New 6-Minute-Walking Test Parameter—Distance/Desaturation Index (DDI) Correctly Diagnoses Short-Term Response to Immunomodulatory Therapy in Hypersensitivity Pneumonitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Diagnosis of HP and Treatment Effect Assessment
2.2. Statistical Analysis
2.3. Regulatory Board Approval
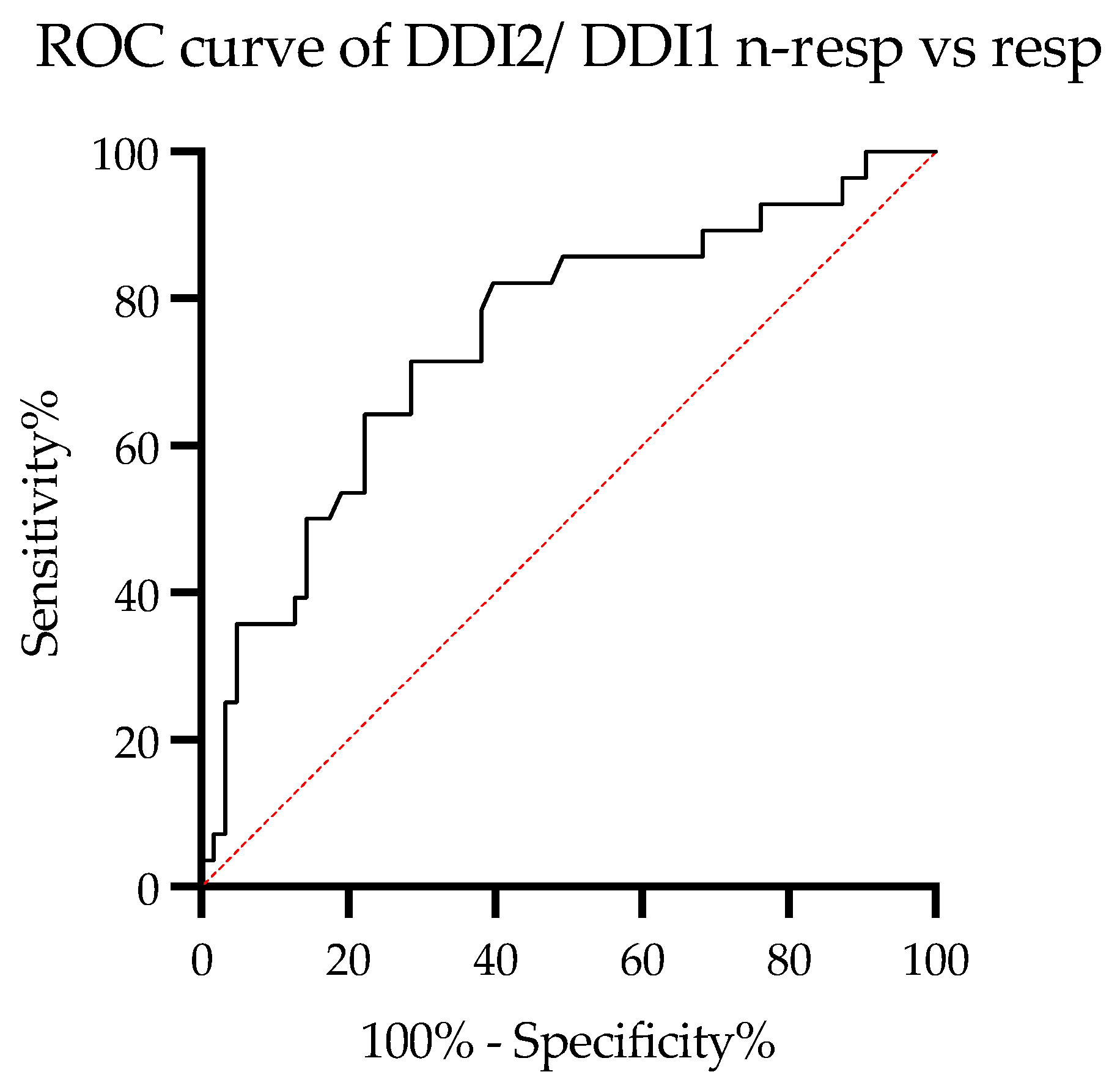
3. Results
Baseline Characteristics of the Study Group
4. Discussion
| ILD-Type | Ref Nº | 6MWD (m) | 6MWT SpO2-1 (%) | 6MWT SpO2-2 (%) | 6MWT SpO2 Desaturation (%) | DSP |
|---|---|---|---|---|---|---|
| IPF | [2] | 240 ± 300 | 95±2 | 89 ± 7 | 7±6 | - |
| IPF | [4] | 392.4 ± 108.5 | - | - | - | - |
| CTD-ILD | [8] | 352.26 ± 57.36 | - | - | - | - |
| PPF | [15] | 357.7 ± 99.2 | - | - | - | - |
| IPF | [22] | 406.9 ± 71.6 | 97.4 ± 1.2 | 89.4 ± 3 | 7.9 ± 2.8 | 364.8 ± 67.2 |
| Different ILDs | [23] | 457.28 ± 98.4 | 95.5 ± 2.23 | 90.5 ± 6.43 | 4.96 ± 5.57 | - |
| Sarcoidosis | [25] | 349 ± 72 | 95.5 ± 2.4 | 91.4 ± 7.3 | - | 320 ± 75 |
| fHP | [26] | 232.22 ± 49.53 | 89.67 ± 3.86 | 80.56 ± 6.20 | - | - |
| IPF | [28] | 355.4 ± 108.2 | 95.6 ± 1.0 | - | 7.1 ± 4.1 | - |
| HP | Present study | 485.7 ± 106.3 | 95.38 ± 2.72 | 87.59 ± 7.38 | 7.82 ± 6.67 | 424.2 ± 110.4 |
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Caminati, A.; Bianchi, A.; Cassandro, R.; Mirenda, M.R.; Harari, S. Walking distance on 6-MWT is a prognostic factor in idiopathic pulmonary fibrosis. Respir. Med. 2009, 103, 117–123. [Google Scholar] [CrossRef] [PubMed]
- du Bois, R.M.; Albera, C.; Bradford, W.Z.; Costabel, U.; Leff, J.A.; Noble, P.W.; Sahn, S.A.; Valeyre, D.; Weycker, D.; King, T.E. 6-Minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2014, 43, 1421–1429. [Google Scholar] [CrossRef]
- Bois, R.M.; Weycker, D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Kartashov, A.; Lancaster, L.; Noble, P.W.; Sahn, S.A.; Szwarcberg, J.; et al. Six-minute-walk test in idiopathic pulmonary fibrosis: Test validation and minimal clinically important difference. Am. J. Respir. Crit. Care Med. 2011, 183, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 61, 2200879. [Google Scholar] [CrossRef] [PubMed]
- Sobiecka, M.; Lewandowska, K.; Kober, J.; Franczuk, M.; Skoczylas, A.; Tomkowski, W.; Kuś, J.; Szturmowicz, M. Can a New Scoring System Improve Prediction of Pulmonary Hypertension in Newly Recognised Interstitial Lung Diseases? Lung 2020, 198, 547–554. [Google Scholar] [CrossRef]
- Waxman, A.; Restrepo-Jaramillo, R.; Thenappan, T.; Ravichandran, A.; Engel, P.; Bajwa, A.; Allen, R.; Feldman, J.; Argula, R.; Smith, P.; et al. Inhaled Treprostinil in Pulmonary Hypertension Due to Interstitial Lung Disease. N. Engl. J. Med. 2021, 384, 325–334. [Google Scholar] [CrossRef]
- Seema, S.; Nagesh, N.J.; Suriyan, S.; Talatam, R.P. Correlation of Six Minute Walk Test and Lung Function Test Variables (% FEV1, % FVC, % DLCO) in Patients with Connective Tissue Disorder—Interstitial Lung Disease. J. Evol. Med. Dent. Sci. 2020, 9, 2690–2694. Available online: https://www.jemds.com/data_pdf/N%20Nalini%20Jayanthi,%20Issue%2037,%20September%2014.pdf (accessed on 4 December 2022).
- Rocha, V.; Paixão, C.; Marques, A. Physical activity, exercise capacity and mortality risk in people with interstitial lung disease: A systematic review and meta-analysis. J. Sci. Med. Sport 2022, 25, 903–910. [Google Scholar] [CrossRef]
- King, T.E.; Albera, C.; Bradford, W.Z.; Costabel, U.; Hormel, P.; Lancaster, L.; Noble, P.W.; Sahn, S.A.; Szwarcberg, J.; Thomeer, M.; et al. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): A multicentre, randomised, placebo-controlled trial. Lancet 2009, 374, 222–228. [Google Scholar] [CrossRef]
- Idiopathic Pulmonary Fibrosis Clinical Research Network. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N. Engl. J. Med. 2010, 363, 620–628. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E., Jr.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Prasse, A.; Kreuter, M.; Johow, J.; Rabe, K.F.; Bonella, F.; Bonnet, R.; Grohe, C.; Held, M.; Wilkens, H.; et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 476–486. [Google Scholar] [CrossRef] [PubMed]
- De Sadeleer, L.J.; Hermans, F.; De Dycker, E.; Yserbyt, J.; Verschakelen, J.A.; Verbeken, E.K.; Verleden, G.M.; Verleden, S.E.; Wuyts, W.A. Impact of BAL lymphocytosis and presence of honeycombing on corticosteroid treatment effect in fibrotic hypersensitivity pneumonitis: A retrospective cohort study. Eur. Respir. J. 2020, 55, 1901983. [Google Scholar] [CrossRef]
- Salisbury, M.L.; Gu, T.; Murray, S.; Gross, B.H.; Chughtai, A.; Sayyouh, M.; Kazerooni, E.A.; Myers, J.L.; Lagstein, A.; Konopka, K.E.; et al. Hypersensitivity Pneumonitis: Radiologic Phenotypes Are Associated with Distinct Survival Time and Pulmonary Function Trajectory. Chest 2019, 155, 699–711. [Google Scholar] [CrossRef]
- Lewandowska, K.B.; Barańska, I.; Sobiecka, M.; Radwan-Rohrenschef, P.; Dybowska, M.; Franczuk, M.; Roży, A.; Skoczylas, A.; Bestry, I.; Kuś, J.; et al. Factors Predictive for Immunomodulatory Therapy Response and Survival in Patients with Hypersensitivity Pneumonitis—Retrospective Cohort Analysis. Diagnostics 2022, 12, 2767. [Google Scholar] [CrossRef]
- Szturmowicz, M.; Barańska, I.; Jędrych, M.E.; Bartoszuk, I.; Radwan-Roehrenschef, P.; Roży, A.; Bestry, I.; Chorostowska-Wynimko, J.; Langfort, R.; Kuś, J. Hypersensitivity pneumonitis recognised in a single pulmonary unit, between 2005 and 2015—Comparison with recently proposed diagnostic criteria. Adv. Respir. Med. 2019, 87, 83–89. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef]
- Przybyłowski, T.; Tomalak, W.; Siergiejko, Z.; Jastrzębski, D.; Maskey-Warzęchowska, M.; Piorunek, T.; Wojda, E.; Boros, P. Wytyczne Polskiego Towarzystwa Chorób Płuc dotyczące podstaw zastosowania, sposobu wykonywania oraz interpretacji testu 6-minutowego chodu (6MWT) Polish Respiratory Society guidelines for the methodology and interpretation of the 6 minute walk test (6MWT). Pneumonol. Alergol. Polska. Wyd. Pol. 2015, 83, 283–297. [Google Scholar] [CrossRef]
- Lettieri, C.J.; Nathan, S.D.; Browning, R.F.; Barnett, S.D.; Ahmad, S.; Shorr, A.F. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir. Med. 2006, 100, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Deng, M.; Liang, X.; Wei, H.; Wu, X. Features and predictive value of 6-min walk test outcomes in interstitial lung disease: An observation study using wearable monitors. BMJ Open 2022, 12, e055077. [Google Scholar] [CrossRef] [PubMed]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; du Bois, R.M.; Fagan, E.A.; Fishman, R.S.; Glaspole, I.; Glassberg, M.K.; Lancaster, L.; et al. Pirfenidone for idiopathic pulmonary fibrosis: Analysis of pooled data from three multinational phase 3 trials. Eur. Respir. J. 2016, 47, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Alhamad, E.H.; Shaik, S.A.; Idrees, M.M.; Alanezi, M.O.; Isnani, A.C. Outcome measures of the 6 minute walk test: Relationships with physiologic and computed tomography findings in patients with sarcoidosis. BMC Pulm. Med. 2010, 10, 42. [Google Scholar] [CrossRef]
- Tony, F.A.; Soliman, Y.M.A.; Salem, H.A. Effect of Oral Methyl Prednisolone on Different Radiological Patterns of Hypersensitivity Pneumonitis. J. Asthma Allergy 2021, 14, 501–511. [Google Scholar] [CrossRef]
- Gupta, N.; Goyal, M.; Vk, H.; Chandran, A.; Sharma, A. 6 Minute Walk Test and Idiopathic Pulmonary Fibrosis: Distance or Desaturation? A Prospective Observational Study. J. Med. Therap. 2017. early view. Available online: http://www.oatext.com/Minute-walk-test-and-idiopathic-pulmonary-fibrosis-distance-or-desaturation-A-prospective-observational-study.php (accessed on 20 November 2022).
- Lama, V.N.; Flaherty, K.R.; Toews, G.B.; Colby, T.V.; Travis, W.D.; Long, Q.; Murray, S.; Kazerooni, E.A.; Gross, B.H.; Lynch, J.P.; et al. Prognostic Value of Desaturation during a 6-Minute Walk Test in Idiopathic Interstitial Pneumonia. Am. J. Respir. Crit. Care Med. 2003, 168, 1084–1090. [Google Scholar] [CrossRef]
- Nathan, S.D.; du Bois, R.M.; Albera, C.; Bradford, W.Z.; Costabel, U.; Kartashov, A.; Noble, P.W.; Sahn, S.A.; Valeyre, D.; Weycker, D.; et al. Validation of test performance characteristics and minimal clinically important difference of the 6-minute walk test in patients with idiopathic pulmonary fibrosis. Respir. Med. 2015, 109, 914–922. [Google Scholar] [CrossRef]
| Parameter | Responders | Non-Responders |
|---|---|---|
| VCmax or TL,co | Any increase or stable | Any decrease |
| AND | AND | |
| Chest X-ray | Stabilization or improvement | Stabilization or worsening |
| Variable | Whole Group N = 91 |
|---|---|
| Age at diagnosis (y), mean (±SD) | 51.6 (±10.32) |
| Males, Nº (%) | 44 (48) |
| Ever smokers, Nº (%) | 37 (40.7) |
| VC max (L), mean (±SD) | 2.97 (±0.97) |
| VC max (% pred.), mean (±SD) | 82.4 (±20.1) |
| TLC (L), mean (±SD) | 5.32 (±1.15) |
| TLC (% pred.), mean (±SD) | 94.7 (±21.7) |
| RV%TLC (% pred.), mean (±SD) | 114.2 (±21.86) |
| TLco (% pred.), mean (±SD) | 48.87 (±14.9) |
| Tiffenau index (%), mean (±SD) | 78 (±10.9) |
| 6MWD (m), mean (±SD) | 480.1 (±106.2) |
| 6MWT SpO2-1 (%), mean (±SD) | 95.4 (±2.3) |
| 6MWT SpO2-2 (%), mean (±SD) | 87.8 (±7.3) |
| 6MWT desaturation (%), mean (±SD) | 7.82 (±6.67) |
| Distance/desaturation index (DDI), mean (±SD) | 155.1 (±172.9) |
| Distance-saturation product (DSP), mean (±SD) | 424.2 (±110.4) |
| Fibrotic HP Nº (%) | 52 (57) |
| Non-fibrotic HP Nº (%) | 39 (43) |
| Prednisone monotherapy Nº (%) | 76 (83.5) |
| Prednisone + azathioprine Nº (%) | 15 (16.5) |
| Parameter | Whole Group | fHP | Non-fHP | p |
|---|---|---|---|---|
| 6MWD (m) | 485.7 (±106.3) | 472.9 (±116.4) | 484.0 (±98.48) | 0.79 |
| 6MWT SpO2-1 (%) | 95.38 (±2.72) | 95.67 (±2.03) | 95.03 (±2.54) | 0.28 |
| 6MWT SpO2-2 (%) | 87.59 (±7.38) | 87.19 (±7.39) | 88.64 (±7.21) | 0.23 |
| 6MWT SpO2 desaturation (%) | 7.82 (±6.67) | 8.48 (±6.554) | 6.39 (±6.548) | 0.05 |
| DDI | 155.1 (±172.9) | 134.4 (±139.8) | 182.8 (±175.3) | 0.07 |
| DSP | 424.2 (±110.4) | 425 (±103.7) | 423.2 (±120.3) | 0.83 |
| Parameter | VC max %pred | TL,co %pred | ||||
|---|---|---|---|---|---|---|
| r | 95% CI | p | r | 95% CI | p | |
| 6MWD | −0.0206 | −0.1897 to 0.2292 | 0.8444 | 0.3616 | 0.1645 to 0.5309 | 0.0004 |
| 6MWT SpO2 desaturation (%) | −0.4463 | −0.5997 to −0.2611 | <0.0001 | −0.6592 | −0.7633 to −0.5217 | <0.0001 |
| DSP | 0.1309 | −0.0809 to 0.3314 | 0.2111 | 0.5134 | 0.3405 to 0.6528 | <0.0001 |
| DDI | 0.3890 | 0.1953 to 0.5534 | 0.0001 | 0.6525 | 0.5131 to 0.7583 | <0.0001 |
| Parameter | Whole Group (n = 91) | p | Responders (n = 63) | p | Non-Responders (n = 28) | p | |||
|---|---|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatment | ||||
| 6MWD (m) | 479 (458–502) | 524 (489–536) | 0.0001 | 479 (453–503) | 524 (475.5–534) | 0.0036 | 479.5 (437–531) | 525.5 (491.5–568) | 0.0112 |
| 6MWT SpO2 1 (%) | 96 (94.9–95.9) | 96 (95.8–96.6) | 0.0017 | 96 (95–96) | 96 (96–96.9) | 0.0015 | 96 (94.1–96.1) | 96 (94.6–96.5) | 0.3347 |
| 6MWT SpO2 2 (%) | 89 (86.3–89.3) | 92 (88.8–91.5) | 0.0171 | 89 (86–89.5) | 92 (88.8–92) | 0.0015 | 88.5 (84.7–91.2) | 92 (87–92.1) | 0.1487 |
| 6MWT desat (%) | 6 (6.23–8.96) | 4 (4.89–7.24) | 0.0073 | 7 (6.3–9.3) | 4 (4.7–7.5) | 0.0093 | 5 (4.2–10.2) | 4 (3.8–8.1) | 0.4797 |
| DSP | 416.5 (401.2–447.2) | 471.6 (440.3–487.0) | <0.0001 | 414.7 (395.8–446.9) | 471.6 (428.8–488.8) | 0.0004 | 421.6 (380.1–481.3) | 470.7 (437.6–511.7) | 0.0118 |
| DDI | 70.7 (119.1–191.1) | 117.7 (155.7–231.9) | 0.0765 | 65.7 (91.8–165) | 144.0 (167.7–264.7) | 0.0019 | 111.0 (132.3–297.8) | 81.9 (85.1–201.5) | 0.2016 |
| Parameter | Treatment Related Changes, Median (95% CI) | p | |
|---|---|---|---|
| Responders | Non-Responders | ||
| 6MWT desat. change (%) | −2 (−4.08–−1.61) | −1.5 (−2.98–0.12) | 0.3475 |
| 6MWT dist change (m) | 51 (36–72) | 10.5 (−61.2–27.9) | 0.0056 |
| DSP post/DSP pre | 1.09 (1.04–1.19) | 1.09 (1.06–1.24) | 0.6428 |
| DDI post/DDI pre | 1.67 (1.85–3.63) | 0.88 (0.7–1.73) | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, K.B.; Sobiecka, M.; Boros, P.W.; Dybowska, M.; Barańska, I.; Jędrych, M.E.; Gładzka, A.; Tomkowski, W.Z.; Szturmowicz, M. New 6-Minute-Walking Test Parameter—Distance/Desaturation Index (DDI) Correctly Diagnoses Short-Term Response to Immunomodulatory Therapy in Hypersensitivity Pneumonitis. Diagnostics 2023, 13, 1109. https://doi.org/10.3390/diagnostics13061109
Lewandowska KB, Sobiecka M, Boros PW, Dybowska M, Barańska I, Jędrych ME, Gładzka A, Tomkowski WZ, Szturmowicz M. New 6-Minute-Walking Test Parameter—Distance/Desaturation Index (DDI) Correctly Diagnoses Short-Term Response to Immunomodulatory Therapy in Hypersensitivity Pneumonitis. Diagnostics. 2023; 13(6):1109. https://doi.org/10.3390/diagnostics13061109
Chicago/Turabian StyleLewandowska, Katarzyna B., Małgorzata Sobiecka, Piotr W. Boros, Małgorzata Dybowska, Inga Barańska, Małgorzata E. Jędrych, Agata Gładzka, Witold Z. Tomkowski, and Monika Szturmowicz. 2023. "New 6-Minute-Walking Test Parameter—Distance/Desaturation Index (DDI) Correctly Diagnoses Short-Term Response to Immunomodulatory Therapy in Hypersensitivity Pneumonitis" Diagnostics 13, no. 6: 1109. https://doi.org/10.3390/diagnostics13061109
APA StyleLewandowska, K. B., Sobiecka, M., Boros, P. W., Dybowska, M., Barańska, I., Jędrych, M. E., Gładzka, A., Tomkowski, W. Z., & Szturmowicz, M. (2023). New 6-Minute-Walking Test Parameter—Distance/Desaturation Index (DDI) Correctly Diagnoses Short-Term Response to Immunomodulatory Therapy in Hypersensitivity Pneumonitis. Diagnostics, 13(6), 1109. https://doi.org/10.3390/diagnostics13061109








