Variability of Sentinel Lymph Node Location in Patients with Trunk Melanoma
Abstract
:1. Introduction
1.1. SLNB Concept
1.2. The Sentinel Lymph Node—A Histopathological Report
2. Materials and Methods
2.1. Study Design
2.2. SLNB Surgical Technique
2.3. Data Collection
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Arisi, M.; Zane, C.; Caravello, S.; Rovati, C.; Zanca, A.; Venturini, M.; Calzavara-Pinton, P. Sun Exposure and Melanoma, Certainties and Weaknesses of the Present Knowledge. Front. Med. 2018, 5, 235. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Han, G.; Duque, M.T.; Morrison, S.; Leong, S.P.; Kashani-Sabet, M.; Vetto, J.; White, R.; Schneebaum, S.; Pockaj, B.; et al. Sentinel Lymph Node Biopsy Is Prognostic in Thickest Melanoma Cases and Should Be Performed for Thick Melanomas. Ann. Surg. Oncol. 2021, 28, 1007–1016. [Google Scholar] [CrossRef]
- Morton, D.L.; Wen, D.-R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch. Surg. 1992, 127, 392–399. [Google Scholar] [CrossRef]
- NCCN Guidelines—Melanoma: Cutaneous, Version 2. 2023. Available online: https://www.nccn.org/guidelines/guidelines-process/transparency-process-and-recommendations/GetFileFromFileManagerGuid?FileManagerGuidId=3171c772-e593-4455-90ec-84154399ea7d (accessed on 5 May 2023).
- Dickson, P.V.; Gershenwald, J.E. Staging and Prognosis of Cutaneous Melanoma. Surg. Oncol. Clin. N. Am. 2011, 20, 1–17. [Google Scholar] [CrossRef]
- Gyorki, D.E.; Sanelli, A.; Herschtal, A.; Lazarakis, S.; McArthur, G.A.; Speakman, D.; Spillane, J.; Henderson, M.A. Sentinel Lymph Node Biopsy in T4 Melanoma: An Important Risk-Stratification Tool. Ann. Surg. Oncol. 2016, 23, 579–584. [Google Scholar] [CrossRef]
- Luke, J.J.; Ascierto, P.A.; Carlino, M.S.; Gershenwald, J.E.; Grob, J.-J.; Hauschild, A.; Kirkwood, J.M.; Long, G.V.; Mohr, P.; Robert, C.; et al. KEYNOTE-716: Phase III study of adjuvant pembrolizumab versus placebo in resected high-risk stage II melanoma. Future Oncol. 2020, 16, 4429–4438. [Google Scholar] [CrossRef] [PubMed]
- Prieto, V.G. Sentinel Lymph Nodes in Cutaneous Melanoma. Clin. Lab. Med. 2017, 37, 417–430. [Google Scholar] [CrossRef]
- Ariyan, S.; Ali-Salaam, P.; Cheng, D.W.; Truini, C. Reliability of Lymphatic Mapping After Wide Local Excision of Cutaneous Melanoma. Ann. Surg. Oncol. 2007, 14, 2377–2383. [Google Scholar] [CrossRef]
- Bluemel, C.; Herrmann, K.; Giammarile, F.; Nieweg, O.E.; Dubreuil, J.; Testori, A.; Audisio, R.A.; Zoras, O.; Lassmann, M.; Chakera, A.H.; et al. EANM practice guidelines for lymphoscintigraphy and sentinel lymph node biopsy in melanoma. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, W.; Hertzenberg, C.; Jewell, W.; Al-Kasspooles, M.F.; Damjanov, I.; Cohen, M.S. Utility of frozen-section analysis of sentinel lymph node biopsy specimens for melanoma in surgical decision making. Am. J. Surg. 2008, 196, 827–832; discussion 832–833. [Google Scholar] [CrossRef] [PubMed]
- Prieto, V.G. Use of frozen sections in the examination of sentinel lymph nodes in patients with melanoma. Semin. Diagn. Pathol. 2008, 25, 112–115. [Google Scholar] [CrossRef]
- Somerset, A.E.; Jameson, M.J. Sentinel Lymph Node Biopsy in Patients with Melanoma. 2021. Available online: https://emedicine.medscape.com/article/854424-overview#a1 (accessed on 10 May 2023).
- Prieto, V.G. Sentinel lymph nodes in cutaneous melanoma: Handling, examination, and clinical repercussion. Arch. Pathol. Lab. Med. 2010, 134, 1764–1769. [Google Scholar] [CrossRef]
- Rhodin, K.E.; Fimbres, D.P.; Burner, D.N.; Hollander, S.; O’connor, M.H.; Beasley, G.M. Melanoma lymph node metastases–moving beyond quantity in clinical trial design and contemporary practice. Front. Oncol. 2022, 12, 1021057. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment—Update 2022. Eur. J. Cancer 2022, 170, 256–284. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.-J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef]
- Kachare, S.D.; Brinkley, J.; Wong, J.H.; Vohra, N.A.; Zervos, E.E.; Fitzgerald, T.L. The Influence of Sentinel Lymph Node Biopsy on Survival for Intermediate-Thickness Melanoma. Ann. Surg. Oncol. 2014, 21, 3377–3385. [Google Scholar] [CrossRef]
- Nakamura, Y. The Role and Necessity of Sentinel Lymph Node Biopsy for Invasive Melanoma. Front. Med. 2019, 6, 231. [Google Scholar] [CrossRef] [PubMed]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Masoud, S.J.; Perone, J.A.; Farrow, N.E.; Mosca, P.J.; Tyler, D.S.; Beasley, G.M. Sentinel Lymph Node Biopsy and Completion Lymph Node Dissection for Melanoma. Curr. Treat. Options Oncol. 2018, 19, 55, Erratum in Curr. Treat. Options Oncol. 2019, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, H.J.; Klop, W.M.C.; Speijers, M.J.; Lohuis, P.J.F.M.; Nieweg, O.E.; Hoekstra, H.J.; Balm, A.J.M. Lymphatic Drainage Patterns from Melanomas on the Shoulder or Upper Trunk to Cervical Lymph Nodes and Implications for the Extent of Neck Dissection. Ann. Surg. Oncol. 2012, 19, 3906–3912. [Google Scholar] [CrossRef]
- Shannon, C.M.; Mehta, N.K.; Li, H.; Nguyen, S.A.; Koochakzadeh, S.; Elston, D.M.; Kaczmar, J.M.; Day, T.A. Anatomic Region of Cutaneous Melanoma Impacts Survival and Clinical Outcomes: A Population-Based Analysis. Cancers 2023, 15, 1229. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Smedby, K.E.; Schultz, I.; Olsson, H.; Ingvar, C.; Hansson, J.; Gillgren, P. Sentinel Node Location in Trunk and Extremity Melanomas: Uncommon or Multiple Lymph Drainage Does Not Affect Survival. Ann. Surg. Oncol. 2014, 21, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Fadaki, N.; Li, R.; Parrett, B.; Sanders, G.; Thummala, S.; Martineau, L.; Cardona-Huerta, S.; Miranda, S.; Cheng, S.-T.; Miller, J.R., 3rd; et al. Is Head and Neck Melanoma Different from Trunk and Extremity Melanomas with Respect to Sentinel Lymph Node Status and Clinical Outcome? Ann. Surg. Oncol. 2013, 20, 3089–3097. [Google Scholar] [CrossRef] [PubMed]
- Morgado, F.J.; Soeiro, P.; Brinca, A.; Pinho, A.; Vieira, R. Does the pattern of lymphatic drainage influence the risk of nodal recurrence in trunk melanoma patients with negative sentinel lymph node biopsy? An. Bras. Dermatol. 2021, 96, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Ribero, S.; Quaglino, P.; Osella-Abate, S.; Sanlorenzo, M.; Senetta, R.; Macrì, L.; Savoia, P.; Macripò, G.; Sapino, A.; Bernengo, M. Relevance of multiple basin drainage and primary histologic regression in prognosis of trunk melanoma patients with negative sentinel lymph nodes. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1132–1137. [Google Scholar] [CrossRef]
- Ribero, S.; Osella-Abate, S.; Pasquali, S.; Rossi, C.R.; Borgognoni, L.; Piazzalunga, D.; Solari, N.; Schiavon, M.; Brandani, P.; Ansaloni, L.; et al. Prognostic Role of Multiple Lymphatic Basin Drainage in Sentinel Lymph Node-Negative Trunk Melanoma Patients: A Multicenter Study from the Italian Melanoma Intergroup. Ann. Surg. Oncol. 2016, 23, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.F.; Uren, R.F.; Shaw, H.M.; McCarthy, W.H.; Quinn, M.J.; O’brien, C.J.; Howman-Giles, R.B. Location of sentinel lymph nodes in patients with cutaneous melanoma: New insights into lymphatic anatomy. J. Am. Coll. Surg. 1999, 189, 195–204. [Google Scholar] [CrossRef]
| Total N = 62 | Upper Trunk Melanoma 39, (62.9%) | Lower Trunk Melanoma
N = 23, (37.1%) | p-Value | |
|---|---|---|---|---|
| Age (years) median (min-max) | 54.5 (33–78) | 55 (34–78) | 53 (33–77) | 0.782 |
| Sex Masculine n,% | 36, (58.1%) | 22, (56.4%) | 14, (60.9%) | 0.731 |
| BMI median (min-max) | 23.9(19.8–30.6) | 23.7 (19.8–30.6) | 24.1 (19.9–29.2) | 0.291 |
| Tumor stage n,% | 0.413 | |||
| pT1 | 20, (32.3%) | 14, (35.9%) | 6, (26.1%) | |
| pT2 | 9, (14.5%) | 4, (10.3%) | 5, (21.7%) | |
| pT3 | 15, (24.2%) | 11, (28.2%) | 4, (17.4%) | |
| pT4 | 18, (29.0%) | 10, (25.6%) | 8, (34.8%) | |
| Breslow mm median (min-max) | 2.3 (0.5–12.5) | 2.3 (0.5–12.5) | 2.3 (0.8–9.3) | 0.511 |
| 1o LN localization n,% | <0.001 | |||
| Cervical | 6, (9.7%) | 6, (15.4%) | 0 | |
| Axillar | 44, (71.0%) | 33, (84.6%) | 11, (47.8%) | |
| Inguinal | 12, (19.4%) | 0 | 12, (52.2%) | |
| 2o LN localization n,% | 0.057 | |||
| N = 20 | ||||
| Cervical | 2, (10.0%) | 2, (14.3%) | 0 | |
| Axillar | 16, (80.0%) | 12, (85.7%) | 4, (66.7%) | |
| Inguinal | 2, (10.0%) | 0 | 2, (33.3%) | |
| 3o LN localization n,% | - | |||
| N = 2 | ||||
| Cervical | 0 | 0 | 0 | |
| Axillar | 2, (100%) | 1, (100%) | 1, (100%) | |
| Inguinal | 0 | 0 | 0 | |
| Positive LN n,% | 16, (25.8%) | 12, (30.8%) | 6, (17.4%) | 0.245 |
| Period to LNB (days) median (min-max) | 29.0 (22.0–40.0) | 29.0 (22.0–40.0) | 28.0 (22.0–39.0) | 0.373 |
| BRAF gene, n,% | 10, (71.4%) | 6, (66.7%) | 4, (80.0%) | 1 |
| N = 14 | ||||
| Anesthesia type n,% | <0.001 | |||
| General | 53, (85.5%) | 39, (100%) | 14, (60.9%) | |
| Local | 9, (14.5%) | 0 | 9, (39.1%) | |
| Surgery duration (minutes) median(min-max) | 115.0 (70.0–200.0) | 120.0 (75.0–195.0) | 110.0 (70.0–200.0) | 0.056 |
| Reintervention n,% | 1, (1.6%) | 1, (2.6%) | 0 | 1 |
| Period of healing melanoma site (days) median (min-max) | 12.0 (10.0–21.0) | 12.0 (10.0–21.0) | 12.0 (10.0–18.0) | 0.833 |
| Period of healing LN (days) median (min-max) | 8.0 (7.0–8.0) | 8.0 (7.0–9.0) | 8.0 (7.0–8.0) | 0.373 |
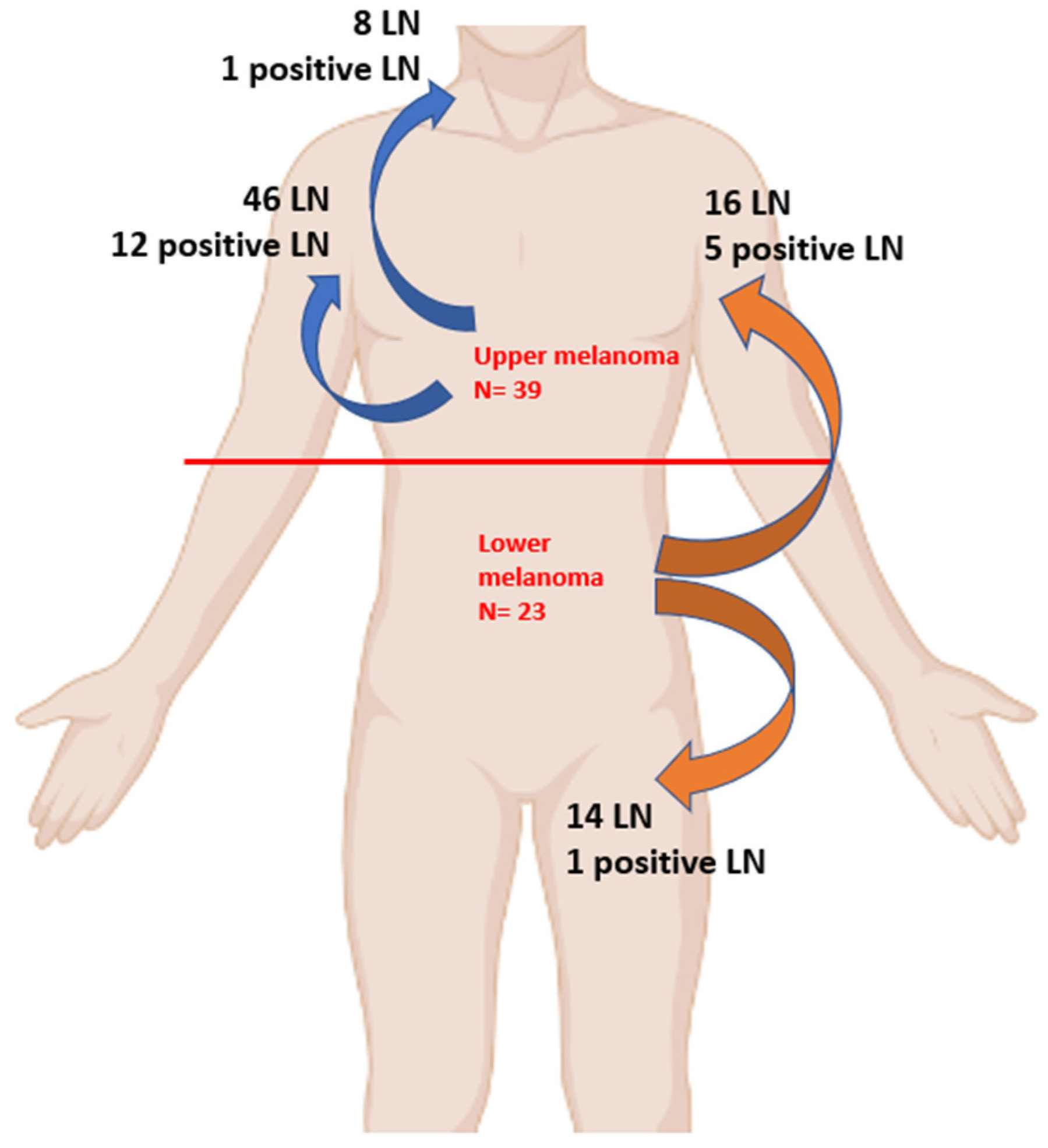
| N = 84 | Cervical LN 8, (9.5%) | Axillar LN 62, (73.8%) | Inguinal LN 14, (16.7%) |
|---|---|---|---|
| Upper Melanoma, N = 54, 64.3% | 8, (100%) | 46, (74.2%) | 0 |
| Positive | 1 | 12 | |
| Lower Melanoma, N = 30, 35.7% | 0 | 16, (25.8%) | 14, (100%) |
| Positive | 5 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobirca, F.; Leventer, M.; Georgescu, D.E.; Dumitrescu, D.A.; Alexandru, C.; Serban, D.; Valeanu, L.; Pătrașcu, T.; Bobircă, A. Variability of Sentinel Lymph Node Location in Patients with Trunk Melanoma. Diagnostics 2023, 13, 2790. https://doi.org/10.3390/diagnostics13172790
Bobirca F, Leventer M, Georgescu DE, Dumitrescu DA, Alexandru C, Serban D, Valeanu L, Pătrașcu T, Bobircă A. Variability of Sentinel Lymph Node Location in Patients with Trunk Melanoma. Diagnostics. 2023; 13(17):2790. https://doi.org/10.3390/diagnostics13172790
Chicago/Turabian StyleBobirca, Florin, Mihaela Leventer, Dragos Eugen Georgescu, Dan Andrei Dumitrescu, Cristina Alexandru, Dragos Serban, Liana Valeanu, Traian Pătrașcu, and Anca Bobircă. 2023. "Variability of Sentinel Lymph Node Location in Patients with Trunk Melanoma" Diagnostics 13, no. 17: 2790. https://doi.org/10.3390/diagnostics13172790
APA StyleBobirca, F., Leventer, M., Georgescu, D. E., Dumitrescu, D. A., Alexandru, C., Serban, D., Valeanu, L., Pătrașcu, T., & Bobircă, A. (2023). Variability of Sentinel Lymph Node Location in Patients with Trunk Melanoma. Diagnostics, 13(17), 2790. https://doi.org/10.3390/diagnostics13172790











