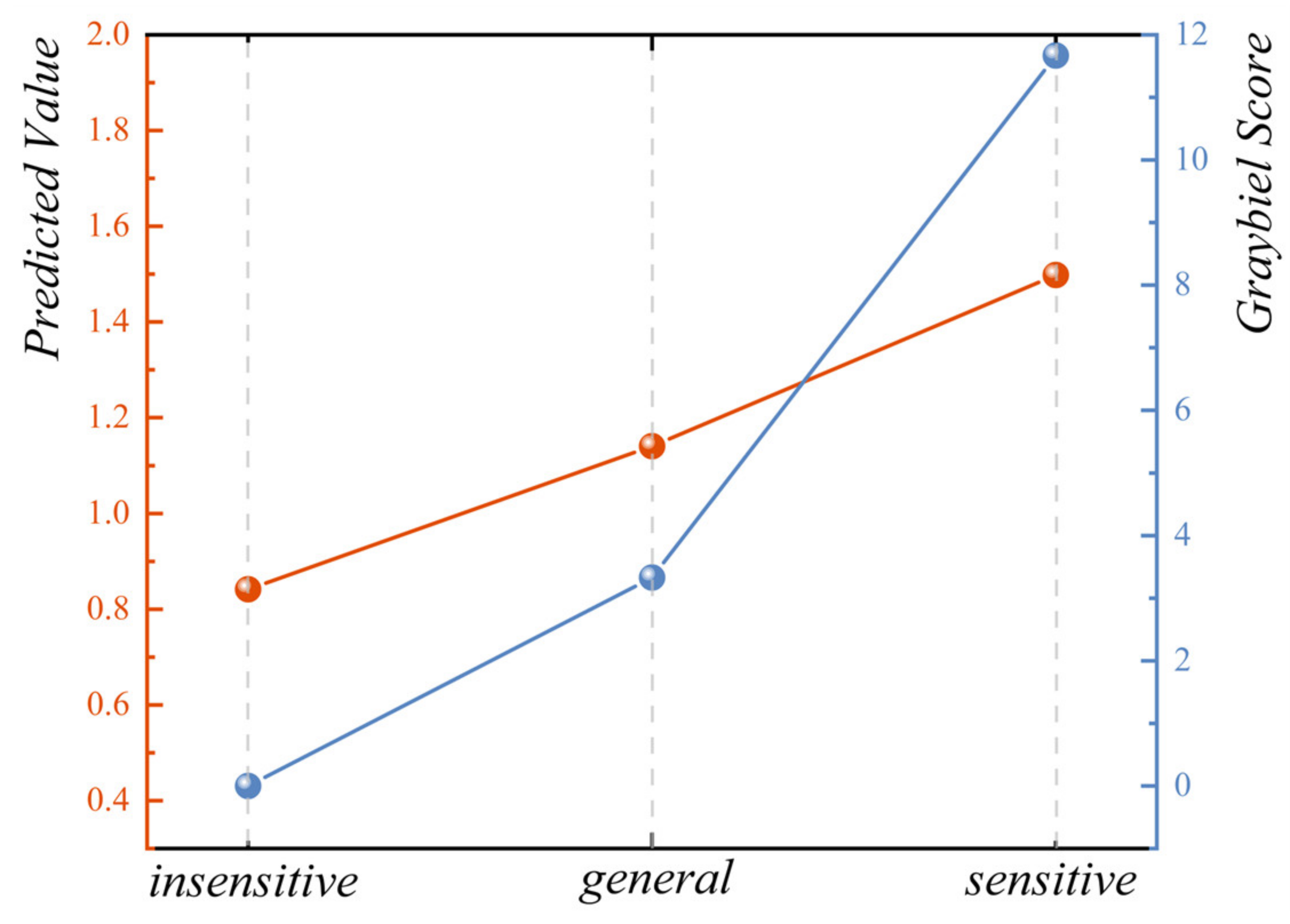
6. Validation of Motion Sickness Evaluation Model from Vehicle Test
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaufeld, M.; Mundt, M.; Forst, S.; Hecht, H. Optical See-through Augmented Reality Can Induce Severe Motion Sickness. Displays 2022, 74, 102283. [Google Scholar] [CrossRef]
- Park, S.; Ha, J.; Kim, L. Effect of Visually Induced Motion Sickness from Head-Mounted Display on Cardiac Activity. Sensors 2022, 22, 6213. [Google Scholar] [CrossRef] [PubMed]
- Stoffregen, T.A.; Riccio, G.E. An Ecological Critique of the Sensory Conflict Theory of Motion Sickness. Ecol. Psychol. 1991, 3, 159–194. [Google Scholar] [CrossRef]
- Thornton, W.E.; Bonato, F. Space Motion Sickness and Motion Sickness: Symptoms and Etiology. Aviat. Space Environ. Med. 2013, 84, 716–721. [Google Scholar] [CrossRef]
- Knox, G.W. Motion Sickness: An Evolutionary and Genetic Basis for the Negative Reinforcement Model. Aviat. Space Environ. Med. 2014, 85, 46–49. [Google Scholar] [CrossRef]
- Mittelstaedt, J.M. Individual Predictors of the Susceptibility for Motion-Related Sickness: A Systematic Review. J. Vestib. Res. 2020, 30, 165–193. [Google Scholar] [CrossRef]
- Huang, K.-C.; John, A.R.; Jung, T.-P.; Tsai, W.-F.; Yu, Y.-H.; Lin, C.-T. Comparing the Differences in Brain Activities and Neural Comodulations Associated With Motion Sickness Between Drivers and Passengers. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1259–1267. [Google Scholar] [CrossRef]
- Warwick-Evans, L.A.; Symons, N.; Fitch, T.; Burrows, L. Evaluating Sensory Conflict and Postural Instability. Theories of Motion Sickness. Brain Res. Bull. 1998, 47, 465–469. [Google Scholar] [CrossRef]
- Wibirama, S.; Nugroho, H.A.; Hamamoto, K. Depth Gaze and ECG Based Frequency Dynamics during Motion Sickness in Stereoscopic 3D Movie. Entertain. Comput. 2018, 26, 117–127. [Google Scholar] [CrossRef]
- Golding, J.F. Predicting Individual Differences in Motion Sickness Susceptibility by Questionnaire. Personal. Individ. Differ. 2006, 41, 237–248. [Google Scholar] [CrossRef]
- Lim, H.K.; Ji, K.; Woo, Y.S.; Han, D.; Lee, D.-H.; Nam, S.G.; Jang, K.-M. Test-Retest Reliability of the Virtual Reality Sickness Evaluation Using Electroencephalography (EEG). Neurosci. Lett. 2021, 743, 135589. [Google Scholar] [CrossRef]
- Keshavarz, B.; Murovec, B.; Mohanathas, N.; Golding, J.F. The Visually Induced Motion Sickness Susceptibility Questionnaire (VIMSSQ): Estimating Individual Susceptibility to Motion Sickness-Like Symptoms When Using Visual Devices. Hum. Factors J. Hum. Factors Ergon. Soc. 2023, 65, 107–124. [Google Scholar] [CrossRef]
- Keshavarz, B.; Hecht, H. Stereoscopic Viewing Enhances Visually Induced Motion Sickness but Sound Does Not. Presence Teleoperators Virtual Environ. 2012, 21, 213–228. [Google Scholar] [CrossRef]
- Reinhard, R.; Rutrecht, H.M.; Hengstenberg, P.; Tutulmaz, E.; Geissler, B.; Hecht, H.; Muttray, A. The Best Way to Assess Visually Induced Motion Sickness in a Fixed-Base Driving Simulator. Transp. Res. Part F Traffic Psychol. Behav. 2017, 48, 74–88. [Google Scholar] [CrossRef]
- De Winkel, K.N.; Irmak, T.; Kotian, V.; Pool, D.M.; Happee, R. Relating Individual Motion Sickness Levels to Subjective Discomfort Ratings. Exp. Brain Res. 2022, 240, 1231–1240. [Google Scholar] [CrossRef]
- Tschan, R.; Wiltink, J.; Best, C.; Bense, S.; Dieterich, M.; Beutel, M.E.; Eckhardt-Henn, A. Validation of the German Version of the Vertigo Symptom Scale (VSS) in Patients with Organic or Somatoform Dizziness and Healthy Controls. J. Neurol. 2008, 255, 1168–1175. [Google Scholar] [CrossRef]
- Jang, K.-M.; Kwon, M.; Nam, S.G.; Kim, D.; Lim, H.K. Estimating Objective (EEG) and Subjective (SSQ) Cybersickness in People with Susceptibility to Motion Sickness. Appl. Ergon. 2022, 102, 103731. [Google Scholar] [CrossRef]
- Chuang, S.-W.; Chuang, C.-H.; Yu, Y.-H.; King, J.-T.; Lin, C.-T. EEG Alpha and Gamma Modulators Mediate Motion Sickness-Related Spectral Responses. Int. J. Neural Syst. 2016, 26, 1650007. [Google Scholar] [CrossRef]
- Park, S.; Mun, S.; Ha, J.; Kim, L. Non-Contact Measurement of Motion Sickness Using Pupillary Rhythms from an Infrared Camera. Sensors 2021, 21, 4642. [Google Scholar] [CrossRef]
- Kiryu, T.; Tada, G.; Toyama, H.; Iijima, A. Integrated Evaluation of Visually Induced Motion Sickness in Terms of Autonomic Nervous Regulation. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 4597–4600. [Google Scholar]
- Li, Y.; Liu, A.; Ding, L. Machine Learning Assessment of Visually Induced Motion Sickness Levels Based on Multiple Biosignals. Biomed. Signal Process Control 2019, 49, 202–211. [Google Scholar] [CrossRef]
- Recenti, M.; Ricciardi, C.; Aubonnet, R.; Picone, I.; Jacob, D.; Svansson, H.Á.R.; Agnarsdóttir, S.; Karlsson, G.H.; Baeringsdóttir, V.; Petersen, H.; et al. Toward Predicting Motion Sickness Using Virtual Reality and a Moving Platform Assessing Brain, Muscles, and Heart Signals. Front. Bioeng. Biotechnol. 2021, 9, 635661. [Google Scholar] [CrossRef] [PubMed]
- Ko, L.-W.; Lee, H.-C.; Tsai, S.-F.; Shih, T.-C.; Chuang, Y.-T.; Huang, H.-L.; Ho, S.-Y.; Lin, C.-T. EEG-Based Motion Sickness Classification System with Genetic Feature Selection. In Proceedings of the 2013 IEEE Symposium on Computational Intelligence, Cognitive Algorithms, Mind, and Brain (CCMB), Singapore, 16–19 April 2013; pp. 158–164. [Google Scholar]
- Hwang, J.-U.; Bang, J.-S.; Lee, S.-W. Classification of Motion Sickness Levels Using Multimodal Biosignals in Real Driving Conditions. In Proceedings of the 2022 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Prague, Czech Republic, 9–12 October 2022; pp. 1304–1309. [Google Scholar]
- Zhang, C.; Li, S.; Li, Y.; Li, S.E.; Nie, B. Analysis of Motion Sickness Associated Brain Activity Using FNIRS: A Driving Simulator Study. IEEE Access 2020, 8, 207415–207425. [Google Scholar] [CrossRef]
- Kinoshita, F.; Okuno, H.; Touyama, H.; Takada, M.; Miyao, M.; Takada, H. Effect of Background Element Difference on Regional Cerebral Blood Flow While Viewing Stereoscopic Video Clips. In Universal Access in Human-Computer Interaction. Design Approaches and Supporting Technologies: 14th International Conference, UAHCI 2020, Held as Part of the 22nd HCI International Conference, HCII 2020, Copenhagen, Denmark, 19–24 July 2020, Proceedings, Part I 22; Springer International Publishing: Cham, Switzerland, 2020; pp. 355–365. [Google Scholar]
- Takada, M.; Tateyama, K.; Kinoshita, F.; Takada, H. Evaluation of Cerebral Blood Flow While Viewing 3D Video Clips. In Universal Access in Human–Computer Interaction. Human and Technological Environments: 11th International Conference, UAHCI 2017, Held as Part of HCI International 2017, Vancouver, BC, Canada, 9–14 July 2017, Proceedings, Part III 11; Springer International Publishing: Cham, Switzerland, 2017; pp. 492–503. [Google Scholar]
- Hoppes, C.W.; Sparto, P.J.; Whitney, S.L.; Furman, J.M.; Huppert, T.J. Functional Near-Infrared Spectroscopy during Optic Flow with and without Fixation. PLoS ONE 2018, 13, e0193710. [Google Scholar] [CrossRef] [PubMed]
- Talsma, T.M.W.; Hassanain, O.; Happee, R.; de Winkel, K.N. Validation of a Moving Base Driving Simulator for Motion Sickness Research. Appl. Ergon. 2023, 106, 103897. [Google Scholar] [CrossRef]
- Leilei, P.; Ruirui, Q.; Shuifeng, X.; Yuqi, M.; Yang, S.; Rong, X.; Li, G.; Yiling, C. Predictive Ability of Motion Sickness Susceptibility Questionnaire for Motion Sickness Individual Difference in Chinese Young Males. Ocean Coast. Manag. 2021, 203, 105505. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, K.; Han, Q.; Wang, Y.; Guo, G.; Ye, L. An FNIRS Labeling Image Feature-Based Customized Driving Fatigue Detection Method. J. Ambient. Intell. Humaniz. Comput. 2022. [Google Scholar] [CrossRef]
- Zapała, D.; Augustynowicz, P.; Tokovarov, M. Recognition of Attentional States in VR Environment: An FNIRS Study. Sensors 2022, 22, 3133. [Google Scholar] [CrossRef]
- Ayaz, H.; Izzetoglu, M.; Shewokis, P.A.; Onaral, B. Sliding-Window Motion Artifact Rejection for Functional Near-Infrared Spectroscopy. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6567–6570. [Google Scholar]
- Asgher, U.; Ahmad, R.; Naseer, N.; Ayaz, Y.; Khan, M.J.; Amjad, M.K. Assessment and Classification of Mental Workload in the Prefrontal Cortex (PFC) Using Fixed-Value Modified Beer-Lambert Law. IEEE Access 2019, 7, 143250–143262. [Google Scholar] [CrossRef]
- Karmakar, S.; Kamilya, S.; Dey, P.; Guhathakurta, P.K.; Dalui, M.; Bera, T.K.; Halder, S.; Koley, C.; Pal, T.; Basu, A. Real Time Detection of Cognitive Load Using FNIRS: A Deep Learning Approach. Biomed. Signal Process Control 2023, 80, 104227. [Google Scholar] [CrossRef]
- Cope, M.; Delpy, D.T. System for Long-Term Measurement of Cerebral Blood and Tissue Oxygenation on Newborn Infants by near Infra-Red Transillumination. Med. Biol. Eng. Comput. 1988, 26, 289–294. [Google Scholar] [CrossRef]
- Yoshino, K.; Oka, N.; Yamamoto, K.; Takahashi, H.; Kato, T. Correlation of Prefrontal Cortical Activation with Changing Vehicle Speeds in Actual Driving: A Vector-Based Functional near-Infrared Spectroscopy Study. Front. Hum. Neurosci. 2013, 7, 895. [Google Scholar] [CrossRef]
- Dufour, J.S.; Reiter, A.; Cox, C.; Weston, E.B.; Markey, M.; Turner, A.; Le, P.; Aurand, A.M.; Simmons, S.; Altman, L.; et al. Motion Sickness Decreases Low Back Function and Changes Gene Expression in Military Aircrew. Clin. Biomech. 2022, 96, 105671. [Google Scholar] [CrossRef]
- Hwang, S.; Sama, M.R.; Kuhn, S.; Erusu, V.; Raiti, J. An Adaptive Tilting Interface to Alleviate Motion Sickness for Passengers in Vehicles. In Proceedings of the 15th International Conference on PErvasive Technologies Related to Assistive Environments, Corfu, Greece, 29 June–1 July 2022; ACM: New York, NY, USA, 2022; pp. 323–324. [Google Scholar]
- Kim, H.J.; Park, K.; Park, Y.-H.; Yang, J.H. Comparative Simulator Experiment on Preventing Motion Sickness by Aromatherapy Inhalation Based on MISC and EEG. In Proceedings of the 2022 IEEE 3rd International Conference on Human-Machine Systems (ICHMS), Orlando, FL, USA, 17–19 November 2022; pp. 1–2. [Google Scholar]
- Li, G.; Onuoha, O.; McGill, M.; Brewster, S.; Chen, C.P.; Pollick, F. Comparing Autonomic Physiological and Electroencephalography Features for VR Sickness Detection Using Predictive Models. In Proceedings of the 2021 IEEE Symposium Series on Computational Intelligence, SSCI 2021—Proceedings, Orlando, FL, USA, 5–7 December 2021. [Google Scholar] [CrossRef]


| Parameters | Value |
|---|---|
| CylinderOrigLength | 580.000000 |
| MaxTravelRange | 295.000000 |
| TopHexagonLongerSideLength | 420.000000 |
| TopHexagonShortSideLength | 120.000000 |
| TopCircumcircleDiamiter | 567.000000 |
| BottomHexagonLongerSideLength | 450.000000 |
| BottomHexagonShortSideLength | 150.000000 |
| BottomCircumcircleDiamiter | 634.000000 |
| PlatformMaxYTravelRange | 304.189148 |
| PlatformMaxRotateAngle | 32.444931 |
| Subject Number | Normal Mode | Comfortable Mode |
|---|---|---|
| 1 | 2.170 | −0.490 |
| 2 | 3.279 | 3.272 |
| DF | SS | MS | F | p | |
|---|---|---|---|---|---|
| Model | 1 | 4.13 | 4.13 | 1.28 | 0.26 |
| Error | 78 | 250.94 | 3.22 | ||
| Total | 79 | 255.08 | |||
| Model | 1 | 35.29 | 35.29 | 21.02 | 1.70 × 10−5 |
| Error | 78 | 130.91 | 1.68 | ||
| Total | 79 | 166.19 | |||
| Model | 1 | 4.05 × 10−5 | 4.05 × 10−5 | 2.19 | 0.14 |
| Error | 78 | 1.44 × 10−3 | 1.84 × 10−5 | ||
| Total | 79 | 1.48 × 10−3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, B.; Zhou, Q. Assessing Passengers’ Motion Sickness Levels Based on Cerebral Blood Oxygen Signals and Simulation of Actual Ride Sensation. Diagnostics 2023, 13, 1403. https://doi.org/10.3390/diagnostics13081403
Ren B, Zhou Q. Assessing Passengers’ Motion Sickness Levels Based on Cerebral Blood Oxygen Signals and Simulation of Actual Ride Sensation. Diagnostics. 2023; 13(8):1403. https://doi.org/10.3390/diagnostics13081403
Chicago/Turabian StyleRen, Bin, and Qinyu Zhou. 2023. "Assessing Passengers’ Motion Sickness Levels Based on Cerebral Blood Oxygen Signals and Simulation of Actual Ride Sensation" Diagnostics 13, no. 8: 1403. https://doi.org/10.3390/diagnostics13081403
APA StyleRen, B., & Zhou, Q. (2023). Assessing Passengers’ Motion Sickness Levels Based on Cerebral Blood Oxygen Signals and Simulation of Actual Ride Sensation. Diagnostics, 13(8), 1403. https://doi.org/10.3390/diagnostics13081403













