Management of Spontaneous Crystalline Lens Luxation in a Patient Diagnosed with Takayasu’s Disease
Abstract
1. Introduction
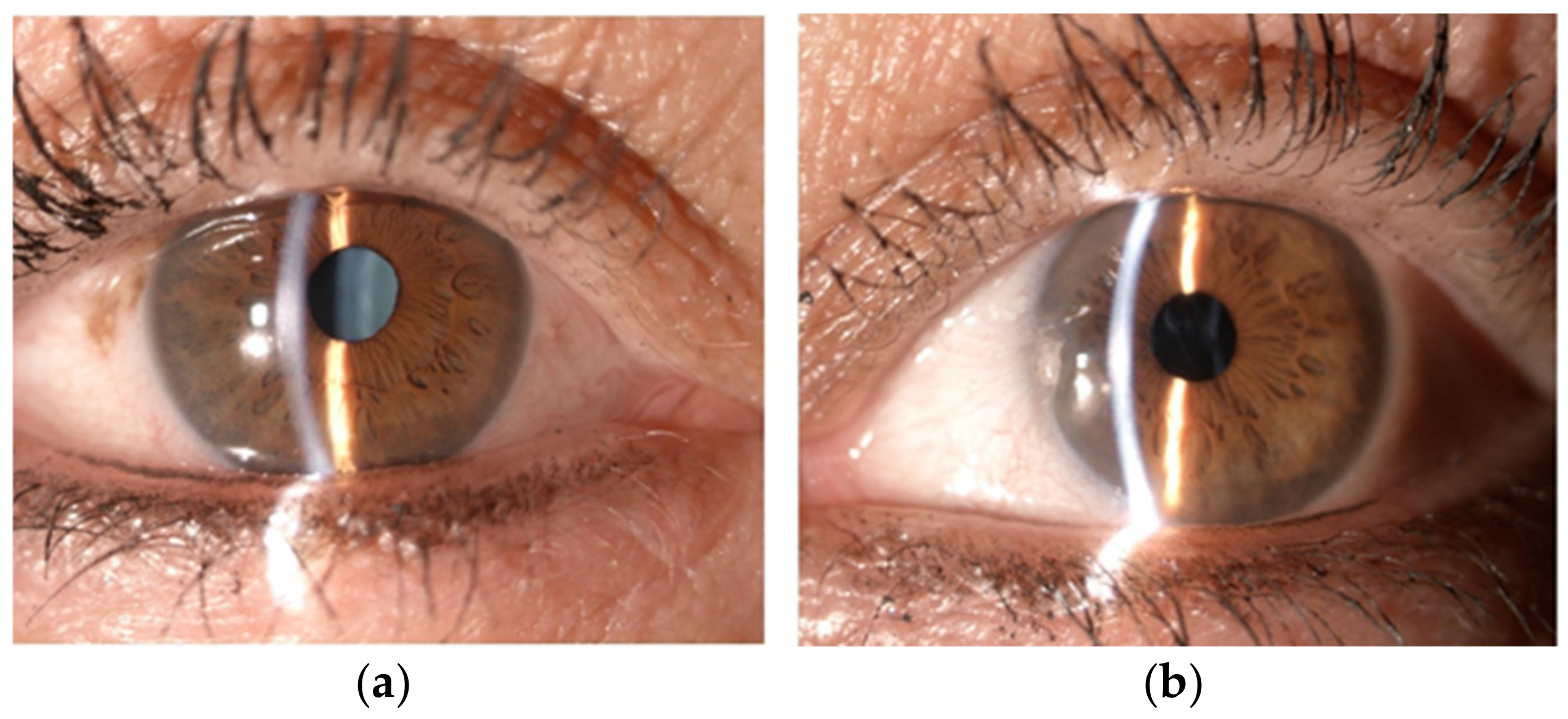
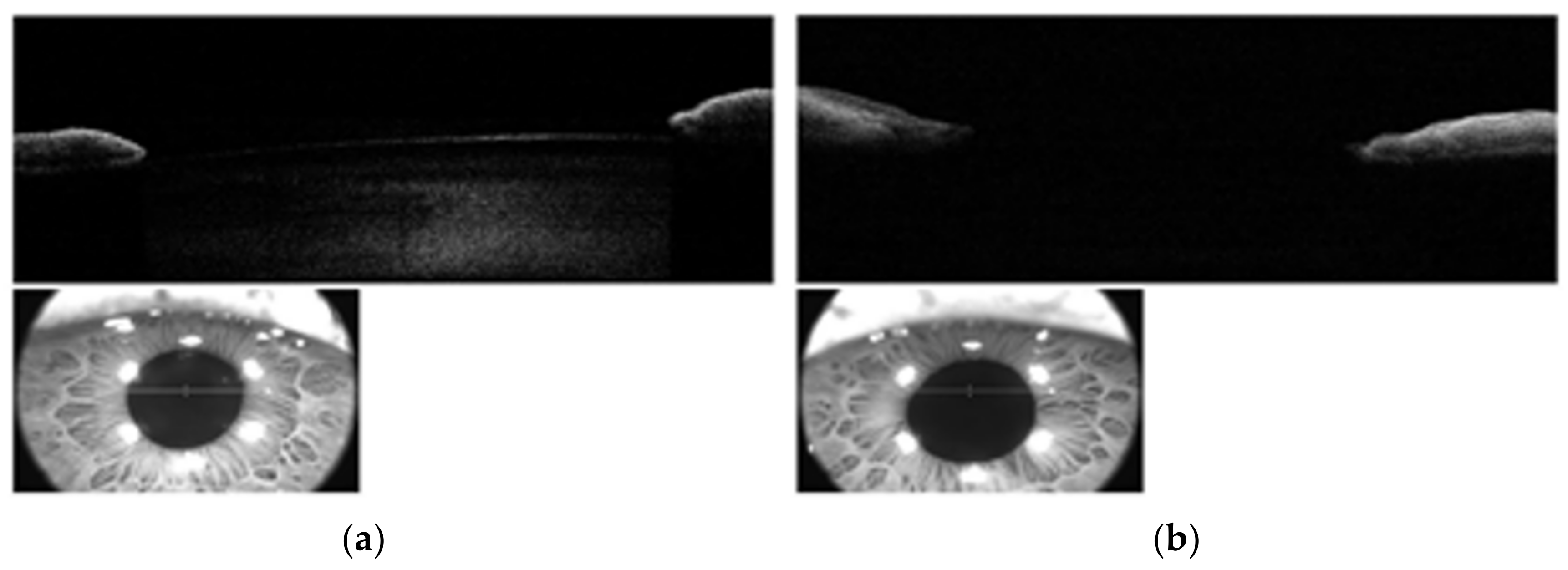
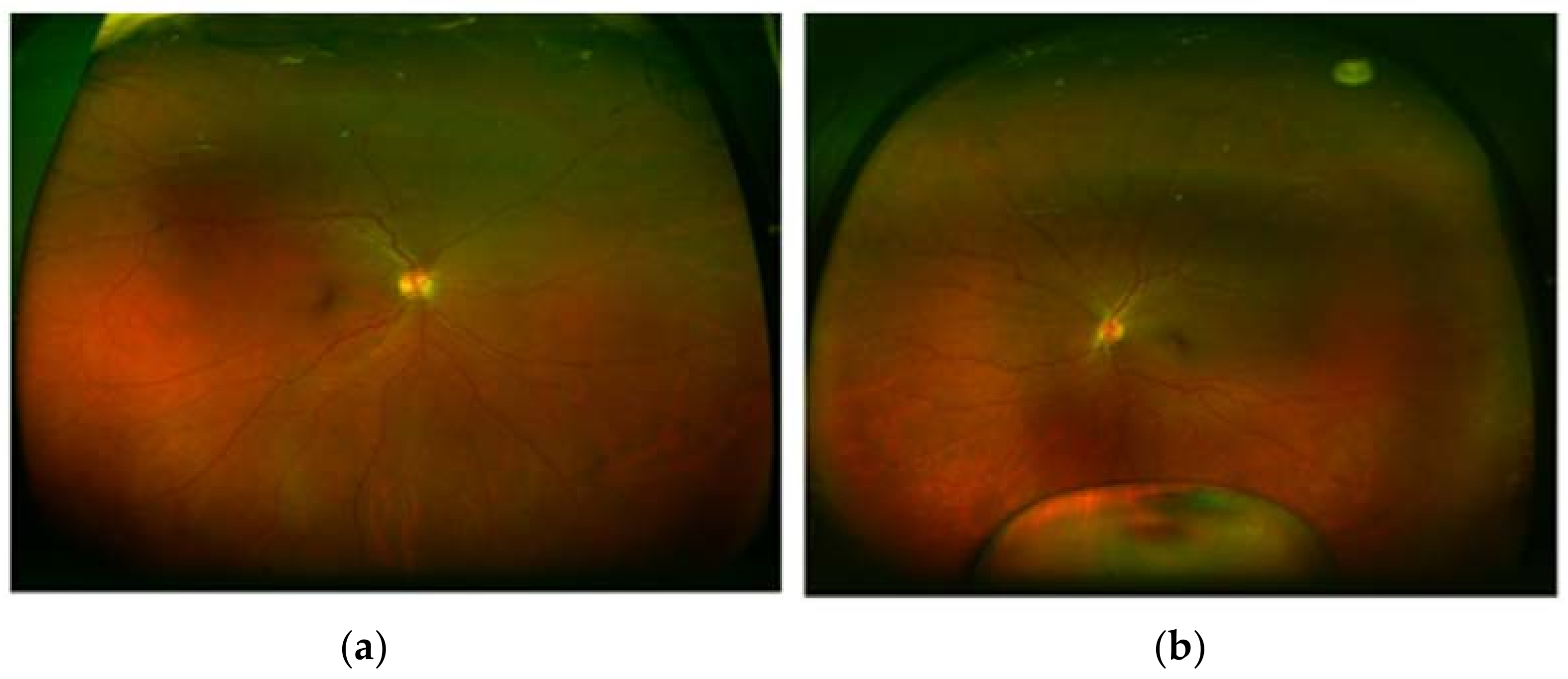
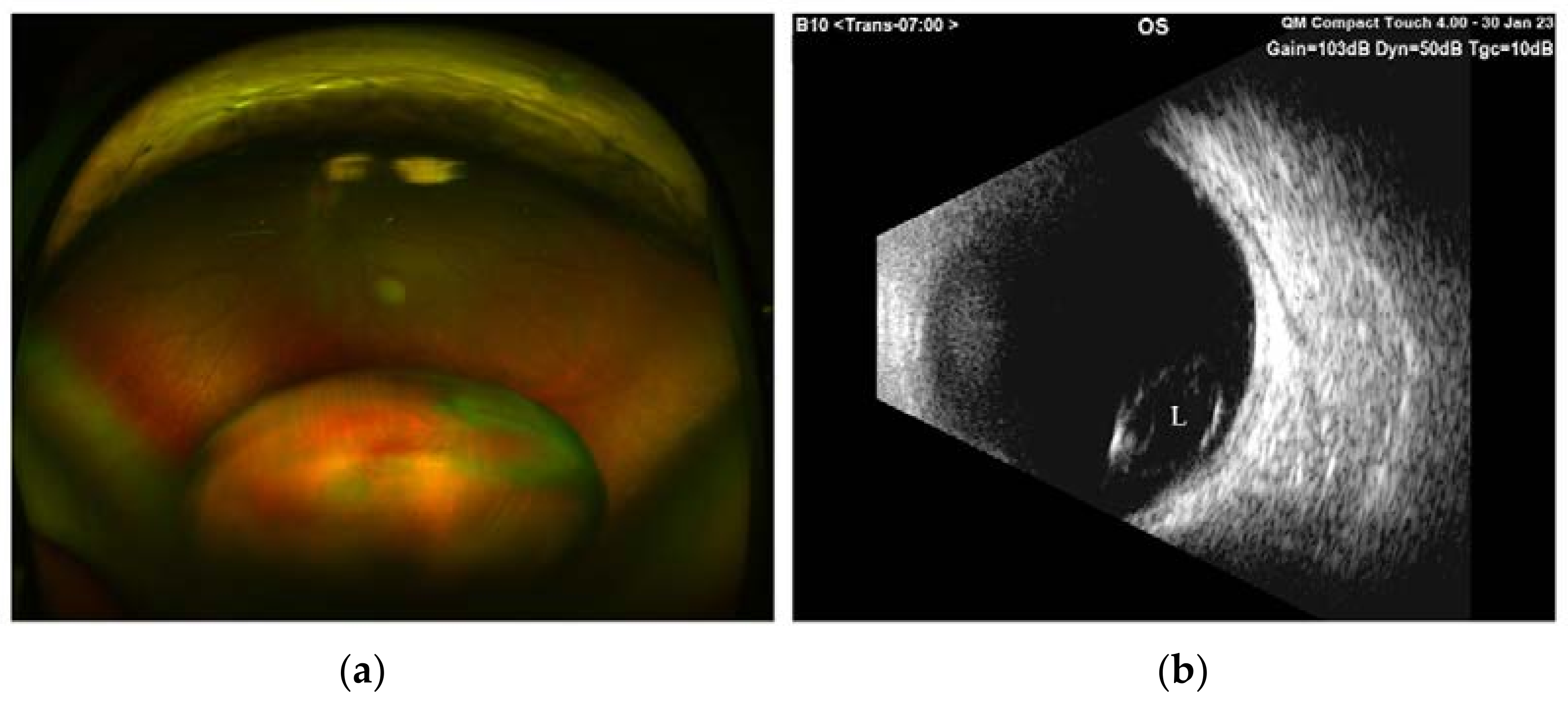
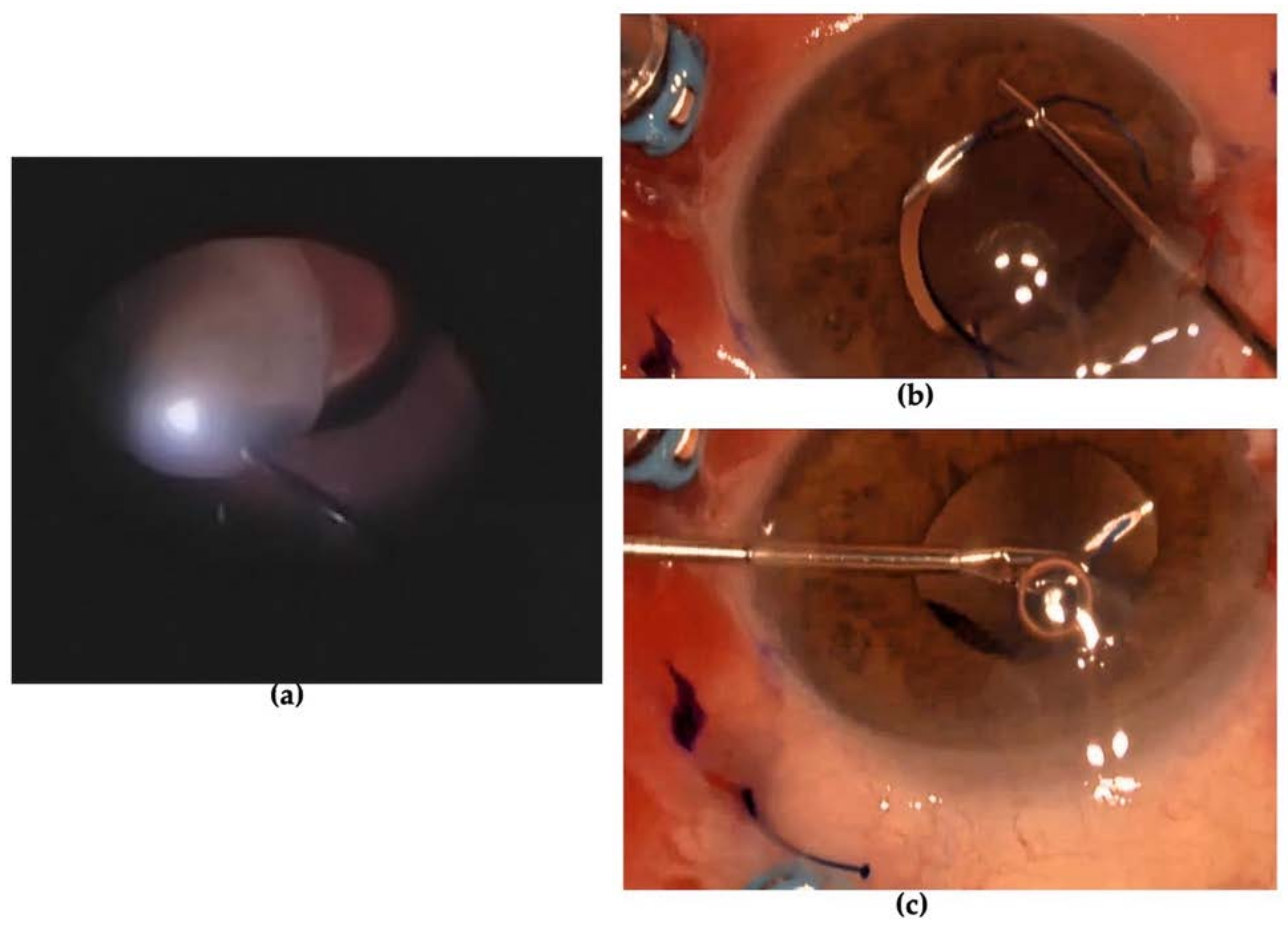
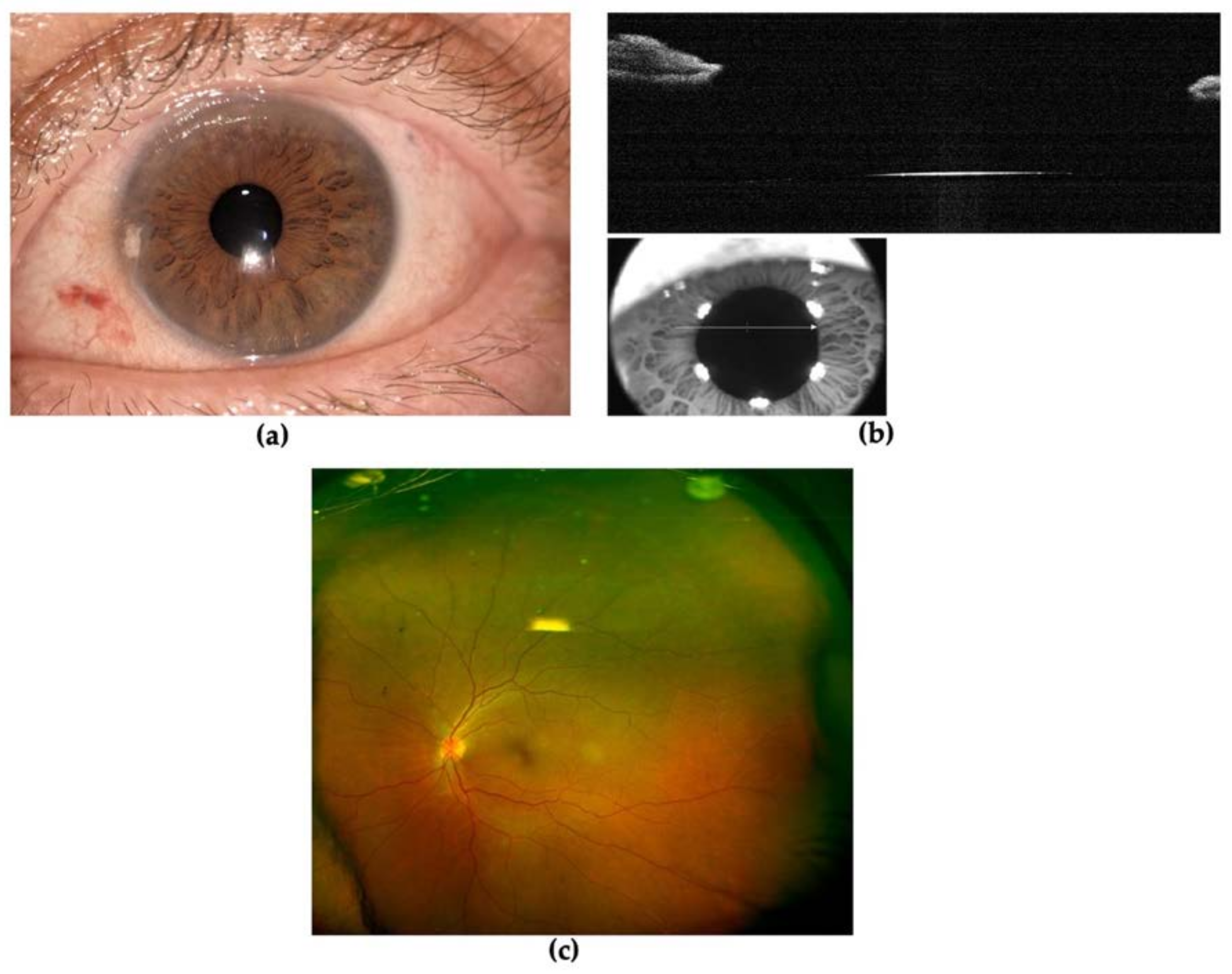
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takayasu, M. A case with curious changes of the central retinal vessels. Acta Soc. Ophthal. Jpn. 1908, 12, 55. [Google Scholar]
- Gupta, V.; Luthra, S.; Shrinkhal, N.; Sinha, S. Takayasu’s arteritis: A unique ophthalmic presentation with CRAO and BRVO. BMJ Case Rep. 2019, 12, e228909. [Google Scholar] [CrossRef] [PubMed]
- Pallangyo, P.; Epafra, E.; Nicholaus, P.; Lyimo, F.; Kazahura, P.; Janabi, M. Bilateral ocular ischemia-induced blindness as a presenting manifestation of Takayasu arteritis: A case report. J. Med. Case Rep. 2017, 11, 153. [Google Scholar] [CrossRef]
- Yamane, S.; Sato, S.; Maruyama-Inoue, M.; Kadonosono, K. Flanged Intrascleral Intraocular Lens Fixation with Double-Needle Technique. Ophthalmology 2017, 124, 1136–1142. [Google Scholar] [CrossRef]
- Ishikawa, K. Diagnostic approach and proposed criteria for the clinical diagnosis of Takayasu’s arteriopathy. J. Am. Coll. Cardiol. 1988, 12, 964–972. [Google Scholar] [CrossRef]
- de Souza, A.W.S.; de Carvalho, J.F. Diagnostic and classification criteria of Takayasu arteritis. J. Autoimmun. 2014, 48-49, 79–83. [Google Scholar] [CrossRef]
- Chun, Y.S.; Park, S.-J.; Kipark, I.; Chung, H.; Lee, J. The clinical and ocular manifestations of Takayasu arteritis. Retina 2001, 21, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.V.; David, S.; Joseph, G.; Horo, S.; Danda, D. Hypoperfusive and hypertensive ocular manifestations in Takayasu arteritis. Clin. Ophthalmol. 2010, 4, 1173–1176. [Google Scholar] [CrossRef]
- Sureja, N.P.; Kalyan, S.; Patel, M.R. Recurrent scleritis as a presenting manifestation of asymptomatic occult Takayasu arteritis. Rheumatol. Adv. Pr. 2020, 5, rkaa065. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, W.H., II. Dislocation of the Lens. Arch. Ophthalmol. 1967, 78, 289–296. [Google Scholar] [CrossRef]
- Aboobakar, I.F.; Johnson, W.M.; Stamer, W.D.; Hauser, M.A.; Allingham, R.R. Major review: Exfoliation syndrome; advances in disease genetics, molecular biology, and epidemiology. Exp. Eye Res. 2017, 154, 88–103. [Google Scholar] [CrossRef]
- Yao, B.; Chen, X.; Liu, G.; Zhao, X. Case report: Spontaneous bilateral intraocular lens dislocation in a patient with homocystinuria. Front. Cardiovasc. Med. 2022, 9, 974842. [Google Scholar] [CrossRef]
- Yang, G.-Y.; Huang, X.; Chen, B.-J.; Xu, Z.-P. Weill-Marchesani-like syndrome caused by an FBN1 mutation with low-penetrance. Chin. Med. J. (Engl.) 2021, 134, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Kirandeep, K.; Bharat, G. Ectopia Lentis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rodrigo, B.-J.; Paulina, L.-L.E.; Francesc, M.D.R.; Eduardo, T.-T.J.; Alejandro, N. Intraocular Lens Subluxation in Marfan Syndrome. Open Ophthalmol. J. 2014, 8, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Asanad, S.; Bayomi, M.; Brown, D.; Buzzard, J.; Lai, E.; Ling, C.; Miglani, T.; Mohammed, T.; Tsai, J.; Uddin, O.; et al. Ehlers-Danlos syndromes and their manifestations in the visual system. Front. Med. 2022, 9, 996458. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Sakai, R.; Shibata, A.; Okada, Y.; Yoshinaga, S.; Kurasawa, T.; Kondo, T.; Amano, K. Concurrent Takayasu Arteritis and Vascular Ehlers–Danlos Syndrome: A Case Report. Front. Cardiovasc. Med. 2022, 9, 805505. [Google Scholar] [CrossRef]
- Mauro, J.; Samson, C.M.; Foster, C.S. Chapter 16: Syphilis. In Diagnosis and Treatment of Uveiti, 2nd ed.; Foster, C.S., Vitale, A.T., Eds.; Jaypee Brothers Medical Publishers: Kolkata, India, 2013; pp. 337–345. [Google Scholar]
- Rathi, A.; Takkar, B.; Sharma, N. Sclerokeratouveitis and lens dislocation in a patient with genital ulcer: Was the great imitator imitated? BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef]
- Das, B.; Pandharpurkar, M. A rare disease with a rarer presentation: Nodular episcleritis in Takayasu’s arteritis. Indian J. Ophthalmol. 2022, 70, 2675. [Google Scholar] [CrossRef]
- Zeng, Y.; Duan, J.; Ge, G.; Zhang, M. Therapeutic Management of Ocular Ischemia in Takayasu’s Arteritis: A Case-Based Systematic Review. Front. Immunol. 2022, 12, 791278. [Google Scholar] [CrossRef]
- Noris, M.; Daina, E.; Gamba, S.; Bonazzola, S.; Remuzzi, G. Interleukin-6 and RANTES in Takayasu Arteritis: A guide for therapeutic decisions? Circulation 1999, 100, 55–60. [Google Scholar] [CrossRef]
- Goel, R.; Kabeerdoss, J.; Ram, B.; Prakash, J.A.J.; Babji, S.; Nair, A.; Jeyaseelan, L.; Jeyaseelan, V.; Mathew, J.; Balaji, V.; et al. Serum Cytokine Profile in Asian Indian Patients with Takayasu Arteritis and its Association with Disease Activity. Open Rheumatol. J. 2017, 11, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, L.; Haroche, J.; Mathian, A.; Gorochov, G.; Amoura, Z. Pathogenesis of Takayasu’s arteritis: A 2011 update. Autoimmun. Rev. 2011, 11, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Alibaz-Oner, F.; Yentür, S.P.; Saruhan-Direskeneli, G.; Direskeneli, H. Serum cytokine profiles in Takayasu’s arteritis: Search for biomarkers. Clin. Exp. Rheumatol. 2015, 33 (Suppl. 89), S32–S35. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrogiuseppe, E.; Pirraglia, M.P.; Sampalmieri, L.; Iannetti, L.; Beccia, A.; Gharbiya, M. Management of Spontaneous Crystalline Lens Luxation in a Patient Diagnosed with Takayasu’s Disease. Diagnostics 2023, 13, 1400. https://doi.org/10.3390/diagnostics13081400
Mastrogiuseppe E, Pirraglia MP, Sampalmieri L, Iannetti L, Beccia A, Gharbiya M. Management of Spontaneous Crystalline Lens Luxation in a Patient Diagnosed with Takayasu’s Disease. Diagnostics. 2023; 13(8):1400. https://doi.org/10.3390/diagnostics13081400
Chicago/Turabian StyleMastrogiuseppe, Elvia, Maria Pia Pirraglia, Lorenzo Sampalmieri, Ludovico Iannetti, Alessandro Beccia, and Magda Gharbiya. 2023. "Management of Spontaneous Crystalline Lens Luxation in a Patient Diagnosed with Takayasu’s Disease" Diagnostics 13, no. 8: 1400. https://doi.org/10.3390/diagnostics13081400
APA StyleMastrogiuseppe, E., Pirraglia, M. P., Sampalmieri, L., Iannetti, L., Beccia, A., & Gharbiya, M. (2023). Management of Spontaneous Crystalline Lens Luxation in a Patient Diagnosed with Takayasu’s Disease. Diagnostics, 13(8), 1400. https://doi.org/10.3390/diagnostics13081400





