Identification of the Prognostic Biomarkers CBX6 and CBX7 in Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. ONCOMINE Database
2.2. The Cancer Genome Atlas Database
2.3. Kaplan–Meier Plotter Database
2.4. TISIDB Analysis
2.5. The Human Protein Atlas Database
2.6. GeneMANIA
2.7. Metascape
2.8. TIMER
2.9. Mutation Data, Heatmaps, and Analysis of Immune Checkpoints and m6A in CBXs
2.10. Tumor-Infiltrating Immune Cell Profile
2.11. Cell Lines
2.12. Western Blotting
2.13. Statistical Analysis
2.14. Multiple Immunohistochemistry
3. Results
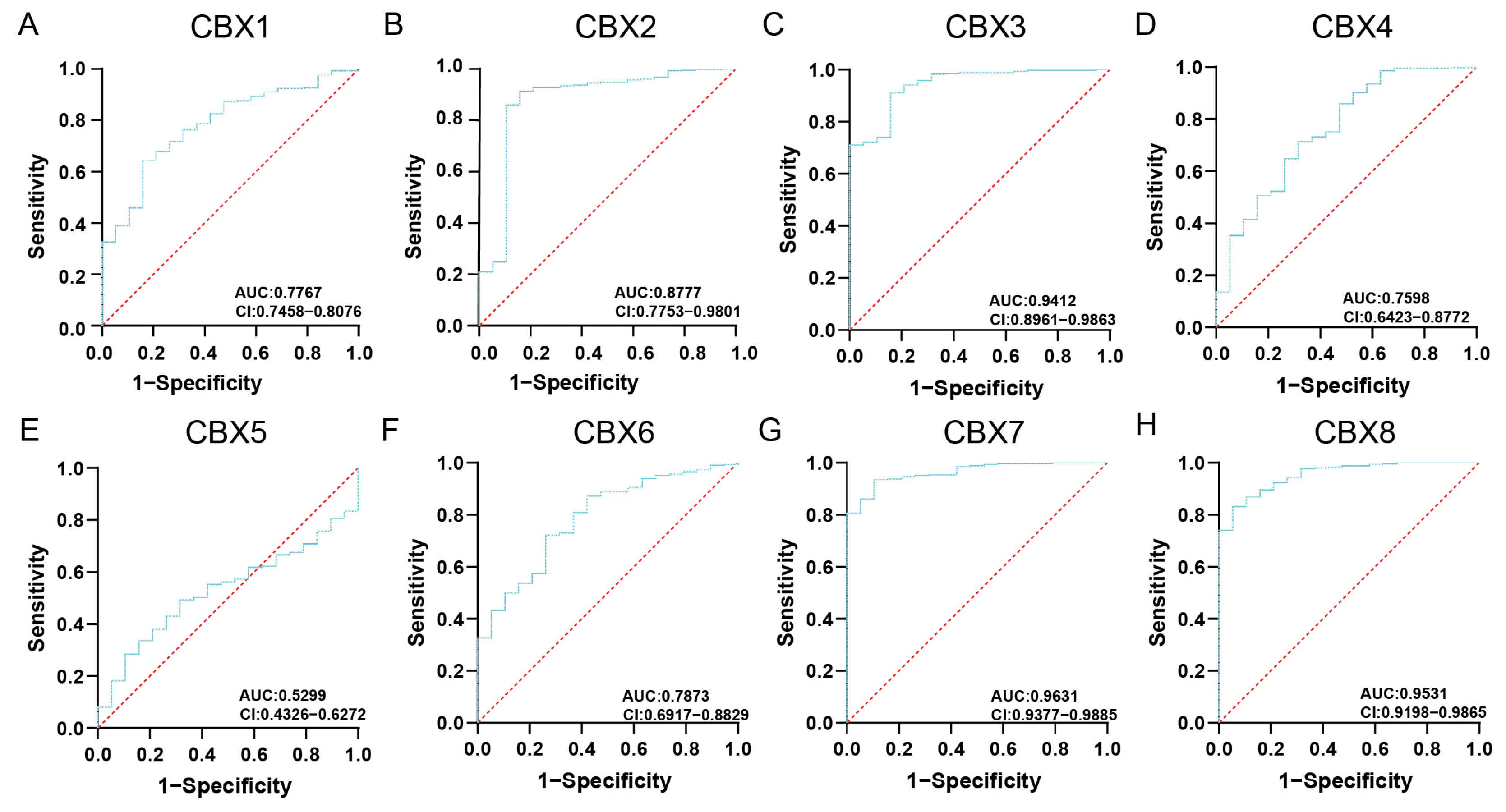
3.1. Expression Levels of CBXs
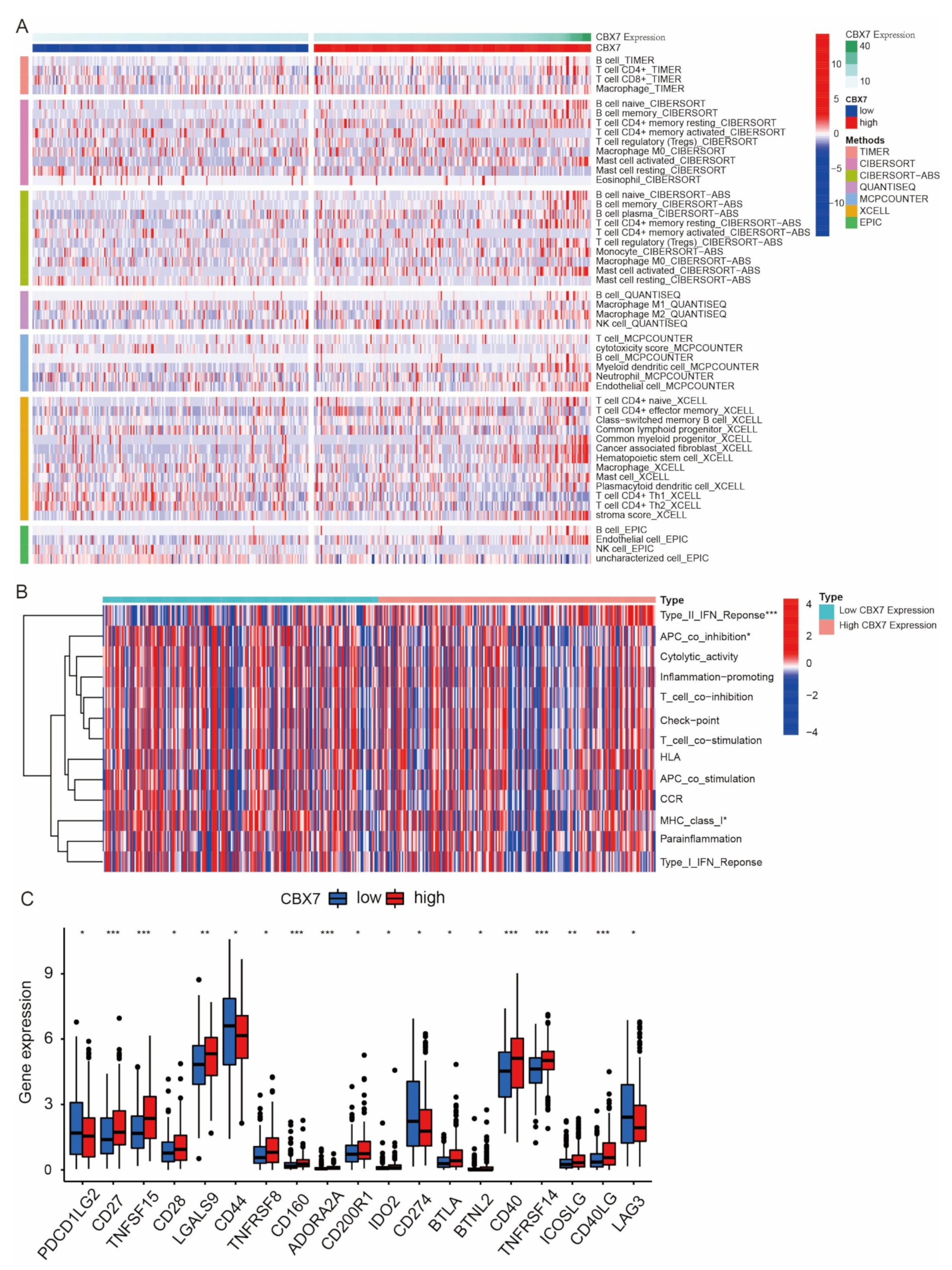
3.2. Association of CBX mRNA Expression with Immune Subtypes in BLCA Patients
3.3. Prognostic Factors Associated with Mortality of BLCA
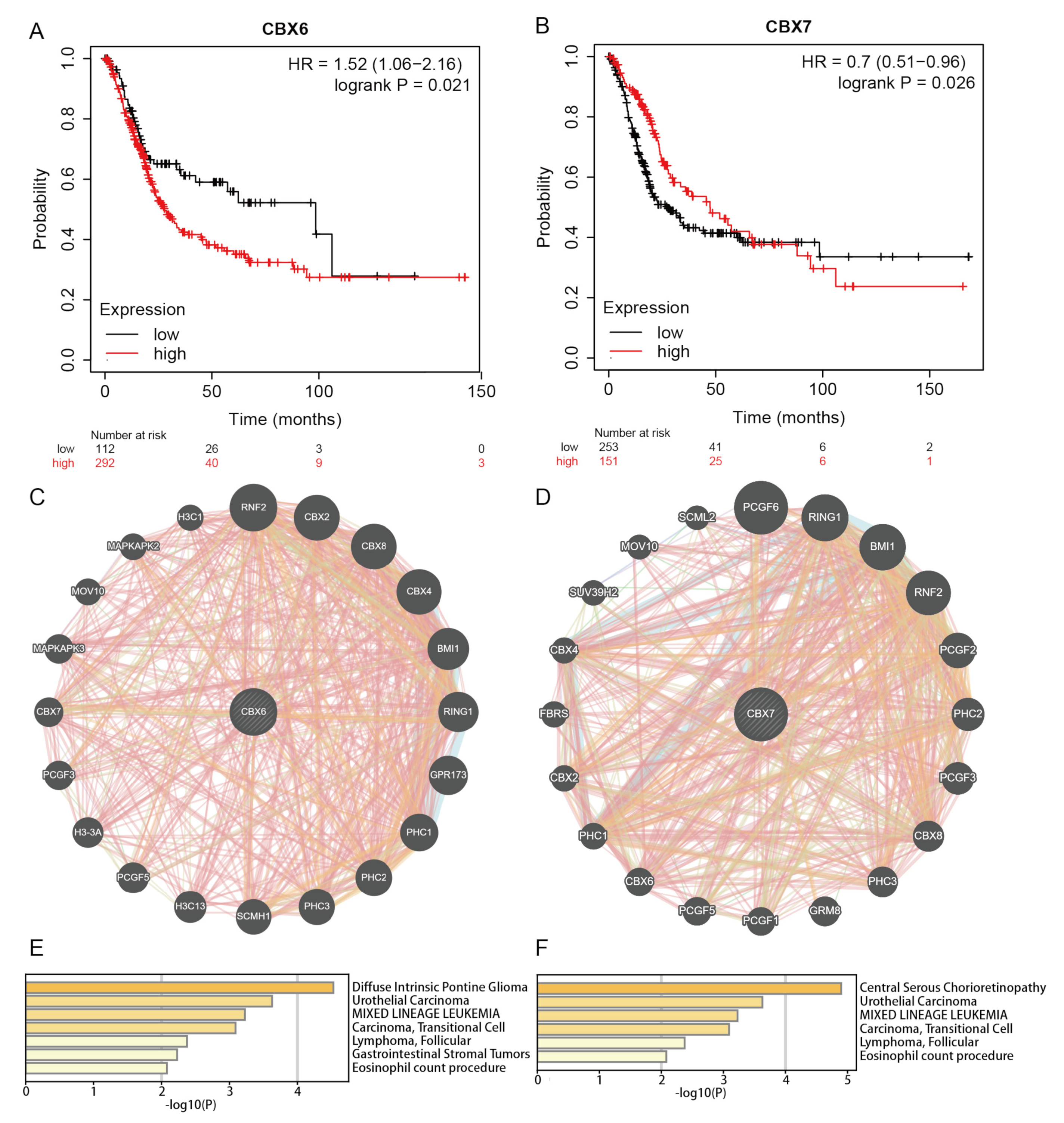
3.4. Prognostic Value of CBXs in BLCA Patients
3.5. Functional Enrichment Analysis of CBXs
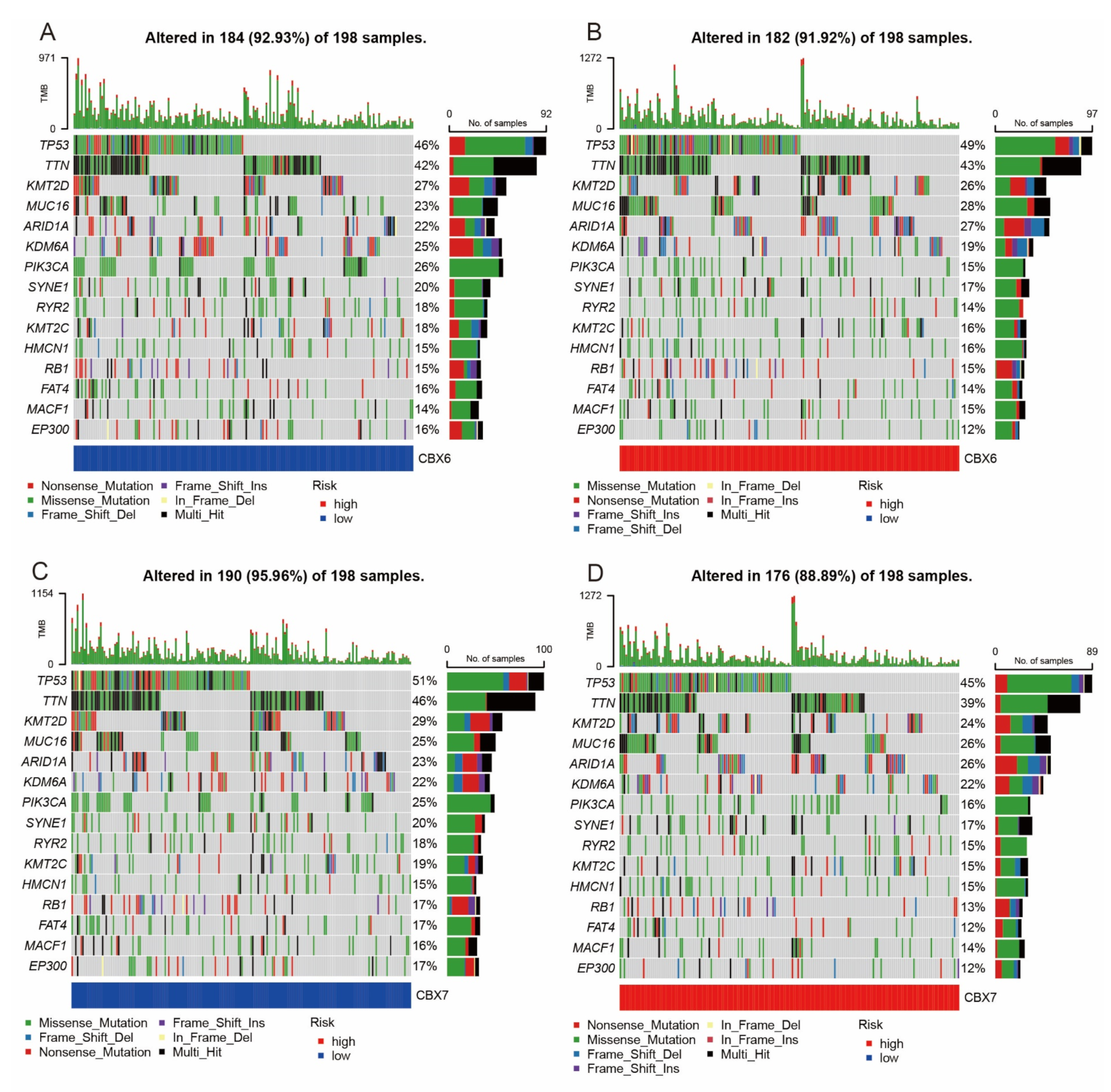
3.6. Mutation Analysis
3.7. Relationship of CBXs and m6A
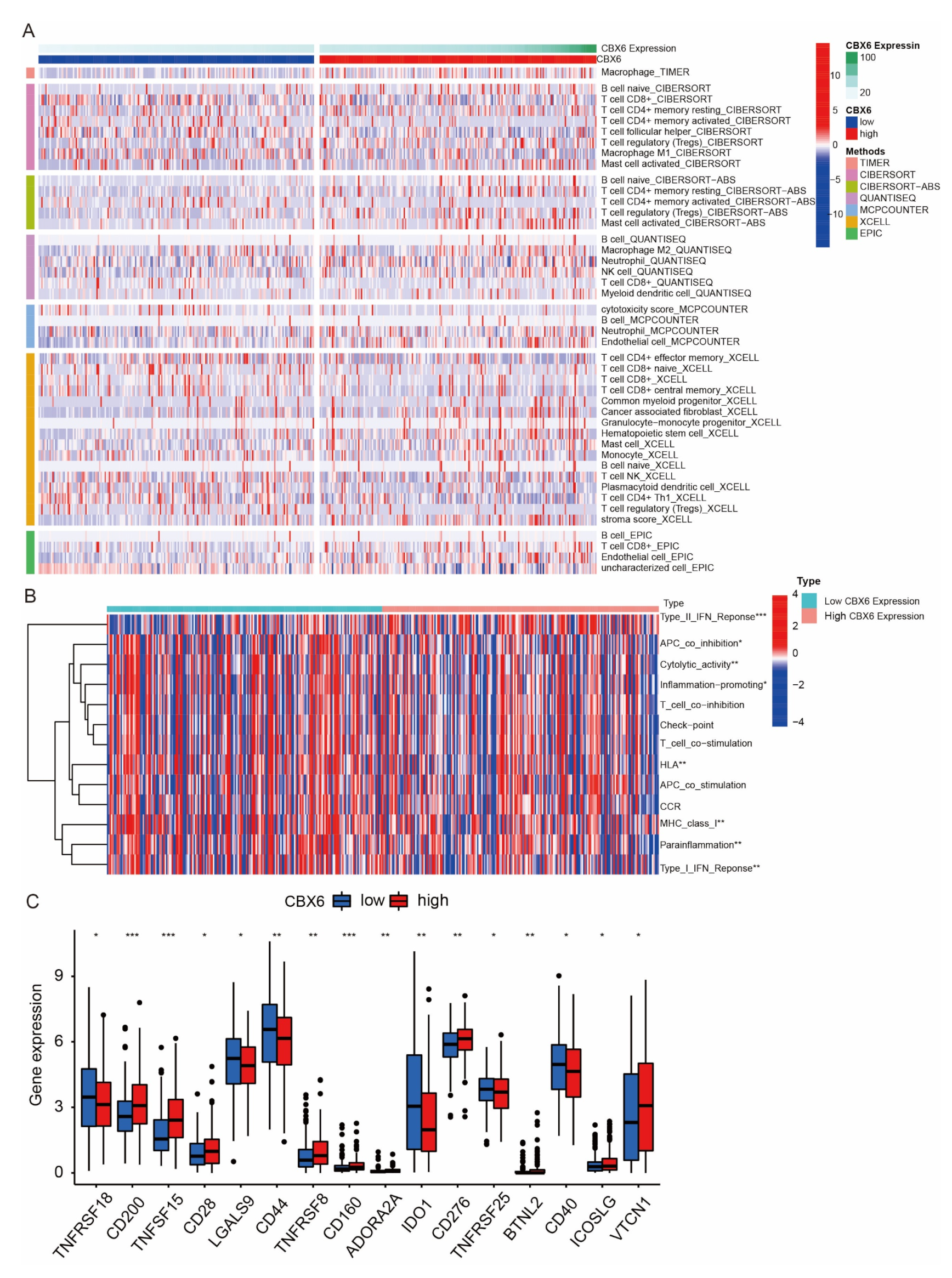
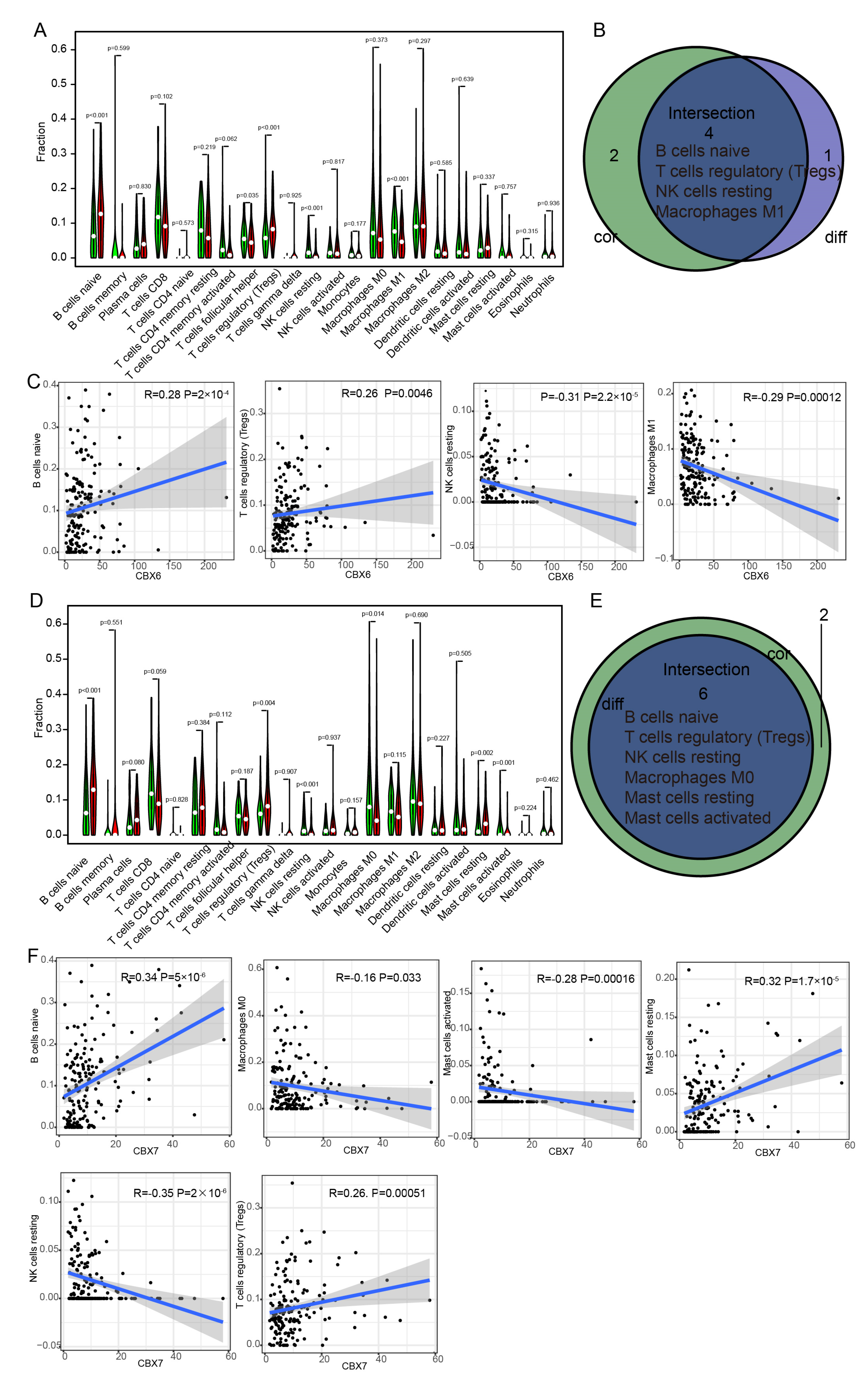
3.8. Immune Cell Infiltration of CBX6 and CBX7 in BLCA Patients
3.9. CBX Family Protein Expression in Normal Urothelial Cells (SV-HUC-1) and BLCA Cell Lines (5637 and T24) Verified Using Western Blotting
3.10. mIHC Results Confirm the Relationship between CBX6 and CBX7 Expression and Immune Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Paner, G.P.; Zehnder, P.; Amin, A.M.; Husain, A.N.; Desai, M.M. Urothelial neoplasms of the urinary bladder occurring in young adult and pediatric patients: A comprehensive review of literature with implications for patient management. Adv. Anat. Pathol. 2011, 18, 79–89. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Malats, N.; Real, F.X. Epidemiology of bladder cancer. Hematol. Oncol. Clin. N. Am. 2015, 29, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Nadal, R.; Bellmunt, J. Management of metastatic bladder cancer. Cancer Treat. Rev. 2019, 76, 10–21. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Lin, T. Lymphatic metastasis of bladder cancer: Molecular mechanisms, diagnosis and targeted therapy. Cancer Lett. 2021, 505, 13–23. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, S.; Ma, J.; Chen, Z.; Song, G.; Rao, D.; Cheng, Y.; Huang, S.; Liu, Y.; Jiang, S.; et al. Spatiotemporal Immune Landscape of Colorectal Cancer Liver Metastasis at Single-Cell Level. Cancer Discov. 2022, 12, 134–153. [Google Scholar] [CrossRef]
- Wang, H.; Mei, Y.; Luo, C.; Huang, Q.; Wang, Z.; Lu, G.M.; Qin, L.; Sun, Z.; Huang, C.W.; Yang, Z.W.; et al. Single-Cell Analyses Reveal Mechanisms of Cancer Stem Cell Maintenance and Epithelial-Mesenchymal Transition in Recurrent Bladder Cancer. Clin. Cancer Res. 2021, 27, 6265–6278. [Google Scholar] [CrossRef]
- Truong, A.S.; Zhou, M.; Krishnan, B.; Utsumi, T.; Manocha, U.; Stewart, K.G.; Beck, W.; Rose, T.L.; Milowsky, M.I.; He, X.; et al. Entinostat induces antitumor immune responses through immune editing of tumor neoantigens. J. Clin. Investig. 2021, 131, e138560. [Google Scholar] [CrossRef]
- Wolf, M.T.; Ganguly, S.; Wang, T.L.; Anderson, C.W.; Sadtler, K.; Narain, R.; Cherry, C.; Parrillo, A.J.; Park, B.V.; Wang, G.; et al. A biologic scaffold-associated type 2 immune microenvironment inhibits tumor formation and synergizes with checkpoint immunotherapy. Sci. Transl. Med. 2019, 11, eaat7973. [Google Scholar] [CrossRef]
- Vincenz, C.; Kerppola, T.K. Different polycomb group CBX family proteins associate with distinct regions of chromatin using nonhomologous protein sequences. Proc. Natl. Acad. Sci. USA 2008, 105, 16572–16577. [Google Scholar] [CrossRef] [PubMed]
- Jangal, M.; Lebeau, B.; Witcher, M. Beyond EZH2: Is the polycomb protein CBX2 an emerging target for anti-cancer therapy? Expert Opin. Ther. Targets 2019, 23, 565–578. [Google Scholar] [CrossRef]
- Zhang, C.; Chang, L.; Yao, Y.; Chao, C.; Ge, Z.; Fan, C.; Yu, H.; Wang, B.; Yang, J. Role of the CBX Molecular Family in Lung Adenocarcinoma Tumorigenesis and Immune Infiltration. Front. Genet. 2021, 12, 771062. [Google Scholar] [CrossRef] [PubMed]
- Mattout, A.; Aaronson, Y.; Sailaja, B.S.; Raghu Ram, E.V.; Harikumar, A.; Mallm, J.P.; Sim, K.H.; Nissim-Rafinia, M.; Supper, E.; Singh, P.B.; et al. Heterochromatin Protein 1beta (HP1beta) has distinct functions and distinct nuclear distribution in pluripotent versus differentiated cells. Genome Biol. 2015, 16, 213. [Google Scholar] [CrossRef] [PubMed]
- Van Wijnen, A.J.; Bagheri, L.; Badreldin, A.A.; Larson, A.N.; Dudakovic, A.; Thaler, R.; Paradise, C.R.; Wu, Z. Biological functions of chromobox (CBX) proteins in stem cell self-renewal, lineage-commitment, cancer and development. Bone 2021, 143, 115659. [Google Scholar] [CrossRef]
- Huang, Z.; Yan, Y.; Zhu, Z.; Liu, J.; He, X.; Dalangood, S.; Li, M.; Tan, M.; Cai, J.; Tang, P.; et al. CBX7 suppresses urinary bladder cancer progression via modulating AKR1B10-ERK signaling. Cell Death Dis. 2021, 12, 537. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Kalyana-Sundaram, S.; Mahavisno, V.; Varambally, R.; Yu, J.; Briggs, B.B.; Barrette, T.R.; Anstet, M.J.; Kincead-Beal, C.; Kulkarni, P.; et al. Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 2007, 9, 166–180. [Google Scholar] [CrossRef]
- Wang, Z.; Jensen, M.A.; Zenklusen, J.C. A Practical Guide to The Cancer Genome Atlas (TCGA). Methods Mol. Biol. 2016, 1418, 111–141. [Google Scholar] [CrossRef]
- Goel, M.K.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274–278. [Google Scholar] [CrossRef]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: An integrated repository portal for tumor-immune system interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef] [PubMed]
- Asplund, A.; Edqvist, P.H.; Schwenk, J.M.; Ponten, F. Antibodies for profiling the human proteome—The Human Protein Atlas as a resource for cancer research. Proteomics 2012, 12, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhang, N.; Chen, M.; Wang, H.; Zhu, D. Targeting the tumour immune microenvironment for cancer therapy in human gastrointestinal malignancies. Cancer Lett. 2019, 458, 123–135. [Google Scholar] [CrossRef]
- Li, P.H.; Kong, X.Y.; He, Y.Z.; Liu, Y.; Peng, X.; Li, Z.H.; Xu, H.; Luo, H.; Park, J. Recent developments in application of single-cell RNA sequencing in the tumour immune microenvironment and cancer therapy. Mil. Med. Res. 2022, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Ning, G.; Huang, Y.L.; Zhen, L.M.; Xu, W.X.; Jiao, Q.; Yang, F.J.; Wu, L.N.; Zheng, Y.Y.; Song, J.; Wang, Y.S.; et al. Transcriptional expressions of Chromobox 1/2/3/6/8 as independent indicators for survivals in hepatocellular carcinoma patients. Aging 2018, 10, 3450–3473. [Google Scholar] [CrossRef]
- Chang, S.C.; Lai, Y.C.; Chen, Y.C.; Wang, N.K.; Wang, W.S.; Lai, J.I. CBX3/heterochromatin protein 1 gamma is significantly upregulated in patients with non-small cell lung cancer. Asia Pac. J. Clin. Oncol. 2018, 14, e283–e288. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhao, L.Y.; He, X.; Yao, R.F.; Lu, F.; Lu, B.N.; Pang, Z.R. CBXs-related prognostic gene signature correlates with immune microenvironment in gastric cancer. Aging 2022, 14, 6227–6254. [Google Scholar] [CrossRef]
- Hu, K.; Yao, L.; Xu, Z.; Yan, Y.; Li, J. Prognostic Value and Therapeutic Potential of CBX Family Members in Ovarian Cancer. Front. Cell Dev. Biol. 2022, 10, 832354. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Qi, Y.; Zuo, Y.; Wang, Q.; Zou, Y.; Schwartz, R.J.; Cheng, J.; Yeh, E.T. SUMO-specific protease 2 is essential for suppression of polycomb group protein-mediated gene silencing during embryonic development. Mol. Cell 2010, 38, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.K.; Lin, H.Y.; Chen, C.F.; Zeng, D. Prognostic values of distinct CBX family members in breast cancer. Oncotarget 2017, 8, 92375–92387. [Google Scholar] [CrossRef]
- Huang, H.; Sun, J.; Li, Z.; Zhang, X.; Li, Z.; Zhu, H.; Yu, X. Impact of the tumor immune microenvironment on the outcome of pancreatic cancer: A retrospective study based on clinical pathological analysis. Gland. Surg. 2022, 11, 472–482. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Mertins, P.; Mani, D.R.; Ruggles, K.V.; Gillette, M.A.; Clauser, K.R.; Wang, P.; Wang, X.; Qiao, J.W.; Cao, S.; Petralia, F.; et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 2016, 534, 55–62. [Google Scholar] [CrossRef]
- Shah, N.; Wang, P.; Wongvipat, J.; Karthaus, W.R.; Abida, W.; Armenia, J.; Rockowitz, S.; Drier, Y.; Bernstein, B.E.; Long, H.W.; et al. Regulation of the glucocorticoid receptor via a BET-dependent enhancer drives antiandrogen resistance in prostate cancer. eLife 2017, 6, e27861. [Google Scholar] [CrossRef]
- Griffiths, B.; Lewis, C.A.; Bensaad, K.; Ros, S.; Zhang, Q.; Ferber, E.C.; Konisti, S.; Peck, B.; Miess, H.; East, P.; et al. Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer Metab. 2013, 1, 3. [Google Scholar] [CrossRef]
- Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors in cancer therapy: A focus on T-regulatory cells. Immunol. Cell Biol. 2018, 96, 21–33. [Google Scholar] [CrossRef]
- Alemohammad, H.; Najafzadeh, B.; Asadzadeh, Z.; Baghbanzadeh, A.; Ghorbaninezhad, F.; Najafzadeh, A.; Safarpour, H.; Bernardini, R.; Brunetti, O.; Sonnessa, M.; et al. The importance of immune checkpoints in immune monitoring: A future paradigm shift in the treatment of cancer. Biomed. Pharmacother. 2022, 146, 112516. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, L.; Di Giorgio, E.; Akimova, T.; Beier, U.H.; Han, R.; Trevisanut, M.; Kalin, J.H.; Cole, P.A.; Hancock, W.W. Inhibiting the coregulator CoREST impairs Foxp3+ Treg function and promotes antitumor immunity. J. Clin. Investig. 2020, 130, 1830–1842. [Google Scholar] [CrossRef] [PubMed]
- Frossi, B.; Mion, F.; Tripodo, C.; Colombo, M.P.; Pucillo, C.E. Rheostatic Functions of Mast Cells in the Control of Innate and Adaptive Immune Responses. Trends Immunol. 2017, 38, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Xie, H.; Fan, Y.; Yang, Z.; Ma, J.; He, D.; Li, L. Infiltrating mast cells promote renal cell carcinoma angiogenesis by modulating PI3K-->AKT-->GSK3beta-->AM signaling. Oncogene 2017, 36, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Shen, T.; Xu, F.; Zhang, J.; Wang, Y.; Wu, J.; Bu, H.; Fu, D.; Fang, B.; Lv, H.; et al. KCNN4 may weaken anti-tumor immune response via raising Tregs and diminishing resting mast cells in clear cell renal cell carcinoma. Cancer Cell Int. 2022, 22, 211. [Google Scholar] [CrossRef]
- Roulleaux Dugage, M.; Nassif, E.F.; Italiano, A.; Bahleda, R. Improving Immunotherapy Efficacy in Soft-Tissue Sarcomas: A Biomarker Driven and Histotype Tailored Review. Front. Immunol. 2021, 12, 775761. [Google Scholar] [CrossRef]
- Gu, L.; Chen, Y.; Li, X.; Mei, Y.; Zhou, J.; Ma, J.; Zhang, M.; Hou, T.; He, D.; Zeng, J. Integrated Analysis and Identification of Critical RNA-Binding Proteins in Bladder Cancer. Cancers 2022, 14, 3739. [Google Scholar] [CrossRef]
- Xu, N.; Ke, Z.B.; Lin, X.D.; Chen, Y.H.; Wu, Y.P.; Chen, Y.; Dong, R.N.; Chen, S.H.; Li, X.D.; Wei, Y.; et al. Development and validation of a molecular prognostic index of bladder cancer based on immunogenomic landscape analysis. Cancer Cell Int. 2020, 20, 302. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, L.; He, S.; Guan, B.; He, A.; Yang, K.; Gong, Y.; Li, X.; Zhou, L. Identification of immune-related LncRNA for predicting prognosis and immunotherapeutic response in bladder cancer. Aging 2020, 12, 23306–23325. [Google Scholar] [CrossRef]





| Coef | HR | 95% CI_1 | 95% CI_u | p-Value | Sig | |
|---|---|---|---|---|---|---|
| Age | 0.035 | 1.035 | 1.018 | 1.053 | 0.000 | *** |
| gendermale | −0.201 | 0.818 | 0.574 | 1.165 | 0.266 | ns |
| raceBlack | 0.57 | 1.769 | 0.712 | 4.394 | 0.219 | ns |
| raceWhite | 0.014 | 1.014 | 0.495 | 2.079 | 0.969 | ns |
| stage2 | 14.538 | 2,060,365 | 0 | Inf | 0.994 | ns |
| stage3 | 15.118 | 3,678,483 | 0 | Inf | 0.993 | ns |
| stage4 | 15.656 | 6,299,742 | 0 | Inf | 0.993 | ns |
| Purity | 0.21 | 1.234 | 0.598 | 2.545 | 0.569 | ns |
| CBX1 | −0.156 | 0.855 | 0.621 | 1.178 | 0.339 | ns |
| CBX2 | −0.081 | 0.922 | 0.78 | 1.089 | 0.340 | ns |
| CBX3 | −0.235 | 0.79 | 0.546 | 1.144 | 0.212 | ns |
| CBX4 | 0.051 | 1.052 | 0.758 | 1.461 | 0.762 | ns |
| CBX5 | 0.284 | 1.328 | 1.059 | 1.666 | 0.014 | * |
| CBX6 | 0.3 | 1.35 | 1.125 | 1.619 | 0.001 | ** |
| CBX7 | −0.409 | 0.664 | 0.536 | 0.824 | 0.000 | *** |
| CBX8 | 0.019 | 1.02 | 0.729 | 1.426 | 0.910 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, L.; Xiong, X.; Kuang, Q.; Peng, M.; Zhu, K.; Luo, P. Identification of the Prognostic Biomarkers CBX6 and CBX7 in Bladder Cancer. Diagnostics 2023, 13, 1393. https://doi.org/10.3390/diagnostics13081393
Li X, Li L, Xiong X, Kuang Q, Peng M, Zhu K, Luo P. Identification of the Prognostic Biomarkers CBX6 and CBX7 in Bladder Cancer. Diagnostics. 2023; 13(8):1393. https://doi.org/10.3390/diagnostics13081393
Chicago/Turabian StyleLi, Xinxin, Lili Li, Xi Xiong, Qihui Kuang, Min Peng, Kai Zhu, and Pengcheng Luo. 2023. "Identification of the Prognostic Biomarkers CBX6 and CBX7 in Bladder Cancer" Diagnostics 13, no. 8: 1393. https://doi.org/10.3390/diagnostics13081393
APA StyleLi, X., Li, L., Xiong, X., Kuang, Q., Peng, M., Zhu, K., & Luo, P. (2023). Identification of the Prognostic Biomarkers CBX6 and CBX7 in Bladder Cancer. Diagnostics, 13(8), 1393. https://doi.org/10.3390/diagnostics13081393






