Use of Autotransfusion following Percutaneous Thrombectomy for Cardiogenic Shock Due to Pulmonary Embolism in a Single Session—A Case Report
Abstract
1. Introduction
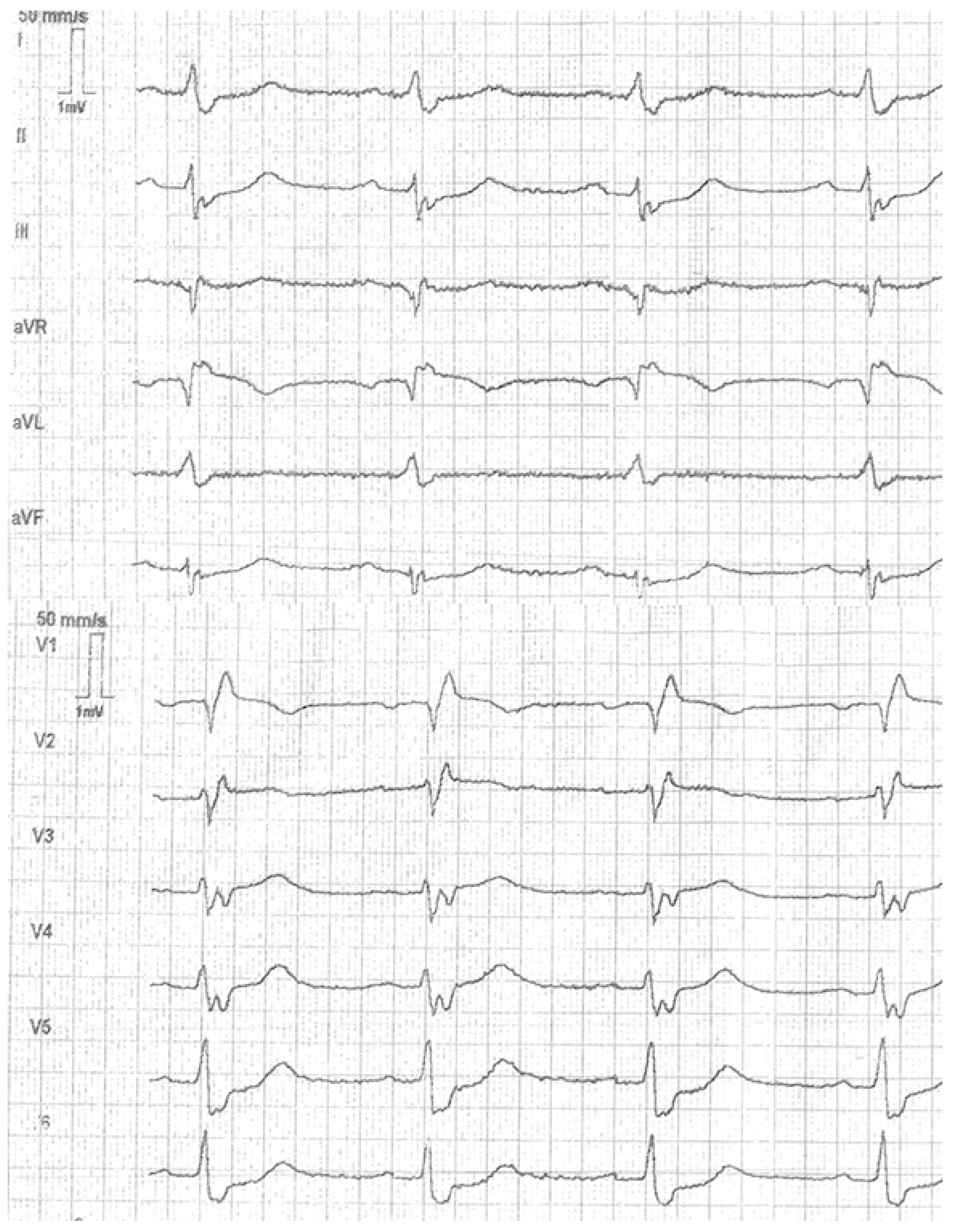
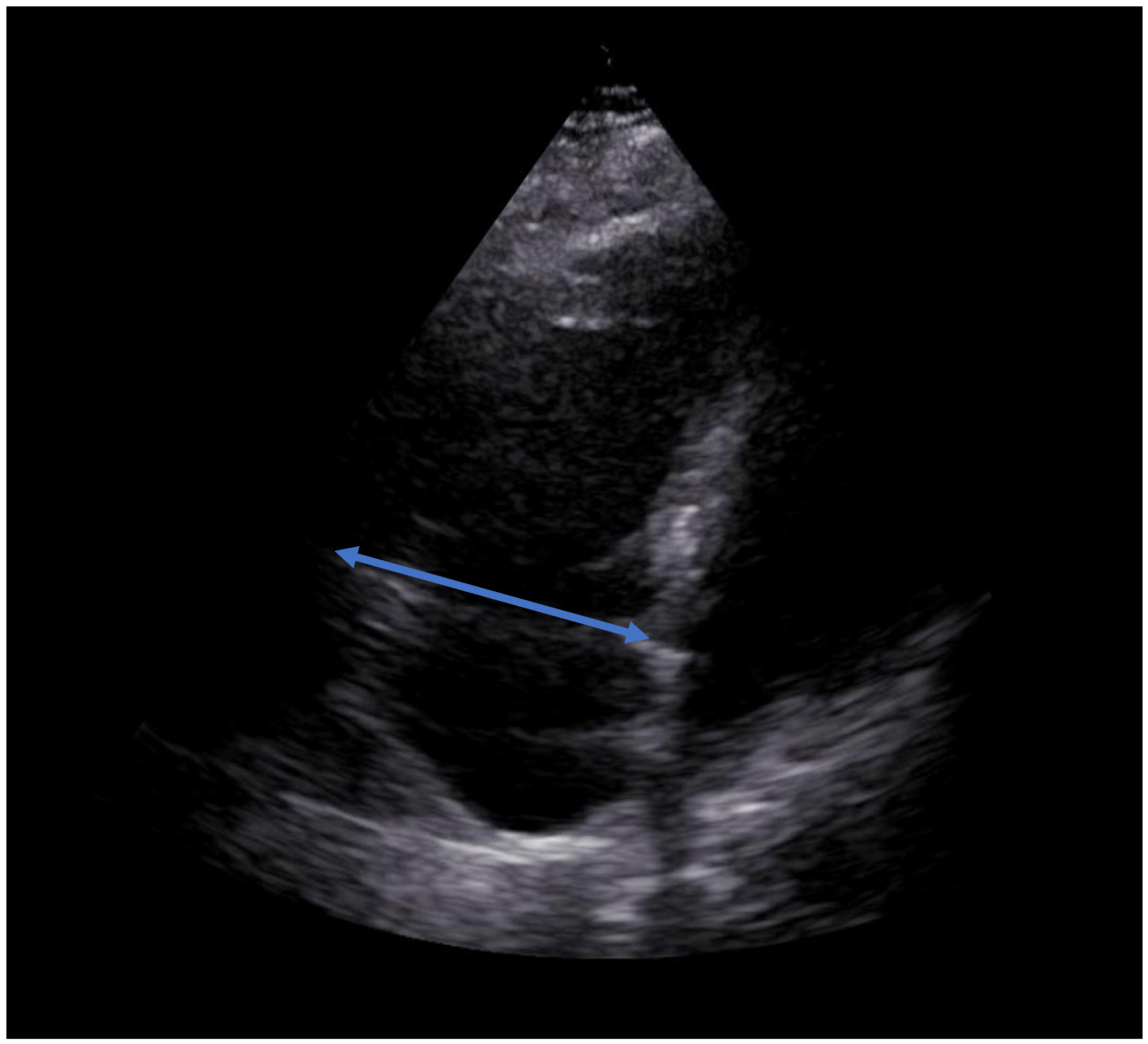
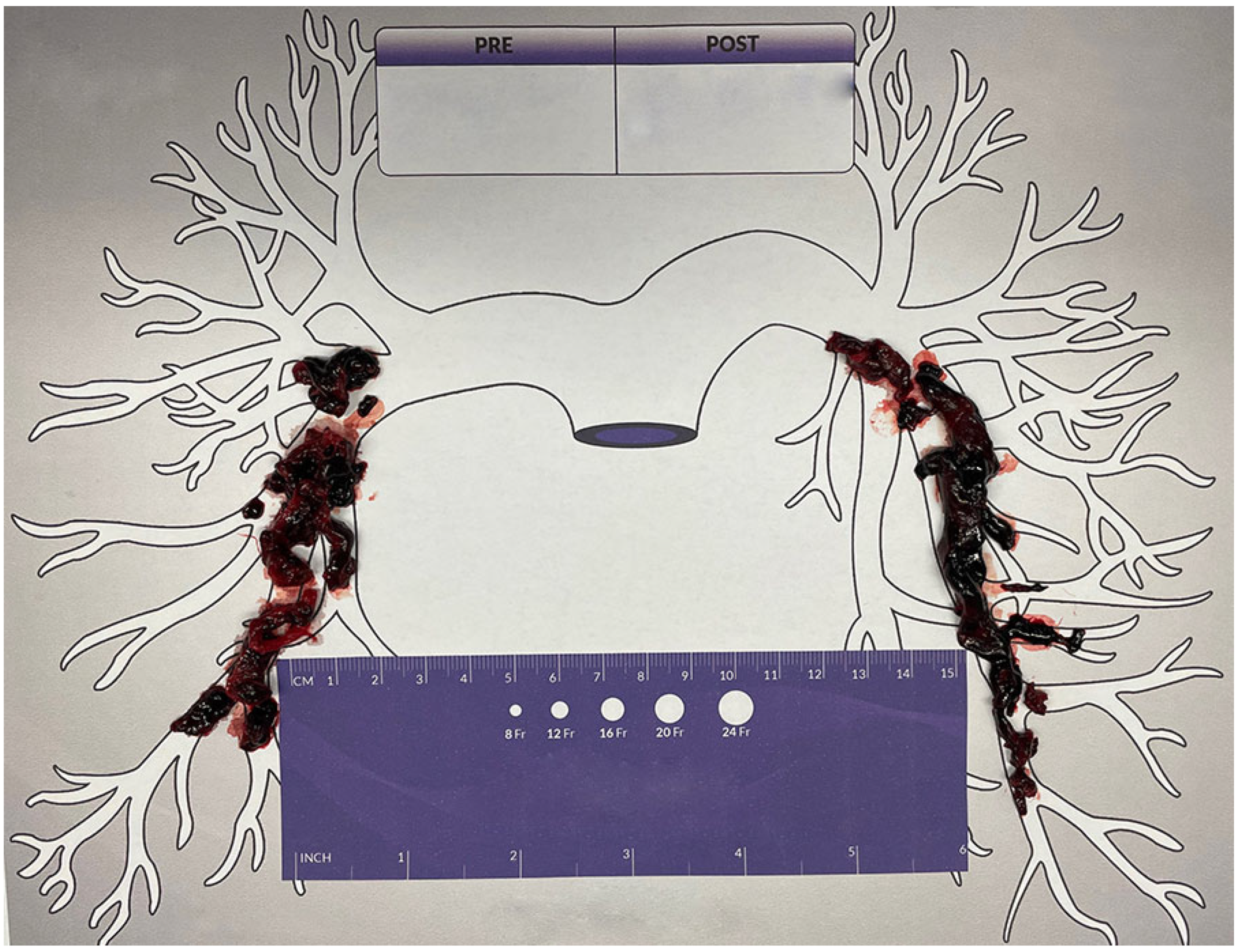
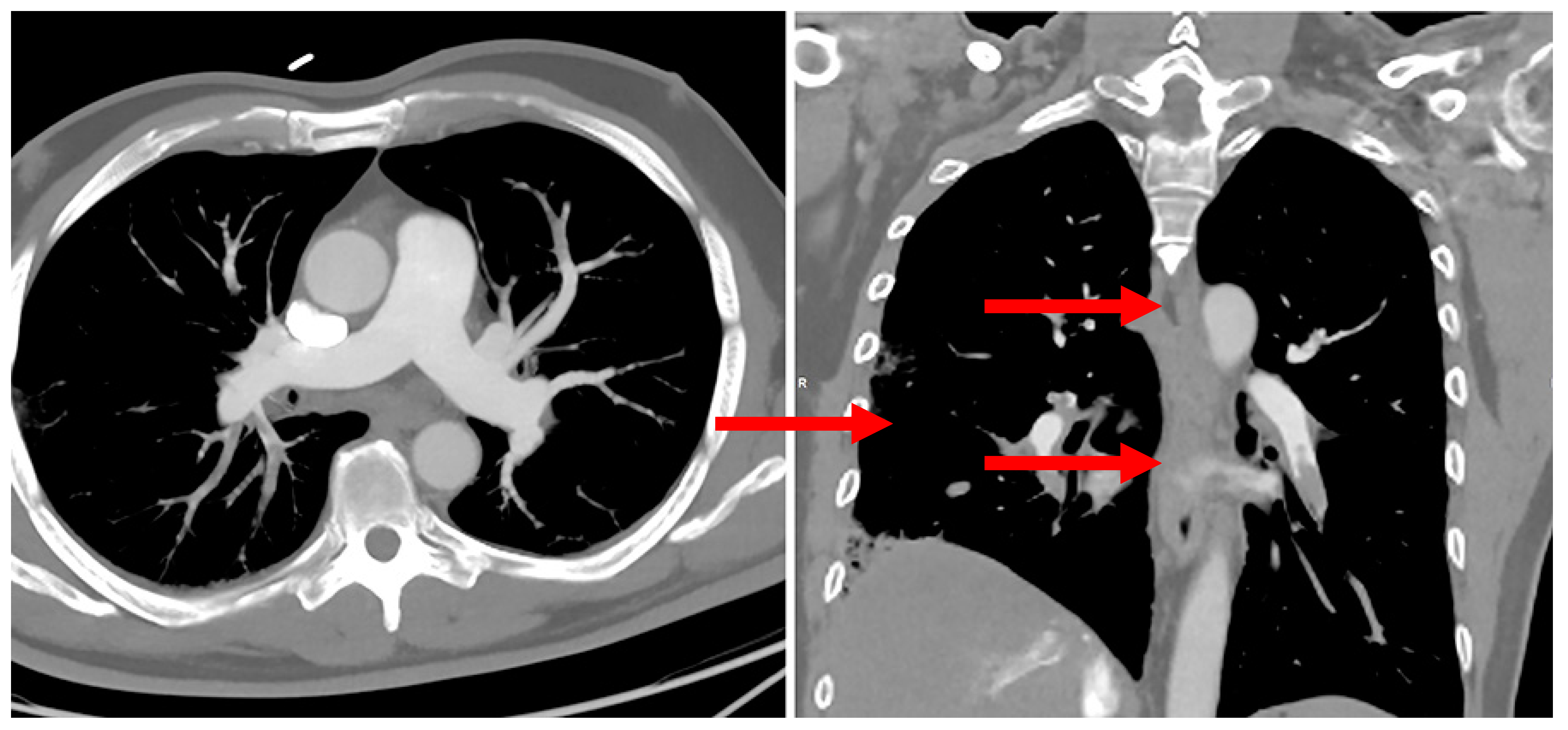
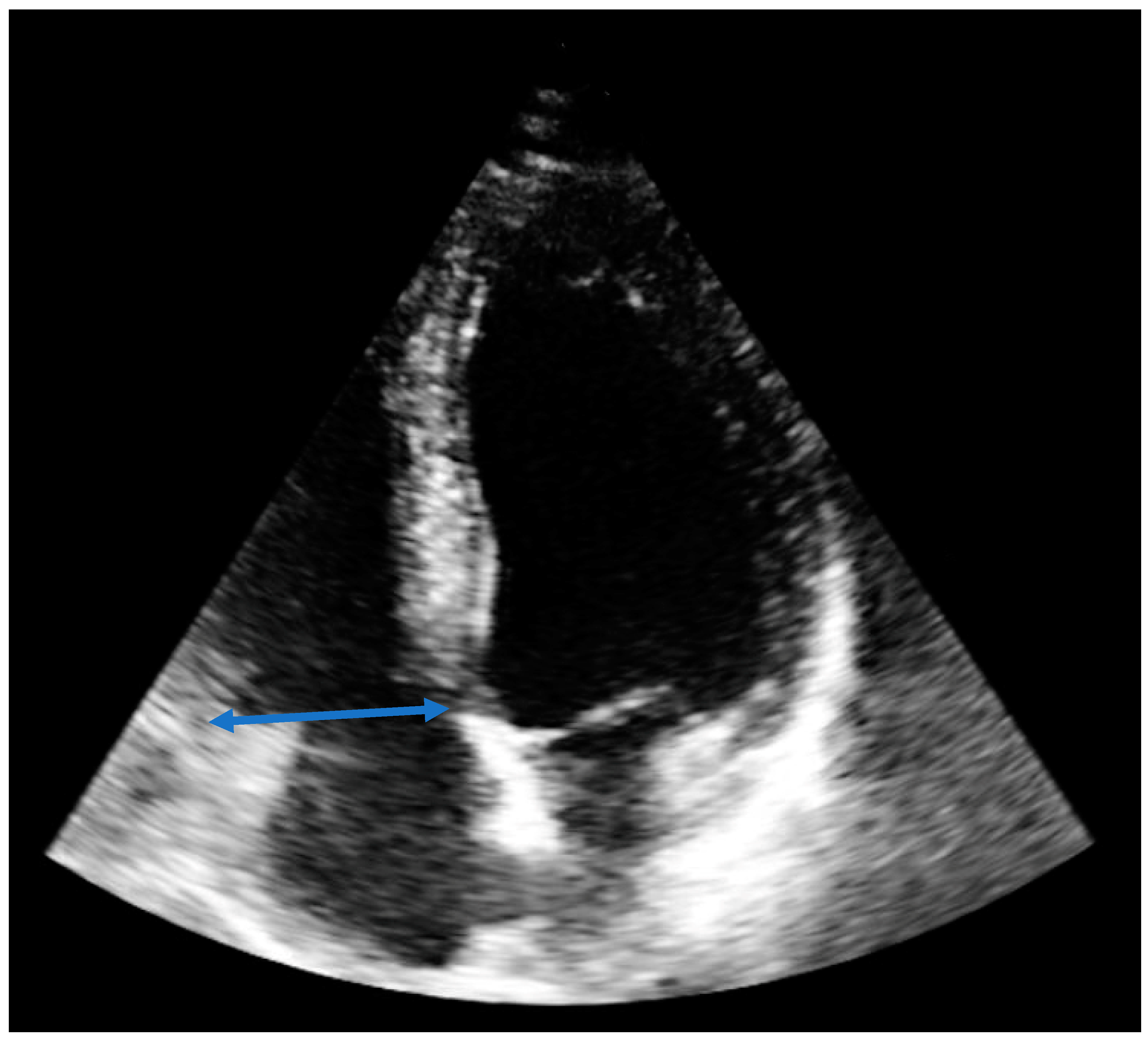
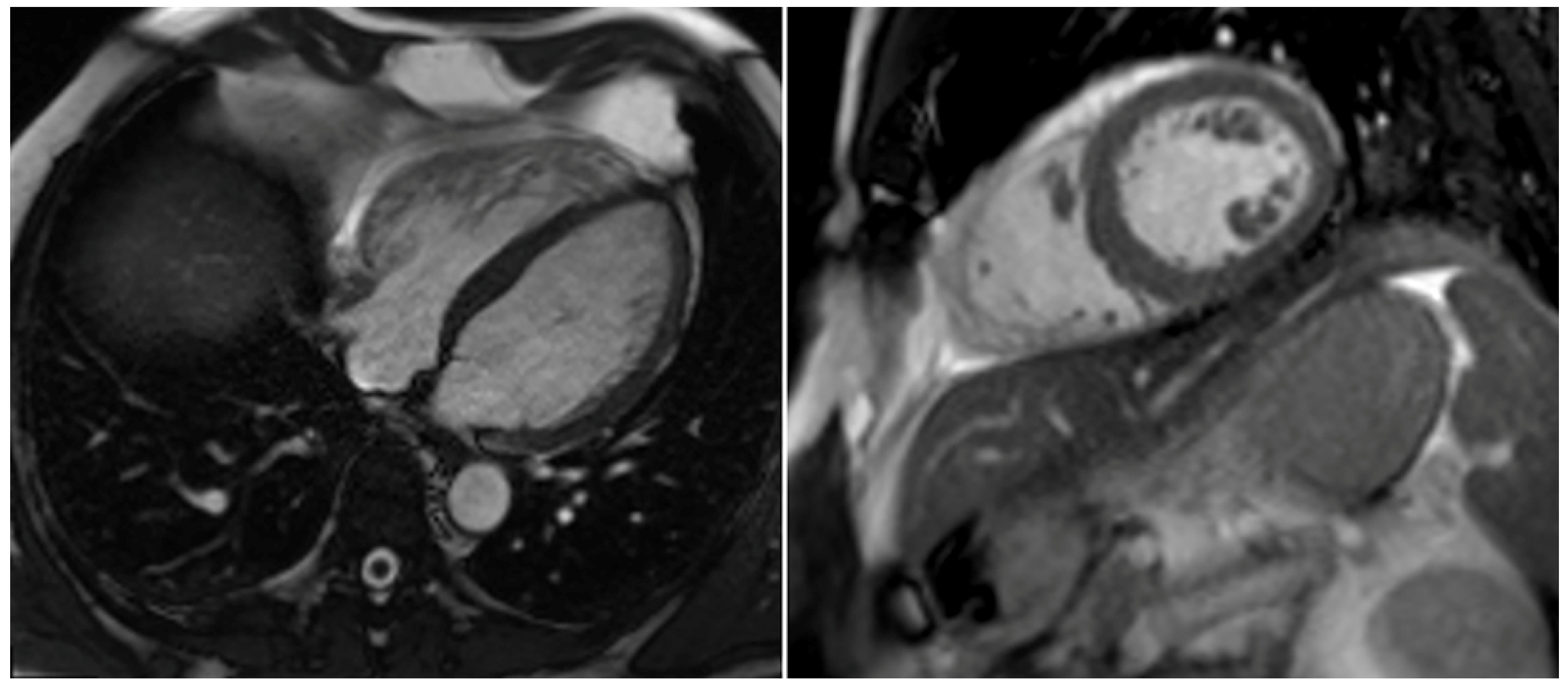
2. Case Report
3. Discussion
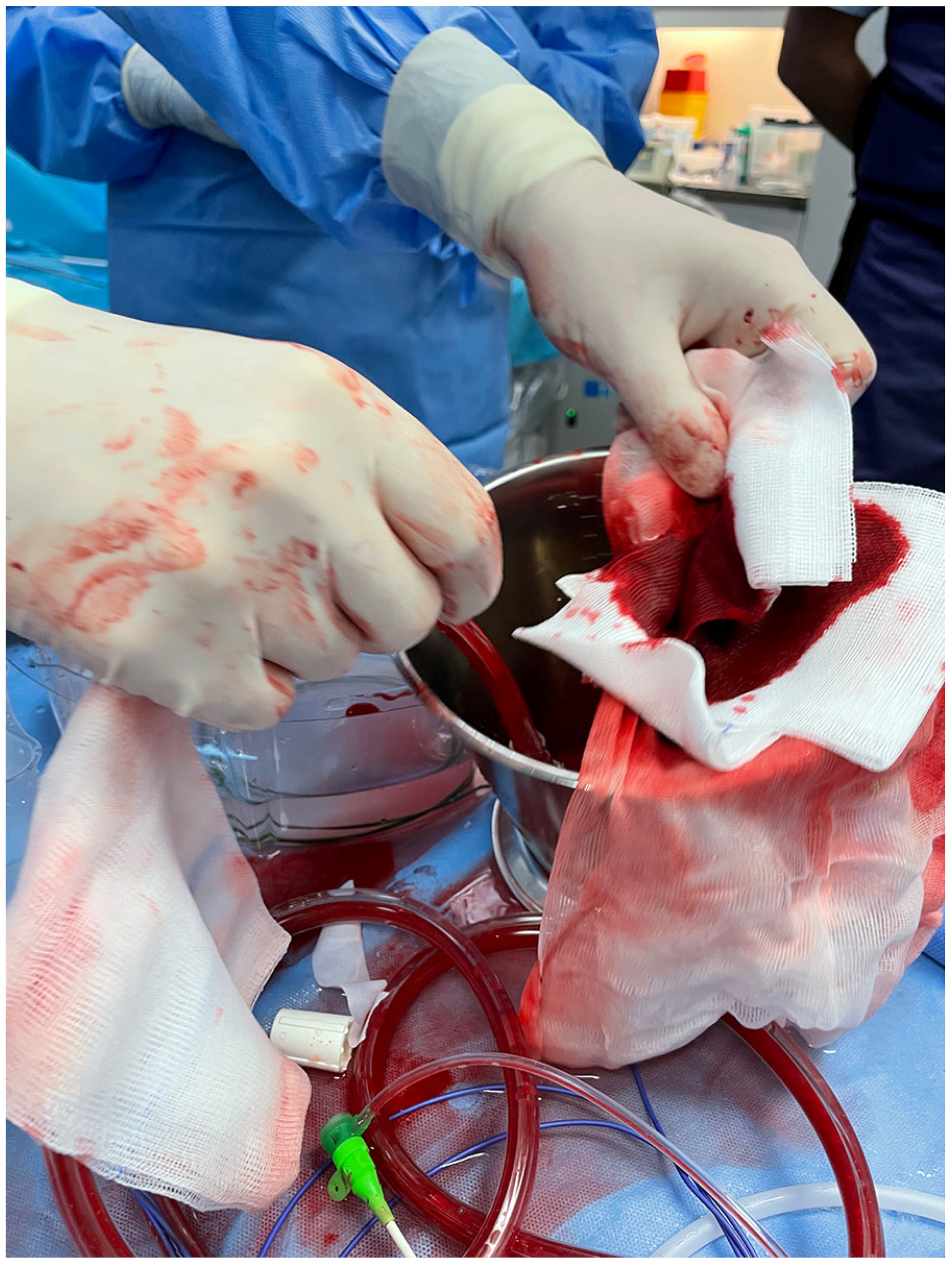
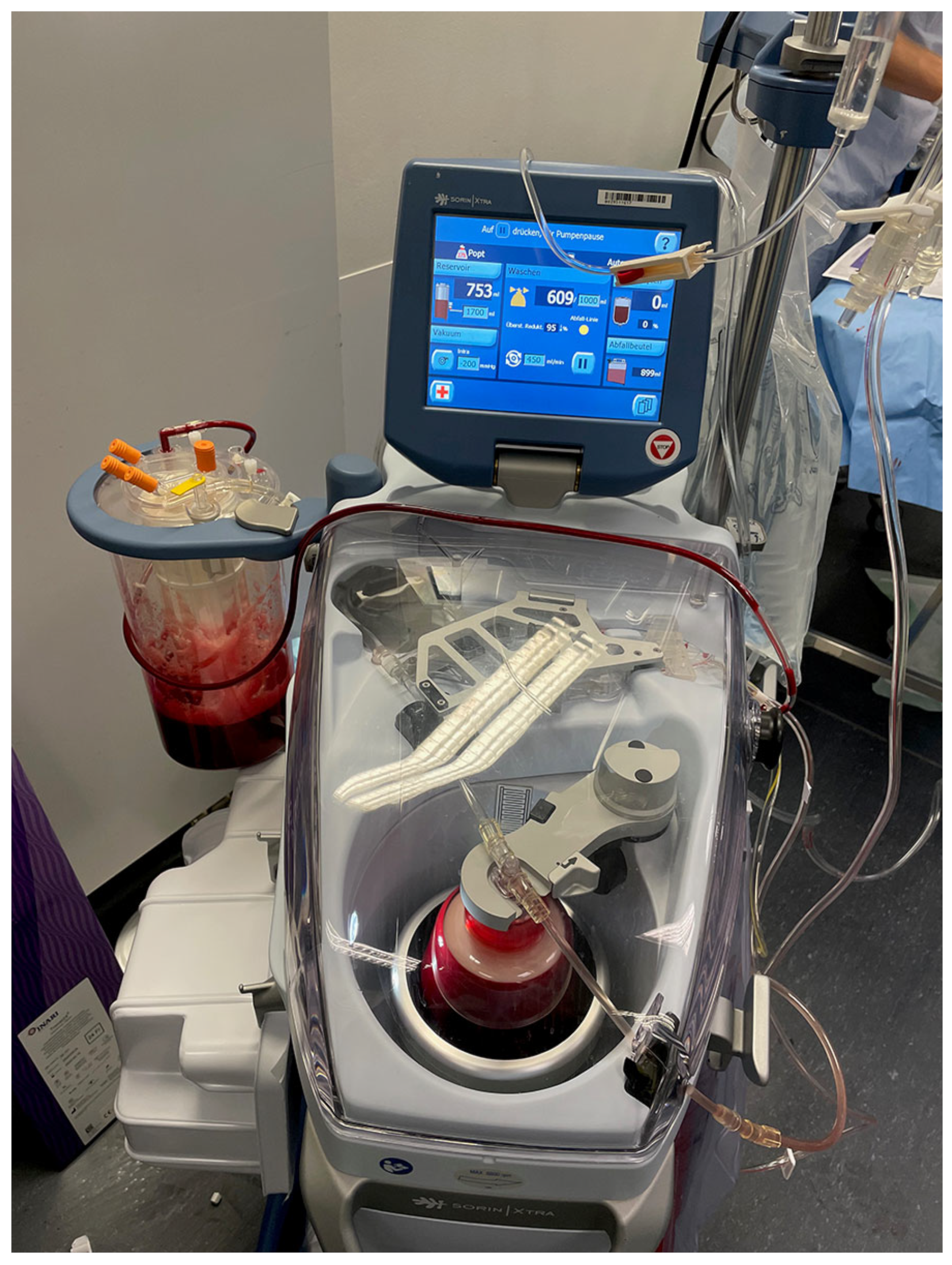
4. Autotransfusion Procedure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorse, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Tarbox, A.K.; Swaroop, M. Symposium: Embolism in the intensive care unit. Int. J. Crit. Illn. Inj. Sci. 2013, 3, 69–72. [Google Scholar] [PubMed]
- Rivera-Lebron, B.; Mcdaniel, M.; Ahrar, K.; Alrifai, A.; Dudzinski, D.M.; Fanola, C.; Blais, D.; Janicke, D.; Melamed, R.; Mohrien, K.; et al. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619853037. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.N.; Devarapally, S.R.; Lee, L.S.; Gupta, N. Catheter Directed Thrombolysis Of Pulmonary Embolism; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ucar, E.Y. Update on Thrombolytic Therapy in Acute Pulmonary Thromboembolism. Eurasian J. Med. 2019, 51, 186–190. [Google Scholar]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Meinel, F.G.; Nance, J.W., Jr.; Schoepf, U.J.; Hoffmann, V.S.; Thierfelder, K.M.; Costello, P.; Goldhaber, S.Z.; Bamberg, F. Predictive Value of Computed Tomography in Acute Pulmonary Embolism: Systematic Review and Meta-analysis. Am. J. Med. 2015, 128, 747–759.e742. [Google Scholar] [CrossRef]
- Agarwal, S.; Saum, J.; Chanamalou, S.; Cole, W.; Patel, S.M. When Harry Met Sally: Single-Session INARI FlowTriever and Impella RP. J. Cardiol. Cases 2021, 23, 57–60. [Google Scholar] [CrossRef]
- Toma, C.; Bunte, M.C.; Cho, K.H.; Jaber, W.A.; Chambers, J.; Stegman, B.; Gondi, S.; Leung, D.A.; Savin, M.; Khandhar, S.; et al. Percutaneous mechanical thrombectomy in a real-world pulmonary embolism population: Interim results of the FLASH registry. Catheter Cardiovasc. Interv. 2022, 99, 1345–1355. [Google Scholar] [CrossRef]
- Tu, T.; Toma, C.; Tapson, V.F.; Adams, C.; Jaber, W.A.; Silver, M.; Khandhar, S.; Amin, R.; Weinberg, M.; Engelhardt, T.; et al. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: The FLARE Study. JACC Cardiovasc. Interv. 2019, 12, 859–869. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Mathbout, M.F.; Hennawi, H.A.; Khedr, A.; Bidwell, K.; Todoran, T.M. Inari large-bore mechanical thrombectomy in intermediate-high risk submassive PE patients: Case series and literature review. Glob. Cardiol. Sci. Pract. 2022, 2022, e202208. [Google Scholar] [CrossRef]
- Nadler, S.B.; Hidalgo, J.H.; Bloch, T. Prediction of blood volume in normal human adults. Surgery 1962, 51, 224–232. [Google Scholar]
- Hooper, N.; Armstrong, T.J. Hemorrhagic Shock; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Carless, P.A.; Henry, D.A.; Moxey, A.J.; O’connell, D.; Brown, T.; Fergusson, D.A. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst. Rev. 2010, 2010, Cd001888. [Google Scholar]
- Klein, A.A.; Bailey, C.R.; Charlton, A.J.; Evans, E.; Guckian-Fisher, M.; Mccrossan, R.; Nimmo, A.F.; Payne, S.; Shreeve, K.; Smith, J.; et al. Association of Anaesthetists guidelines: Cell salvage for peri-operative blood conservation 2018. Anaesthesia 2018, 73, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.H.; Coelho, G.R.; Feitosa Neto, B.A.; Nogueira, E.A.; Teixeira, C.C.; Mesquita, D.F. Liver transplantation in Jehovah’s Witnesses patients in a center of northeastern Brazil. Arq. Gastroenterol. 2013, 50, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.V.; Nagababu, E.; Johnson, D.J.; Kebaish, K.M.; Lipsitz, J.A.; Dwyer, I.M.; Zuckerberg, G.S.; Barodka, V.M.; Berkowitz, D.E.; Frank, S.M. 2,3-Diphosphoglycerate Concentrations in Autologous Salvaged Versus Stored Red Blood Cells and in Surgical Patients After Transfusion. Anesth. Analg. 2016, 122, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, D.; Wasylewski, A.H.; Rassat, J.; Thys, J. Red blood cell survival and morphology during and after intraoperative autotransfusion. Acta Anaesthesiol. Belg. 1984, 35, 43–49. [Google Scholar]
- Waters, J.H. Indications and contraindications of cell salvage. Transfusion 2004, 44 (Suppl. S12), 40S–44S. [Google Scholar] [CrossRef]
- Ottesen, S.; Froysaker, T. Use of Haemonetics Cell Saver for autotransfusion in cardiovascular surgery. Scand. J. Thorac. Cardiovasc. Surg. 1982, 16, 263–268. [Google Scholar] [CrossRef]
- Esper, S.A.; Waters, J.H. Intra-operative cell salvage: A fresh look at the indications and contraindications. Blood Transfus. 2011, 9, 139–147. [Google Scholar]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernandez-Mondejar, E.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Nardi, G.; et al. Management of bleeding and coagulopathy following major trauma: An updated European guideline. Crit. Care 2013, 17, R76. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Melnyk, V.; Facco, F.L.; Waters, J.H.; Smith, K.J. Cost-effectiveness Analysis of Intraoperative Cell Salvage for Obstetric Hemorrhage. Anesthesiology 2018, 128, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.H. Intraoperative blood recovery. ASAIO J. 2013, 59, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.H.; Lukauskiene, E.; Anderson, M.E. Intraoperative blood salvage during cesarean delivery in a patient with beta thalassemia intermedia. Anesth. Analg. 2003, 97, 1808–1809. [Google Scholar] [CrossRef]



| Preinterventional | Immediately after the Procedure | At Discharge 6 Days after the Procedure | |
|---|---|---|---|
| Clinical data | |||
| Dyspnea [NYHA] | IV | II–III | 0 |
| Arterial blood pressure [systolic/diastolic/mean, mmHg] | 73/44/70 | 103/70/84 | 122/61/81 |
| Heart rate [beats/minute] | 100 | 81 | 64 |
| SpO2 [%] | 90 | 95 | 97 |
| Oxygen supplementation [liters/minute] | 10 | 3 | 0 |
| Respiratory rate [breaths/minute] | 29 | 16 | 12–14 |
| Echocardiographic data | |||
| RVEDD [mm] | 65 | 49 | 37 |
| TAPSE [mm] | 12 | 16 | 21 |
| TASV [cm/s] | 8 | 12 | 17 |
| sPAP [mmHg] | 55 | 15 | 14 |
| LVEF [%] | 43 | 54 | 56 |
| D- Sign | Yes | No | No |
| McConnell‘s sign | Yes | Yes | No |
| Systolic notching of PWD over pulmonary valve | Yes | Slightly | No |
| PAC hemodynamics | |||
| PAP [systolic/diastolic/mean, mmHg] | 44/23/29 | 25/10/16 | Not available |
| Laboratory data | |||
| D-Dimer [µg/L] | 41,531 | Not available | Not available |
| Troponin [pg/mL] | 75.20 | 505 | 21.8 |
| Myoglobin [µg/L] | 80 | 36 | Not available |
| NT-Pro-BNP [pg/mL] | 207 | 1062 | <50 |
| Arterial lactate [mmol/L] | 3.5 | 1.5 | Not available |
| Hb [mmol/l] | 10 | 8.2 | 9.7 |
| Thrombocytes [Gpt/l] | 175 | 142 | 315 |
| Hematocrit [%] | 47 | 38 | 42 |
| Therapy data | |||
| Noradrenalin [µg/kg/min] | 0.47 | 0 | 0 |
| Dobutamine [µg/kg/min] | 8.3 | 0 | 0 |
| Unfractionated intravenous heparin | Yes | Yes | No |
| NOAC | No | No | Yes (Apixaban) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haertel, F.; Baez, L.; Franz, M.; Bogoviku, J.; Klein, F.; Dannberg, G.; Schulze, P.C.; Möbius-Winkler, S. Use of Autotransfusion following Percutaneous Thrombectomy for Cardiogenic Shock Due to Pulmonary Embolism in a Single Session—A Case Report. Diagnostics 2023, 13, 1392. https://doi.org/10.3390/diagnostics13081392
Haertel F, Baez L, Franz M, Bogoviku J, Klein F, Dannberg G, Schulze PC, Möbius-Winkler S. Use of Autotransfusion following Percutaneous Thrombectomy for Cardiogenic Shock Due to Pulmonary Embolism in a Single Session—A Case Report. Diagnostics. 2023; 13(8):1392. https://doi.org/10.3390/diagnostics13081392
Chicago/Turabian StyleHaertel, Franz, Laura Baez, Marcus Franz, Jurgen Bogoviku, Friederike Klein, Gudrun Dannberg, P. Christian Schulze, and Sven Möbius-Winkler. 2023. "Use of Autotransfusion following Percutaneous Thrombectomy for Cardiogenic Shock Due to Pulmonary Embolism in a Single Session—A Case Report" Diagnostics 13, no. 8: 1392. https://doi.org/10.3390/diagnostics13081392
APA StyleHaertel, F., Baez, L., Franz, M., Bogoviku, J., Klein, F., Dannberg, G., Schulze, P. C., & Möbius-Winkler, S. (2023). Use of Autotransfusion following Percutaneous Thrombectomy for Cardiogenic Shock Due to Pulmonary Embolism in a Single Session—A Case Report. Diagnostics, 13(8), 1392. https://doi.org/10.3390/diagnostics13081392






