Abstract
(1) Introduction: Erdheim–Chester disease (ECD) is a life-threatening condition and often a diagnostic challenge. It has recently been classified as a hematopoietic tumour, and the cases of ECD reported in the literature has dramatically increased during the last 15 years. (2) Methods: We describe the case of a 57-year-old male patient with severe gynecomastia, with a detailed description of his diagnostic iter and consequent surgical operation. We provide the first systematic review of the literature of breast involvement in ECD, following PRISMA guidelines, including 13 studies and 16 patients. (3) Results: Our report resulted to be the first case of gynecomastia as a single clinical and imaging feature of ECD described in English literature. A total of 81.3% of patients included were female. Among them, 76.9% had unilateral and nodular presentation, while male patients presented bilateral heterogeneous breast enlargement. Globally, 87.5% expressed breast alterations as their first manifestations of ECD. Only 50% presented skeletal involvement. (4) Conclusion: The reported case represents a unique addition to the literature. We found two different patterns in ECD-related breast involvement between male and female patients, an unusual M/F ratio, and a lower rate of bone involvement. Breast involvement is frequently the first clinical feature; therefore, breast caregivers should be aware of this dangerous and most likely underestimated condition.
1. Introduction
The Erdheim–Chester disease (ECD) is a dangerous and rare type of histiocytosis, firstly described by William Chester and Jakob Erdheim in 1930 as a lipoid granulomatosis, while the first reported case on PubMed is dated 1978 [1]. Since then, less than 1000 cases have been reported in the global literature, with a wide spectrum of symptoms related only to its multi-organ involvement (e.g., papilledema, hypogonadism, hypopituitarism, Horner syndrome, testicular pain, ataxia, scalp nodules, renal artery stenosis).
Its pathogenesis is currently still poorly understood. Finding BRAF v600e mutation in more than 50% of ECD patients [2] has led to the formulation of a clonal neoplastic origin for this disease, and the clinical efficacy of Vemurafenib (V600E-mutated BRAF protein inhibitor) in patients who harbour this mutation seems to confirm this theory [3,4].
On the other hand, the evidence of a dominant Th1 immune response (due to low serum IL-4 levels, and high IFN-alpha, IL-7, IL-12 levels) and the therapeutic efficacy of alpha-interferon, anakinra [5,6], infliximab [7], and steroids [8,9] seem to be consistent with a dysregulated inflammatory pattern.
While the pathogenesis is unclear, it is widely accepted that the diagnosis is based on the detection of migration and infiltration of lipid laden CD68+/Cd1a(-) histiocytes, which may occur in different tissues [10], resulting in fibrosis and even deep modifications of the target organ’s anatomy, with a variable grade of function impairment.
Different affected areas may express different signs and symptoms, and this may result in several patterns of clinical presentation.
Skeleton involvement is the most common type (PET/CT are commonly performed in patients suspected for ECD) as it appears in 96% of patients [11], but many other organs can also be involved, such as: the central nervous system [12,13], cardiovascular system [14,15], lungs [16], kidneys [17], adrenal glands (and a great variety of endocrine manifestations as well) [18], skin [19], gastrointestinal tract [20,21,22], skeletal muscle [23,24,25,26], and thyroid [27].
Hemophagocytosis has also been reported [28]. Before the introduction of IFN-alpha in the treatment of ECD, the mean survival after diagnosis was calculated to be 19.2 months. Today, the mortality rate is 26%, and a five-year survival rate is 68%.
Breast involvement is currently considered rare, with no percentages available in the current literature, and with barely two other cases reported in men [29,30].
ECD is currently included in the 2016 World Health Organisation (WHO) classification of hematopoietic tumours [31], and the diagnosis has been confirmed through the discovery of CD68+, CD163+, FXIIIa+, and CD1a- cells corresponding to “non-LCH” histiocytes, which has been grouped among the “L” (Langerhans) group of the 2016 revised histiocytosis classification of the Histiocyte Society [32].
2. Materials and Methods
2.1. Case Study
A.G., male and 57 years old, came to our unit of plastic and reconstructive surgery presenting a severe bilateral gynaecomastia (Figure 1).
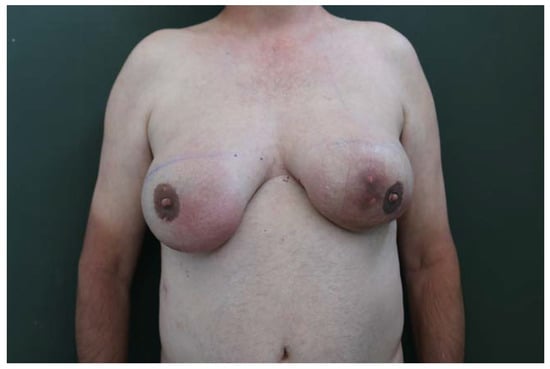
Figure 1.
Grade 4 bilateral gynecomastia (according to Rohrich classification) with an asymmetric development of the glands.
From the age of 50, he had noticed an increase in the volume of his breasts, describing the rate of growth as being faster during the first two years, and slower, but progressive, in the remaining five years.
The patient underwent mammography, mammary ultrasound, agobiopsy (TRU-CUT), and serum exams in order to examine his clinical condition from 2015 to 2016 (Figure 2).
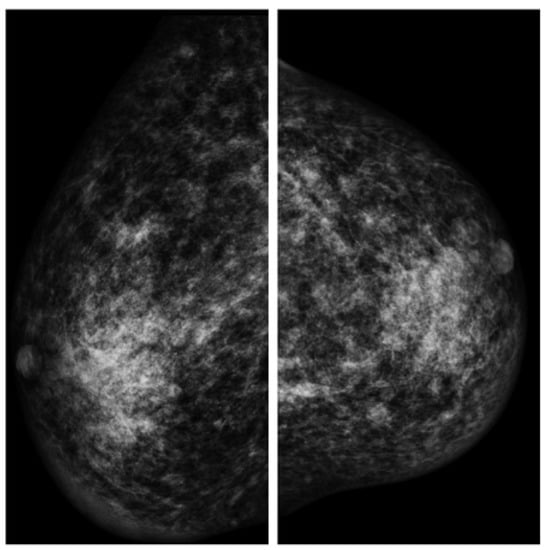
Figure 2.
Mammography findings (left and right breast).
The malignant nature of the tissue was excluded, since no suspicious calcifications came to evidence in the mammary glands, but the presence of hemosiderinic pigment was confirmed by agobiopsy. No hormonal abnormalities were detected (the levels of prolactine, beta-HCG, and estradiol were within the expected range in three different exams).
Several mammary simple cysts, with diameters ranging between 5 and 30 mm, suggestive of fibroadenomas, and a dense fibroglandular breast parenchyma were reported by the radiologist, who described these radiological findings as uneven in a “normal” gynecomastia.
In 2017, the patient was committed to our unit for this unusual gynecomastia, and during the pre-operative imaging exams of the chest and a further bioptical pulmonary exam, a squamous cell carcinoma on the right upper lobe of the lung was incidentally discovered (G2ypT1bN1).
Four cycles of chemotherapy with gemcitabine, vinorelbine, and cisplatin-based neoadjuvant chemotherapy were set up and the progression of gynecomastia stopped during therapy. At this point, a paraneoplastic nature of the gynecomastia seemed to have been the most probable diagnosis to endocrinologists, thoracic surgeons, and the general practitioner, due to a single increase in prolactin levels (25.6 pg/mL; normal range: 2–13 pg/mL) found in 2017 during pre-operative serum exams, and due to the concomitant lung cancer. Nevertheless, the real nature of the condition was still unclear. A lobectomy of the right upper lobe was performed in November 2017, followed by adjuvant chemotherapy, which led the patient to heal from cancer. Finally, the patient returned to our care for the treatment of a paraneoplastic gynecomastia.
Upon clinical examination, the man’s breasts presented a grade 4 bilateral gynecomastia (according to Rohrich et al.) [33] with an asymmetric development of the glands (Figure 1) and hyperpigmentation of the involved skin areas. Jugulum–NAC distance was measured (17 cm on the right breast; 17.5 cm on the left breast). A high circumareolar skin tension was detectable, in particular on the left breast, where a cutaneous ulceration was evident. On breast palpation, a hard consistency of the glands and a parenchymal nodule in the external upper quadrants (about 2 cm) were detectable.
Finally, neither mastodynia, nor inducible or spontaneous galactorrhoea was evident.
The patient expressed a strong psychological discomfort with regard to his severe chest feminisation, along with a high-grade of social and sexual impairment.
We scheduled a subcutaneous mastectomy with a free NAC graft.
An atypical macroscopic appearance of the mammary glands, partially indistinguishable from fat tissue, and resulting in a tough, dense mass with several small (<1 cm), orange calcified concretions, together with areas of fat necrosis, were found intraoperatively. The mass resulted closely attached to pectoralis major muscle fascia and to the skin and subcutaneous tissue, and this made a clean surgical dissection very hard to perform (Figure 3).
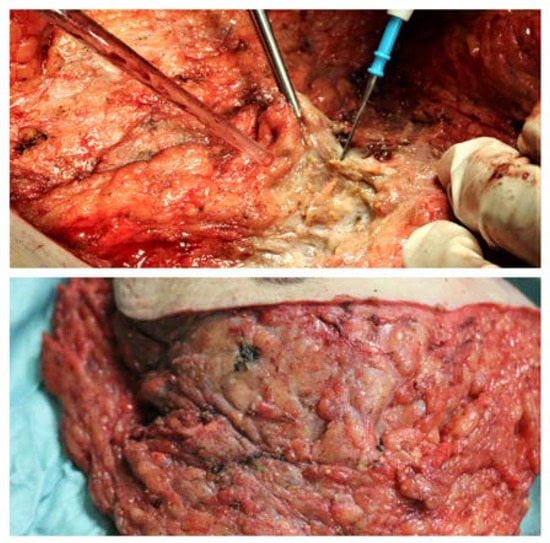
Figure 3.
Intraoperative findings: (Above) The mass was deeply attached to the pectoralis major muscle fascia, and so it was difficult to identify a clean cleavage plan. An atypical appearance of mammary glands, partially indistinguishable from fat tissue, resulting in a single tough, dense mass with several small (<1 cm) orange calcified concretions, and fat necrosis areas. (Below) An atypical appearance of mammary glands, partially indistinguishable from fat tissue, resulting in a single dense mass with small orange calcified concretions, and fat necrosis areas as well.
Skin excess and both mammary masses were removed en bloc, approximately 1480 g from the left side and 1240 g from the right side (Figure 4) and, based on the intraoperative and previous histological findings of inflammatory reactions in 2016, the tissues were sent for further histological and immunohistochemical analyses.
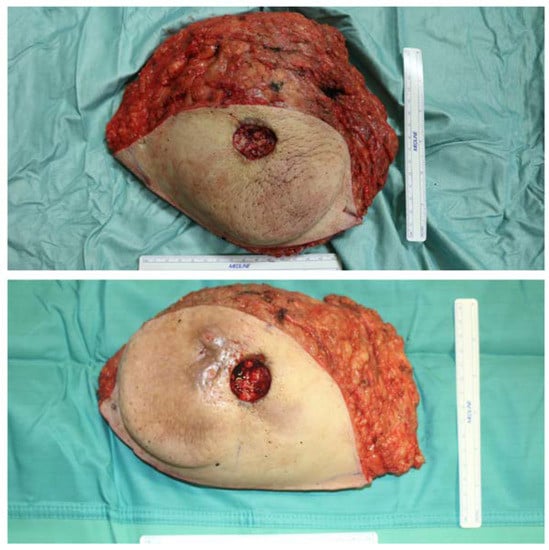
Figure 4.
Appearance of excised breasts; size compared to a 15 cm measuring stick.
Two Redon suction drains were placed and the patient was kept under observation for two days, renewing the compressive dressings once a day.
The patient was then dismissed and evaluated after a week, and is nowadays in follow-up (last postoperative control was performed 12 months after the operation).
Given the high probability of bone involvement in the syndrome, we considered it necessary to investigate the skeletal system through a positron emission tomography, which is able to determine the spreading and the activity of the disease by conducting a whole body assessment of all lesions in a single session. In addition, it is superior to other imaging modalities [34]. In particular, technetium DPD is specific in the detection of bone anomalies.
Total body CT scans performed for lung cancer staging were re-evaluated by the radiologists in order to detect other abnormalities related to the ECD once it was confirmed.
The typical molecular pattern of this particular type of histiocytosis (BRAF v600e) was also investigated.
Based on the peculiar clinical features of our patient, we performed a comprehensive systematic review, including studies that reported cases of Erdheim–Chester disease with breast involvement.
2.2. Systematic Review
2.2.1. Study Selection
Following the preferred reporting items for systematic reviews and meta-analyses guidelines [35], the review included reports of ECD involving the breast, in both male and female patients. Studies were included if they met the following criteria: manuscripts were case reports or series, or described a case report in the text; cases were histologically diagnosed with ECD with breast involvement (regardless of the sex), and studies were written and published in English. Studies were excluded if they were literature reviews, or at least without a description of a case report.
2.2.2. Variables of Interest
Data from the studies selected included: author, age, sex, location, breast features, breast involvement as the first sign of ECD, long bones involvement, relevant clinical history/features.
2.2.3. Data Sources and Search Strategy
An extensive review was conducted on 1 October 2022, for all articles including Erdheim–Chester disease with breast involvement, and the research was performed in 2 different databases: PubMed and Medline. The keywords for the research strategy were “Erdheim–Chester” AND (“mammary glands” OR “mammary”) OR “Erdheim–Chester” AND (“breast” OR “breasts”) OR Erdheim–Chester AND (“gynecomastia” OR “gynaecomastia”). The cases were selected by 2 independent reviewers, in a 2-step inclusion process: step 1: review of title and abstract (or summary) and step 2: a full-text review.
3. Results
The patient returned for an early postoperative check one week later, showing good clinical conditions. Signs of ischemic suffering of both NACs were evident, while the inframammary wound was in a satisfactory state after an appropriate wound-care and management of NAC’s skin necrosis [36,37,38,39,40].
The patient is still in follow-up after our surgery, has no further alterations to his clinical setup, and presents the cicatricial remnants of a bilateral nipple necrosis, which, however, did not affect the evident improvement of the quality of his life (Figure 5). Total body CT scans were negative for radiological signs such as hairy kidneys, coated aorta, pericardial fibrosis, and interstitial lung involvement.
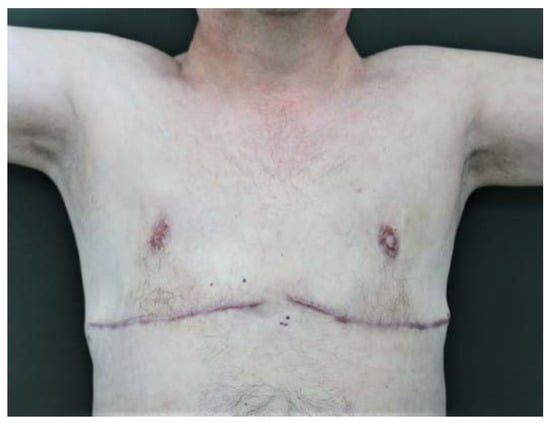
Figure 5.
The sixth-month postoperative clinical follow-up.
The positron emission tomography/CT with technetium DPD excluded any bone involvement.
The histological examination of the breast masses reported aggregates of foamy macrophages, scattered touton (giant) cells, admixed with small lymphocytes and fibrous tissue, and focal hemosiderin depositions.
The findings of migration and infiltration of lipid laden CD68+/Cd1a(-) histiocytes confirmed the diagnosis of ECD.
The molecular pattern BRAF v600e was not present.
No other cases of gynaecomastia without skeletal or systemic involvement have been found in the English scientific literature. A total of 24 articles were found. Among these articles, only 12 met the inclusion criteria. The flowchart is presented in Figure 6. The selected manuscripts were published between 1995 and 2017, ten of them being edited from 2003 onwards. Ten cases have been reported from 2014. We added to the review our case report. The total amount of patients included was therefore 16. The results are briefly described in Table 1. The mean age of the patients was 50.88 years old (range: 32–78 years old, SD: 11.82), with a high predominance of female patients (13: 81.3%). The present study contains the first and only case of isolated gynecomastia as a unique feature of Erdheim–Chester disease. Only eight patients (50%) presented bone involvement, and 100% of these had at least femoral or tibial involvement. Among female patients, ten (76.9%) had unilateral breast involvement, with the tendency for a single or multiple nodular presentation, and four of them had concomitant weight loss and/or fatigue. Among male patients, three (100%) had gynecomastia with a heterogeneous enlargement of both breasts. Cutaneous breast ulcerations and scattered microcalcifications in the breast parenchyma were present in two patients. In the other male patient, a histological examination of breast parenchyma was not performed and/or reported, but an ECD-related hyperprolactinemia was described. In the two patients in which CD68+/Cd1a(-) histiocytes were found in the breast, the gynecomastia was described to have been onset since the age of 50, with a progressive development of severe gynecomastia during the VI decade of life. Globally, 14 patients (87.5%) had breast signs as the first manifestation of ECD.
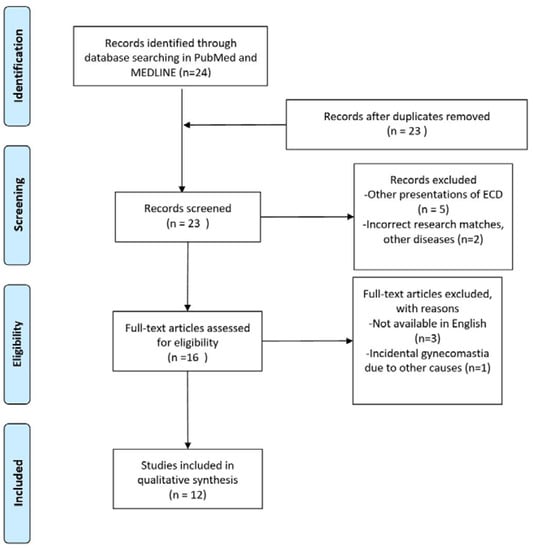
Figure 6.
PRISMA flow diagram.

Table 1.
Breast involvement in Erdheim–Chester disease in the English literature.
4. Discussion
4.1. Case Report
Erdheim–Chester disease is a rare multisystem pathology that consists the infiltration of histiocytes that can potentially colonise any tissue and organ.
The most frequent involvement is in the skeletal system, while the mammary one is rather rare.
It is not currently established whether there is a trophism of the histiocytes towards any specific type of organ. Consequently, specific markers that are able to predict the disease involvement of a particular tissue are currently unavailable. The isolated involvement of the breast in a male patient has never been reported in the English literature.
The patient reported in the present study came to our attention solely because of gynecomastia, and once the diagnosis of Erdheim–Chester disease was confirmed, no other clinical manifestation of that pathology was evident; thus, the case reported in the present study can be considered the first and only case of gynaecomastia as a single clinical and instrumental feature of Erdheim–Chester Disease. The finding of lung cancer in this case can be considered merely an incidentaloma, as well as a disturbing factor in the diagnostic process.
The peculiar and severe gynaecomastia reported was challenging to treat surgically, as a clean dissection was almost impossible to perform. The need for a radical excision of both mammary glands in order to restore harmony and balance to the patient’s body, as well as for the return to a normal working and sexual life, led us to perform a subcutaneous mastectomy with a single inframammary scar and a free nipple-areolar complex transplant.
This led to NAC necrosis, which did not impair the patient’s satisfaction towards the surgical operation, and resulted in a deep improvement of his psychological condition.
4.2. Review
Considering that, from 1930 to 2022, more than 1500 cases were published in the world medical literature or were included in the ECD Global Alliance Registry [10,48], and considering the number of reported patients in this review, the percentage of breast involvement would be close to ~0.1%; however, taking merely into account the cases reported in the global literature (~800) [49], the percentage rises to 2%.
Moreover, it is likely that this percentage again underrates the percentage of breast involvement, since 10 patients out of 16 have been reported from 2014 to 2022.
The majority of patients reported were female (81.3%), but according to the best evidence, ECD has a male predominance (3:1 ratio; 70–75% in two different cohorts) [50,51].
We would suggest two different explanations of this incongruence: either (1) the Erdheim–Chester disease has a peculiar breast pattern influenced by hormones, or, more likely, (2) most of the cases of ECD-related gynecomastia remains unknown or classified as idiopathic because of the absence of other relevant features of the disease or their incorrect evaluation.
The systematic review shows that among patients with breast involvement ECD, bone involvement is much lower than as reported in the literature (50% vs. 95%). Remarkably, it is possible to identify two distinct presentation patterns between males and females: nodular in women with, in some cases (4), an association with a decline in general conditions, i.e., feelings of fatigue and weight loss; and widely infiltrating in men, up to severe gynecomastia conditions, such as the one described by us. These data require additional confirmations and, given the scarcity of reports and the lack of knowledge about the disease, it is not yet possible to formulate conclusive statements.
Most of the reports date back to a limited period of time, i.e., from 2003 until present day, which is consistent with what was stated by other authors, showing that from 2003 to 2013, there is a large increase in the number of ECD reports due to an improved awareness of the pathology, which improved the diagnostic sensibility [52].
This aspect, along with the high percentage of patients in which ECD was discovered following breast semeiotics (87.5%), should lead to a higher consideration for the possibility of ECD, even without other clinical features, among cases of idiopathic gynecomastia (according to Narula and Carlson [53], after the diagnostic evaluation was completed, about 25% of patients were found to have idiopathic gynaecomastia) and breast nodules that are not part of the classic pathologies, given the potential lethality of the disease and the distinct therapeutic opportunities.
5. Conclusions
This reported case is a unique addition to the literature and represents a diagnostic and therapeutic challenge.
In retrospect, the diagnostic therapeutic moments were found to be preserved mainly by the plastic surgeon (who could represent this case as a generic breast caregiver) reflecting on the history described in this article, since there are no other organs involved besides the breast, which, however, remained the only true concern of the patient even during chemotherapy for lung cancer. Overall, we consider the data of our review to be unique in the literature and useful in particular to the breast unit operators, who are not always on guard against this rare, serious, and life-threatening disease [54,55,56,57] among the possible causes of isolated or idiopathic gynecomastia [58]—when ECD occurs in men—or mammary nodules—when it occurs in women. Last but not least, the aim of the study is to provide the first data as the basis for further prospective studies and reports on a disease, the Erdheim–Chester, and one of its manifestations, which has been underestimated.
Author Contributions
Conceptualisation, F.R.G. and G.N.; methodology, F.R.G. and R.C.; validation, G.N., R.C. and L.G.; formal analysis, M.P. and M.M.E.A.; data curation, F.R.G. and S.B.; writing—original draft preparation, F.R.G. and M.P.; writing—review and editing, R.C. and G.M.; supervision, G.N. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Atkins, H.L.; Klopper, J.F.; Ansari, A.N.; Iwai, J. Lipid (cholesterol) granulomatosis (Chester-Erdheim disease) and congenital megacalices. Clin. Nucl. Med. 1978, 3, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Haroche, J.; Charlotte, F.; Arnaud, L.; von Deimling, A.; Helias-Rodzewicz, Z.; Hervier, B.; Cohen-Aubart, F.; Launay, D.; Lesot, A.; Mokhtari, K.; et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood 2012, 120, 2700–2703. [Google Scholar] [CrossRef] [PubMed]
- Haroche, J.; Cohen-Aubart, F.; Emile, J.F.; Arnaud, L.; Maksud, P.; Charlotte, F.; Cluzel, P.; Drier, A.; Hervier, B.; Benameur, N.; et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood 2013, 121, 1495–1500. [Google Scholar] [CrossRef]
- Blombery, P.; Wong, S.Q.; Lade, S.; Prince, H.M. Erdheim-Chester disease harboring the BRAF V600E mutation. J. Clin. Oncol. 2012, 30, e331–e332. [Google Scholar] [CrossRef]
- Killu, A.M.; Liang, J.J.; Jaffe, A.S. Erdheim-Chester disease with cardiac involvement successfully treated with anakinra. Int. J. Cardiol. 2013, 167, e115–e117. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.; Pariente, D.; Lecron, J.C.; Delwail, A.; Taoufik, Y.; Meinzer, U. Treatment of pediatric Erdheim-Chester disease with interleukin-1-targeting drugs. Arthritis Rheum. 2011, 63, 4031–4032. [Google Scholar] [CrossRef]
- Dagna, L.; Corti, A.; Langheim, S.; Guglielmi, B.; De Cobelli, F.; Doglioni, C.; Fragasso, G.; Sabbadini, M.G.; Ferrarini, M. Tumor necrosis factor alpha as a master regulator of inflammation in Erdheim-Chester disease: Rationale for the treatment of patients with infliximab. J. Clin. Oncol. 2012, 30, e286–e290. [Google Scholar] [CrossRef]
- Badzek, S.; Misir-Krpan, A.; Krajina, Z.; Radman, I.; Stern-Padovan, R.; Dotlic, S. Erdheim-Chester disease and concomitant tuberculosis successfully treated with chemotherapy and long-term steroids. Coll. Antropol. 2007, 31, 621–623. [Google Scholar]
- Chen, M.T.; Wang, S.M.; Lin, S.Y.; Ting, I.W.; Liu, J.H.; Kuo, H.L.; Huang, C.C. Pericardial effusion as a crucial presentation of Erdheim-Chester disease in a hemodialysis patient: An overlooked diagnosis. Clin. Nephrol. 2012, 78, 81–84. [Google Scholar] [CrossRef]
- Haroche, J.; Cohen Aubart, F.; Amoura, Z. Erdheim-Chester disease. Blood 2020, 135, 1311–1318. [Google Scholar] [CrossRef]
- Haroche, J.; Arnaud, L.; Amoura, Z. Erdheim-Chester disease. Curr. Opin. Rheumatol. 2012, 24, 53–59. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A.; Pandey, S.; Kumar, R.; Singh, I.; Kumari, N. Isolated Langerhans Cell Histiocytosis Masquerading as Intradural Extramedullary Meningioma: Review on Histiocytic Disorders of Spine. J. Pediatr. Neurosci. 2019, 14, 46–51. [Google Scholar]
- Sánchez-Villalobos, J.M.; Jimeno-Almazán, A.; López-Peña, C.; Hernández-Hortelano, E.; Martínez-Francés, A.; Pérez-Vicente, J.A. Erdheim-Chester disease mimicking multiple sclerosis or a new association? Mult. Scler. Relat. Disord. 2019, 30, 94–97. [Google Scholar] [CrossRef]
- Ghotra, A.S.; Thompson, K.; Lopez-Mattei, J.; Bawa, D.; Hernandez, R.; Banchs, J.; Palaskas, N.; Iliescu, C.; Kim, P.; Yusuf, S.W.; et al. Cardiovascular manifestations of Erdheim-Chester disease. Echocardiography 2019, 36, 229–236. [Google Scholar] [CrossRef]
- Ding, F.; Chahine, J.; Deshwal, H.; Ghosh, S.; Tan, C.; Simpfendorfer, C.; Cremer, P.; Jellis, C.; Arrossi, A.V.; Klein, A.L. Mysterious quad of constrictive pericarditis, recurrent pleural effusions, bone involvement and interstitial lung disease. Oxf. Med. Case Rep. 2019, 2019, omz015. [Google Scholar] [CrossRef]
- Josan, E.S.; Green, J.W.; Zaidi, S.I.M.; Mehta, J.B. Isolated pulmonary involvement in Erdheim-Chester disease. Lung India 2017, 34, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Scolaro, J.C.; Peiris, A.N. The Hairy Kidney of Erdheim-Chester Disease. Mayo Clin. Proc. 2018, 93, 671. [Google Scholar] [CrossRef] [PubMed]
- Courtillot, C.; Laugier Robiolle, S.; Cohen Aubart, F.; Leban, M.; Renard-Penna, R.; Drier, A.; Charlotte, F.; Amoura, Z.; Touraine, P.; Haroche, J. Endocrine Manifestations in a Monocentric Cohort of 64 Patients With Erdheim-Chester Disease. J. Clin. Endocrinol. Metab. 2016, 101, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kobic, A.; Shah, K.K.; Schmitt, A.R.; Goyal, G.; Go, R.S.; Guo, R.; Rech, K.L.; Sartori-Valinotti, J.C. Erdheim-Chester Disease: Expanding the spectrum of cutaneous manifestations. Br. J. Dermatol. 2019, 182, 405–409. [Google Scholar] [CrossRef]
- Christophi, G.P.; Sharma, Y.; Farhan, Q.; Jain, U.; Walker, T.; Sayuk, G.S.; Rubin, D.C. Erdheim-Chester Disease presenting with histiocytic colitis and cytokine storm. J. Gastrointest. Liver Dis. 2017, 26, 183–187. [Google Scholar] [CrossRef]
- De Souza Maciel Rocha Horvat, N.; Coelho, C.R.; Roza, L.C.; de Souza, R.C.; Costa, Y.B.; de Oliveira, E.C.; de Souza Rocha, M.; Baroni, R.H. Spectrum of abdominal imaging findings in histiocytic disorders. Abdom. Imaging 2015, 40, 2738–2746. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yaakov, G.; Munteanu, D.; Sztarkier, I.; Fich, A.; Schwartz, D. Erdheim Chester—A rare disease with unique endoscopic features. World J. Gastroenterol. 2014, 20, 8309–8311. [Google Scholar] [CrossRef]
- Tan, A.P.; Tan, L.K.; Choo, I.H. Erdheim-Chester disease involving breast and muscle: Imaging findings. AJR Am. J. Roentgenol. 1995, 164, 1115–1117. [Google Scholar] [CrossRef]
- Barnes, P.J.; Foyle, A.; Hache, K.A.; Langley, R.G.; Burrell, S.; Juskevicius, R. Erdheim-Chester disease of the breast: A case report and review of the literature. Breast J. 2005, 11, 462–467. [Google Scholar] [CrossRef]
- Provenzano, E.; Barter, S.J.; Wright, P.A.; Forouhi, P.; Allibone, R.; Ellis, I.O. Erdheim-Chester disease presenting as bilateral clinically malignant breast masses. Am. J. Surg. Pathol. 2010, 34, 584–588. [Google Scholar] [CrossRef]
- Ambrosini, V.; Savelli, F.; Merli, E.; Zompatori, M.; Nanni, C.; Allegri, V.; Fanti, S. F-18 FDG PET/CT detects muscle involvement in Erdheim-Chester disease. Clin. Nucl. Med. 2012, 37, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.T.; Patel, P.; Hernandez, A.; Grandinetti, L.M.; Huen, A.C.; Marks, S.; Ho, J.; Monaco, S.E.; Jaffe, R.; Picarsic, J. Langerhans cell histiocytosis and Erdheim-Chester disease, both with cutaneous presentations, and papillary thyroid carcinoma all harboring the BRAF(V600E) mutation. J. Cutan. Pathol. 2016, 43, 270–275. [Google Scholar] [CrossRef]
- Rao, R.N.; Chang, C.C.; Uysal, N.; Presberg, K.; Shidham, V.B.; Tomashefski, J.F., Jr. Fulminant multisystem non-langerhans cell histiocytic proliferation with hemophagocytosis: A variant form of Erdheim-Chester disease. Arch. Pathol. Lab. Med. 2005, 129, e39–e43. [Google Scholar] [CrossRef]
- Loh, W.J.; Sittampalam, K.; Tan, S.C.; Chandran, M. Symptomatic empty sella syndrome: An unusual manifestation of Erdheim-Chester disease. Endocrinol. Diabetes Metab. Case Rep. 2015, 2015, 140122. [Google Scholar] [CrossRef]
- Ferrozzi, F.; Bova, D.; Tognini, G.; Zuccoli, G. Pseudotumoral bilateral involvement of the breast in Erdheim-Chester disease: CT appearance. J. Comput. Assist. Tomogr. 2000, 24, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues: International Agency for Research on Cancer; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Emile, J.F.; Abla, O.; Fraitag, S.; Horne, A.; Haroche, J.; Donadieu, J.; Requena-Caballero, L.; Jordan, M.B.; Abdel-Wahab, O.; Allen, C.E.; et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 2016, 127, 2672–2681. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Ha, R.Y.; Kenkel, J.M.; Adams, W.P., Jr. Classification and management of gynecomastia: Defining the role of ultrasound-assisted liposuction. Plast. Reconstr. Surg. 2003, 111, 909–925. [Google Scholar] [CrossRef]
- García-Gómez, F.J.; Acevedo-Báñez, I.; Martínez-Castillo, R.; Tirado-Hospital, J.L.; Cuenca-Cuenca, J.I.; Pachón-Garrudo, V.M.; Álvarez-Pérez, R.M.; García-Jiménez, R.; Rivas-Infante, E.; García-Morillo, J.S.; et al. The role of 99m F-FD.G.; F-DOPA PET/CT and Tc bone scintigraphy imaging in Erdheim-Chester disease. Eur. J. Radiol. 2015, 84, 1586–1592. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cuomo, R.; Giardino, F.R.; Neri, A.; Nisi, G.; Brandi, C.; Zerini, I.; Jingjian, H.; Grimaldi, L. Optimization of Prepectoral Breast Reconstruction. Breast Care 2021, 16, 36–42. [Google Scholar] [CrossRef]
- Casella, D.; Fusario, D.; Cassetti, D.; Pesce, A.L.; De Luca, A.; Guerra, M.; Cuomo, R.; Ribuffo, D.; Neri, A.; Marcasciano, M. Controlateral Symmetrisation in SRM for Breast Cancer: Now or Then? Immediate versus Delayed Symmetrisation in a Two-Stage Breast Reconstruction. Curr. Oncol. 2022, 29, 9391–9400. [Google Scholar] [CrossRef]
- Cuomo, R.; Giardino, F.R.; Nisi, G.; Jingjian, H.; Diluiso, G.; Tresoldi, M.; Gorizio, P.; Brandi, C.; Grimaldi, L. Fat graft for reducing pain in chronic wounds. Wound Repair Regen. 2020, 28, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, R.; Nisi, G.; Brandi, C.; Giardino, F.R.; Grimaldi, L. Future Directions to Limit Surgical Site Infections. J. Invest. Surg. 2020, 33, 759–761. [Google Scholar] [CrossRef]
- Cuomo, R.; Grimaldi, L.; Nisi, G.; Zerini, I.; Giardino, F.R.; Brandi, C. Ultraportable Devices for Negative Pressure Wound Therapy: First Comparative Analysis. J. Invest. Surg. 2021, 34, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Andrade, V.P.; Nemer, C.C.; Prezotti, A.N.; Goulart, W.S. Erdheim-Chester disease of the breast associated with Langerhans-cell histiocytosis of the hard palate. Virchows Arch. 2004, 445, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Lemos, L.B.; Qu, Z.; Garg, K.; Papasozomenos, S. Pseudoneoplastic proliferation of histiocytes with paclitaxel-induced ultrastructural changes in a mastectomy specimen. Ann. Diagn. Pathol. 2004, 8, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Kiryu, S.; Yamada, H.; Hosoi, M.; Kurokawa, M.; Morikawa, T.; Shibahara, J.; Ohtomo, K. Erdheim-Chester disease with an 18F-fluorodeoxyglucose-avid breast mass and BRAF V600E mutation. Jpn. J. Radiol. 2014, 32, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yan, Q.; Rohr, J.; Wang, Y.; Fan, L.; Wang, Z. Erdheim-Chester disease involving the breast—A rare but important differential diagnosis. Hum. Pathol. 2015, 46, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Basara, I.; Yavuz, E.; Balci, P.; Tuna, E.B.; Sari, I. Erdheim Chester disease presented isolated breast and axillary involvement. JBR-BTR 2015, 98, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Roverano, S.; Drago, C.; Gallo, J.; Ortiz, A.; Migliore, N.; Paira, S. Erdheim-Chester Disease of the Breast Without Systemic Involvement. J. Clin. Rheumatol. 2017, 23, 228–230. [Google Scholar] [CrossRef]
- Binyousef, R.F.; Al-Gahmi, A.M.; Khan, Z.R.; Rawah, E. A rare case of Erdheim-Chester disease in the breast. Ann. Saudi Med. 2017, 37, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://erdheim-chester.org/patient-registry/ (accessed on 1 October 2022).
- Goyal, G.; Heaney, M.L.; Collin, M.; Cohen Aubart, F.; Vaglio, A.; Durham, B.H.; Hershkovitz-Rokah, O.; Girschikofsky, M.; Jacobsen, E.D.; Toyama, K.; et al. Erdheim-Chester disease: Consensus recommendations for the evaluation, diagnosis, and treatment in the molecular era. Blood 2020, 135, 1929–1945. [Google Scholar] [CrossRef]
- Estrada-Veras, J.I.; O’Brien, K.J.; Boyd, L.C.; Dave, R.H.; Durham, B.H.; Xi, L.; Malayeri, A.A.; Chen, M.Y.; Gardner, P.J.; Enriquez, J.R.A.; et al. The clinical spectrum of Erdheim-Chester disease: An observational cohort study. Blood Adv. 2017, 1, 357–366. [Google Scholar] [CrossRef]
- Cohen-Aubart, F.; Emile, J.F.; Carrat, F.; Hélias-Rodzewicz, Z.; Taly, V.; Charlotte, F.; Cluzel, P.; Donadieu, J.; Idbaih, A.; Barète, S.; et al. Phenotypes and survival in Erdheim-Chester disease: Results from a 165-patient cohort. Am. J. Hematol. 2018, 93, E114–E117. [Google Scholar] [CrossRef]
- Haroche, J.; Cohen-Aubart, F.; Arnaud, L.; Hervier, B.; Charlotte, F.; Drier, A.; Gorochov, G.; Grenier, P.A.; Cluzel, P.; Maksud, P.; et al. Erdheim-Chester disease. Rev. Med. Interne 2014, 35, 715–722. [Google Scholar] [CrossRef]
- Narula, H.; Carlson, H. Gynaecomastia—Pathophysiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2014, 10, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Haroche, J.; Amoura, Z.; Charlotte, F.; Salvatierra, J.; Wechsler, B.; Graux, C.; Brousse, N.; Piette, J.C. Imatinib mesylate for platelet-derived growth factor receptor-beta-positive Erdheim-Chester histiocytosis. Blood 2008, 111, 5413–5415. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, M.S.; Calleja, A.; Panse, P.; Appleton, C.; Jaroszewski, D.E.; Tazelaar, H.D.; Mookadam, F. Multimodality imaging showing complete cardiovascular involvement by Erdheim-Chester disease. Eur. J. Echocardiogr. 2010, 11, E25. [Google Scholar] [CrossRef] [PubMed]
- Drier, A.; Haroche, J.; Savatovsky, J.; Godeneche, G.; Dormont, D.; Chiras, J.; Amoura, Z.; Bonneville, F. Cerebral, facial, and orbital involvement in Erdheim-Chester disease: CT and MR imaging findings. Radiology 2010, 255, 586–594. [Google Scholar] [CrossRef]
- Arnaud, L.; Hervier, B.; Neel, A.; Hamidou, M.A.; Kahn, J.E.; Wechsler, B.; Perez-Pastor, G.; Blomberg, B.; Fuzibet, J.G.; Dubourguet, F.; et al. CNS involvement and treatment with interferon-alpha are independent prognostic factors in Erdheim-Chester disease: A multicenter survival analysis of 53 patients. Blood 2011, 117, 2778–2782. [Google Scholar] [CrossRef]
- Narula, H.S.; Carlson, H.E. Gynecomastia. Endocrinol. Metab. Clin. N. Am. 2007, 36, 497–519. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).