Characterization of Muscle Weakness Due to Myasthenia Gravis Using Shear Wave Elastography
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Data Collection
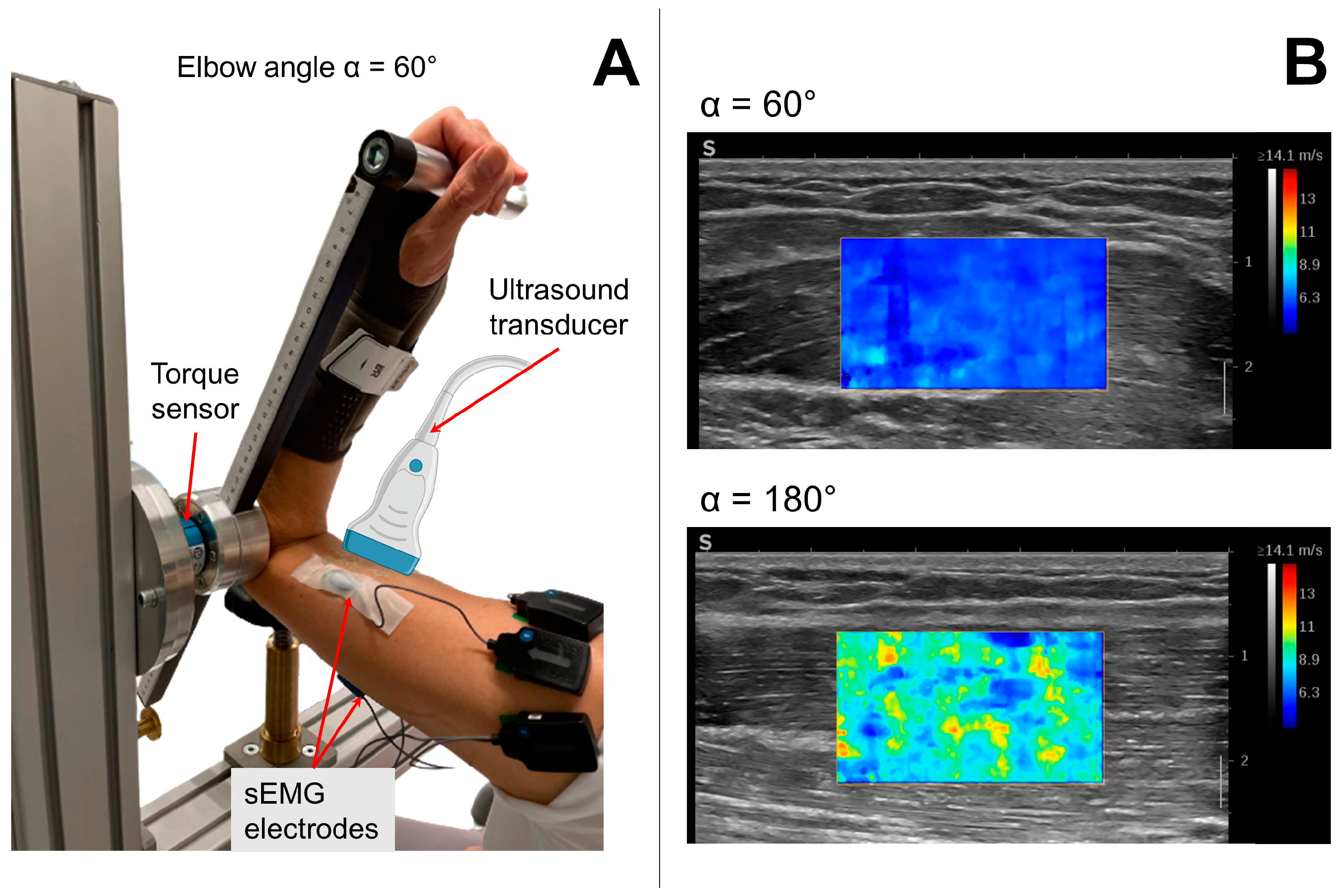
2.3. Experimental Measurements
2.4. Experimental Protocol
2.5. Data Analysis
2.6. Statistics
3. Results
3.1. Anthropometrics
3.2. Active Muscle Characteristics
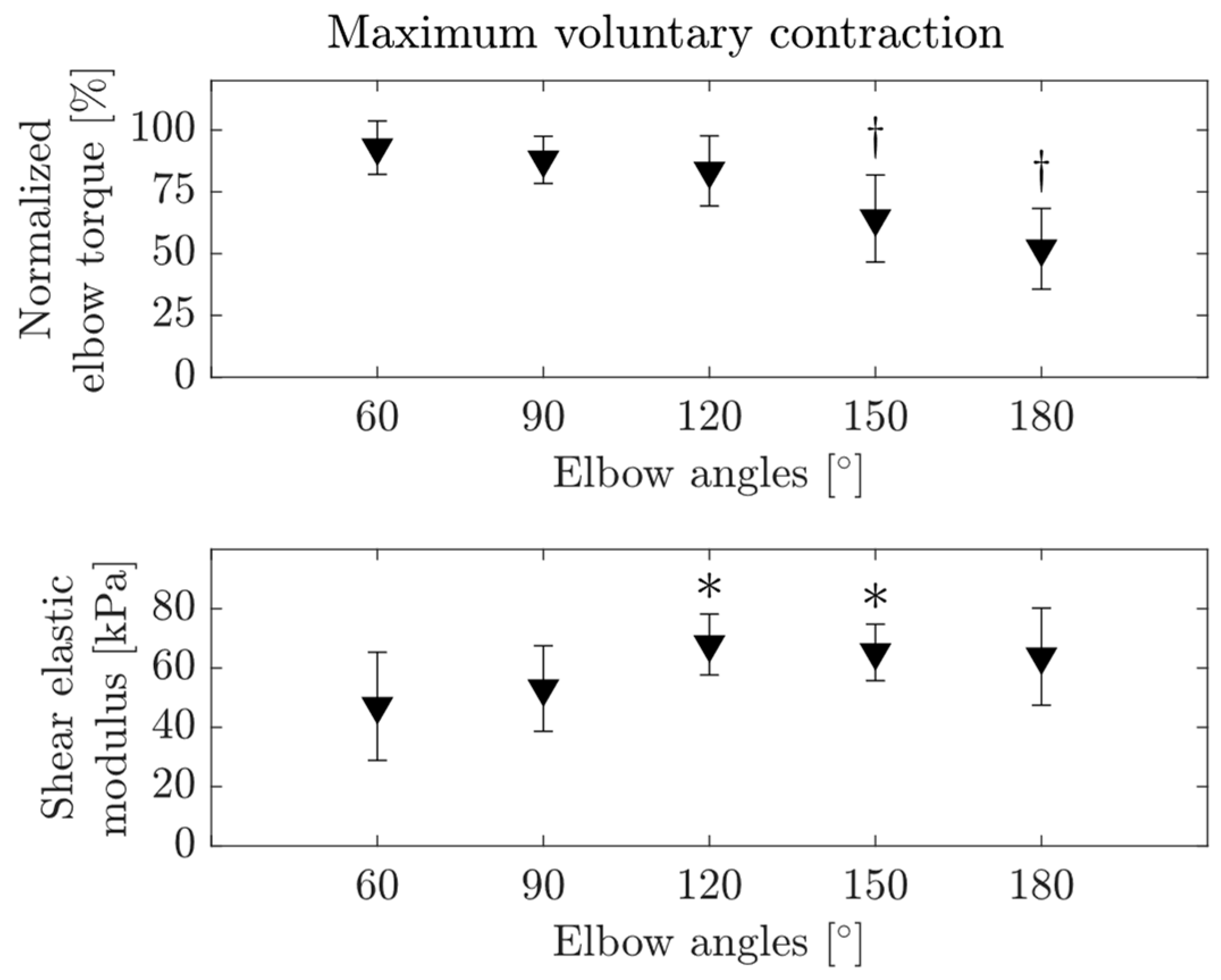
3.2.1. MVC
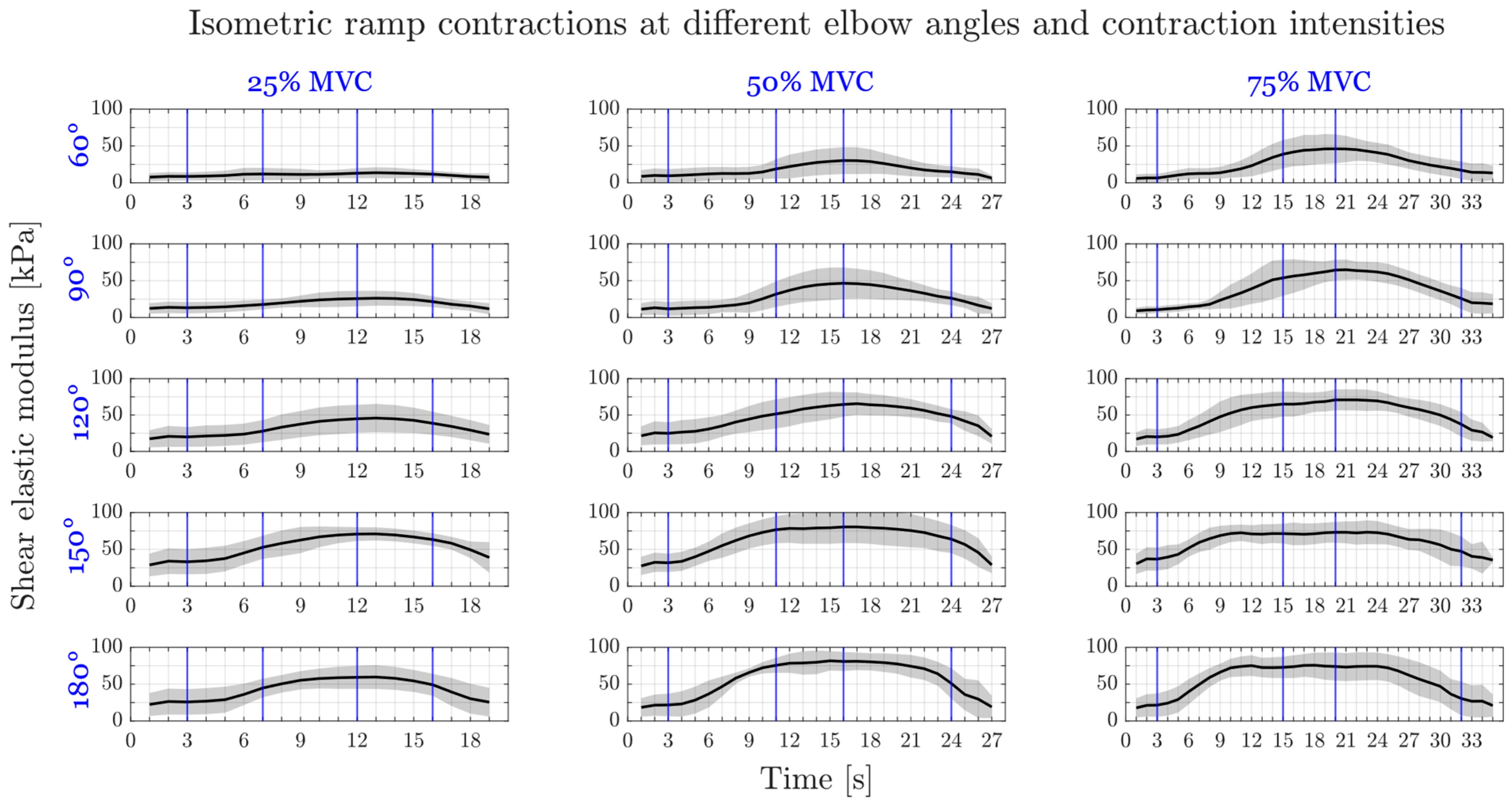
3.2.2. Submaximal Ramp Contractions
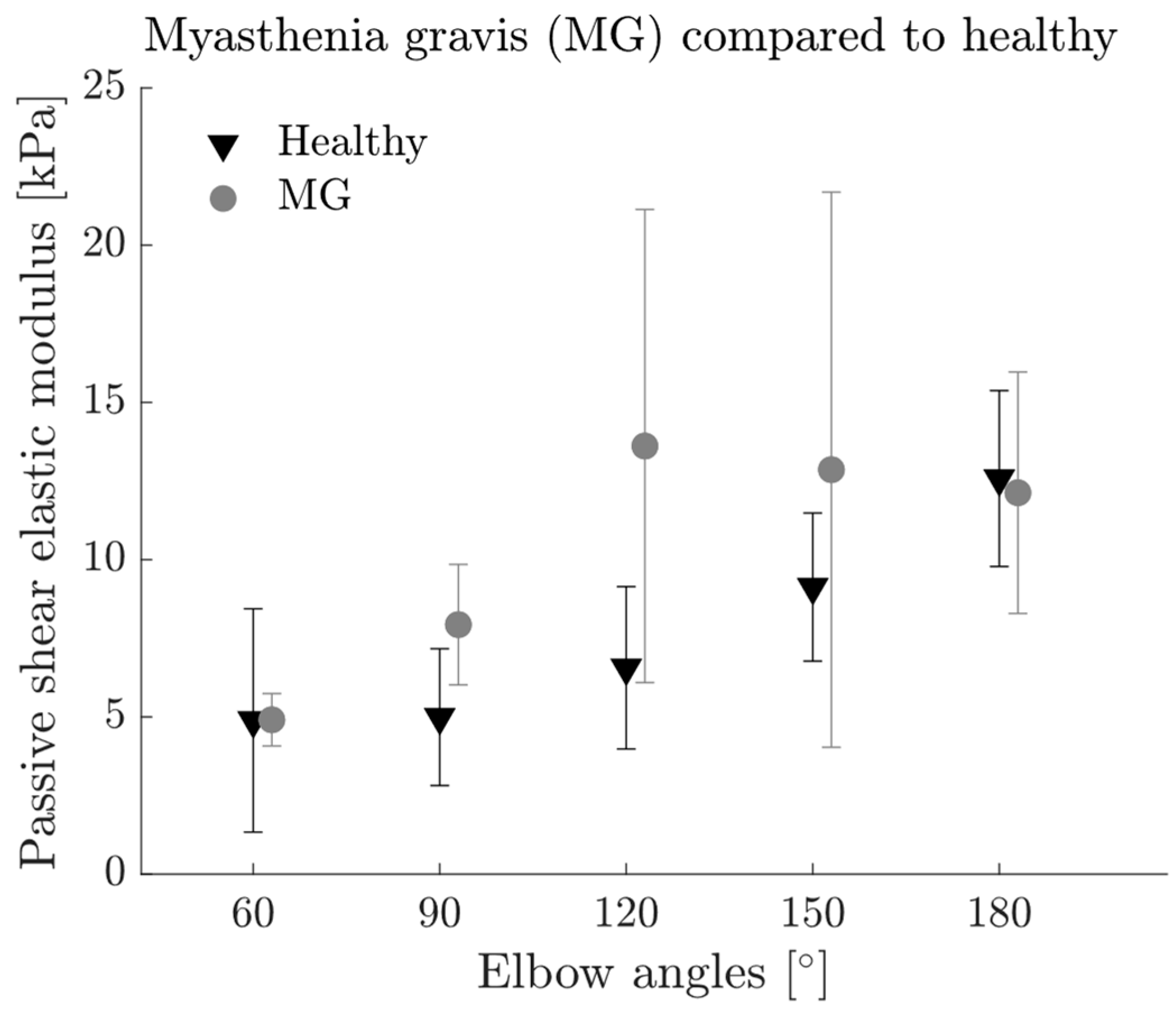
3.3. Passive Muscle Characteristics
3.4. Associations between the Physiological, Mechanical and Electrical Properties Tested
3.5. Comparisons between MG Patients and Healthy Group
4. Discussion
4.1. Towards In Vivo Muscle Force–Length Characteristics
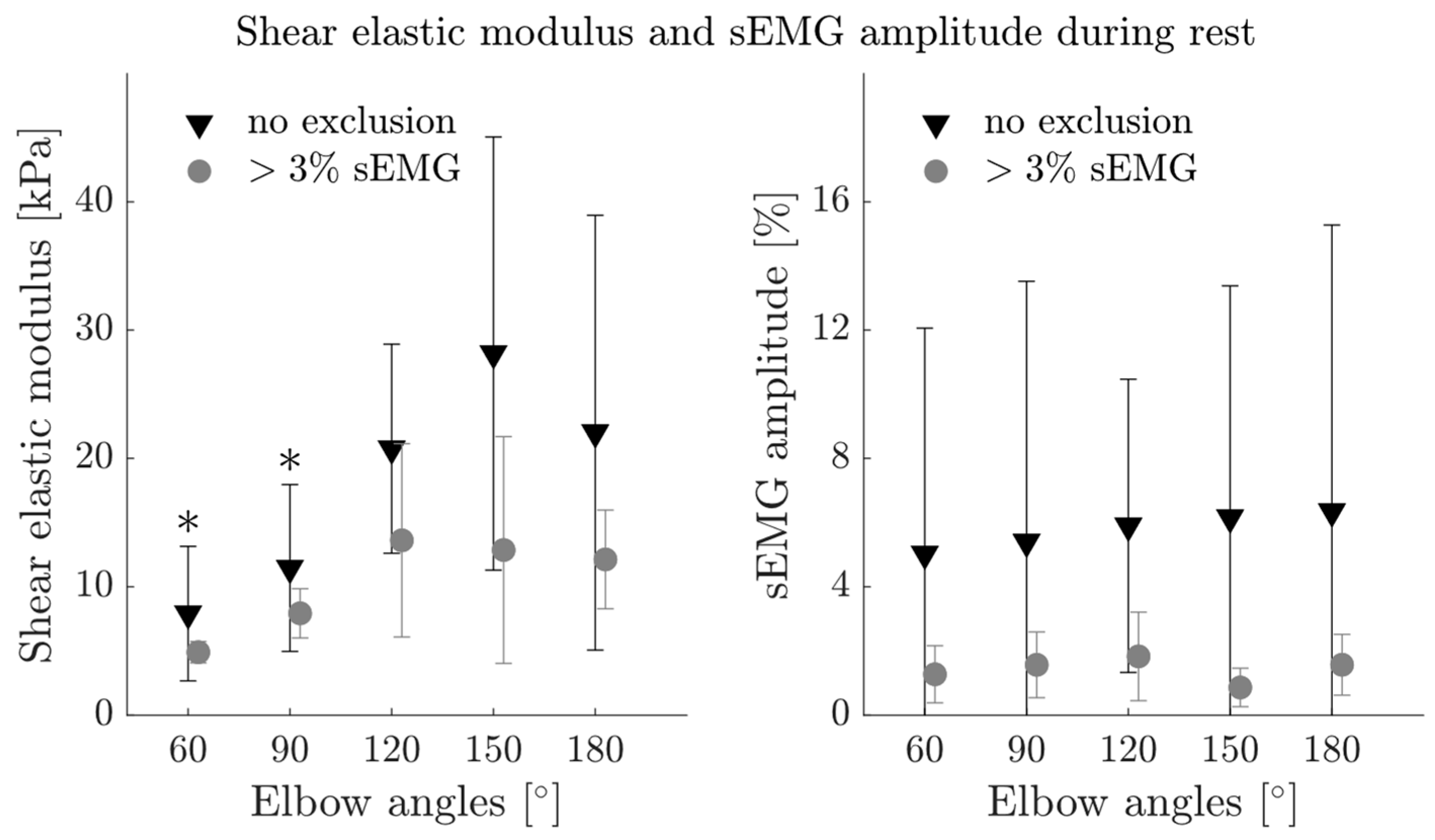
4.2. MG Patients Show Increased Muscle Activity during Rest
4.3. Detecting Muscle Weakness in MG Using SWE
4.4. Implications, Limitations and Clinical Use
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salari, N.; Fatahi, B.; Bartina, Y.; Kazeminia, M.; Fatahian, R.; Mohammadi, P.; Shohaimi, S.; Mohammadi, M. Global Prevalence of Myasthenia Gravis and the Effectiveness of Common Drugs in Its Treatment: A Systematic Review and Meta-Analysis. J. Transl. Med. 2021, 19, 516. [Google Scholar] [CrossRef]
- Deenen, J.C.W.; Horlings, C.G.C.; Verschuuren, J.J.G.M.; Verbeek, A.L.M.; van Engelen, B.G.M. The Epidemiology of Neuromuscular Disorders: A Comprehensive Overview of the Literature. J. Neuromuscul. Disord. 2015, 2, 73–85. [Google Scholar] [CrossRef]
- Sieb, J.P. Myasthenia Gravis: An Update for the Clinician. Clin. Exp. Immunol. 2013, 175, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Parthan, A.; Lauher-Charest, M.; Broderick, L.; Law, N.; Barnett, C. Understanding the Symptom Burden and Impact of Myasthenia Gravis from the Patient’s Perspective: A Qualitative Study. Neurol. Ther. 2022, 12, 107–128. [Google Scholar] [CrossRef]
- Ruiter, A.M.; Verschuuren, J.J.G.M.; Tannemaat, M.R. Fatigue in Patients with Myasthenia Gravis. A Systematic Review of the Literature. Neuromuscul. Disord. 2020, 30, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Cantor, F. Central and Peripheral Fatigue: Exemplified by Multiple Sclerosis and Myasthenia Gravis. PM R 2010, 2, 399–405. [Google Scholar] [CrossRef]
- Barnett, C.; Herbelin, L.; Dimachkie, M.M.; Barohn, R.J. Measuring Clinical Treatment Response in Myasthenia Gravis. Neurol. Clin. 2018, 36, 339. [Google Scholar] [CrossRef]
- Besinger, U.A.; Toyka, K.V.; Hömberg, M.; Heininger, K.; Hohlfeld, R.; Fateh-Moghadam, A. Myasthenia Gravis: Long-Term Correlation of Binding and Bungarotoxin Blocking Antibodies against Acetylcholine Receptors with Changes in Disease Severity. Neurology 1983, 33, 1316–1321. [Google Scholar] [CrossRef]
- Jaretzki, A.; Barohn, R.J.; Ernstoff, R.M.; Kaminski, H.J.; Keesey, J.C.; Penn, A.S.; Sanders, D.B. Myasthenia Gravis: Recommendations for Clinical Research Standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 2000, 55, 16–23. [Google Scholar] [CrossRef]
- Eby, S.F.; Song, P.; Chen, S.; Chen, Q.; Greenleaf, J.F.; An, K.N. Validation of Shear Wave Elastography in Skeletal Muscle. J. Biomech. 2013, 46, 2381–2387. [Google Scholar] [CrossRef]
- Ateş, F.; Andrade, R.J.; Freitas, S.R.; Hug, F.; Lacourpaille, L.; Gross, R.; Yucesoy, C.A.; Nordez, A. Passive Stiffness of Monoarticular Lower Leg Muscles Is Influenced by Knee Joint Angle. Eur. J. Appl. Physiol 2018, 118, 585–593. [Google Scholar] [CrossRef]
- Maïsetti, O.; Hug, F.; Bouillard, K.; Nordez, A. Characterization of Passive Elastic Properties of the Human Medial Gastrocnemius Muscle Belly Using Supersonic Shear Imaging. J. Biomech. 2012, 45, 978–984. [Google Scholar] [CrossRef]
- Zimmer, M.; Kleiser, B.; Marquetand, J.; Ateş, F. Shear Wave Elastography Characterizes Passive and Active Mechanical Properties of Biceps Brachii Muscle in Vivo. J. Mech. Behav. Biomed. Mater. 2023, 137, 105543. [Google Scholar] [CrossRef]
- Ateş, F.; Hug, F.; Bouillard, K.; Jubeau, M.; Frappart, T.; Couade, M.; Bercoff, J.; Nordez, A. Muscle Shear Elastic Modulus Is Linearly Related to Muscle Torque over the Entire Range of Isometric Contraction Intensity. J. Electromyogr. Kinesiol. 2015, 25, 703–708. [Google Scholar] [CrossRef]
- Bouillard, K.; Hug, F.; Guével, A.; Nordez, A. Shear Elastic Modulus Can Be Used to Estimate an Index of Individual Muscle Force during a Submaximal Isometric Fatiguing Contraction. J. Appl. Physiol. 2012, 113, 1353–1361. [Google Scholar] [CrossRef]
- Crutison, J.; Sun, M.; Royston, T.J. The Combined Importance of Finite Dimensions, Anisotropy, and Pre-Stress in Acoustoelastography. J. Acoust. Soc. Am. 2022, 151, 2403. [Google Scholar] [CrossRef]
- Wang, A.B.; Perreault, E.J.; Royston, T.J.; Lee, S.S.M. Changes in Shear Wave Propagation within Skeletal Muscle during Active and Passive Force Generation. J. Biomech. 2019, 94, 115–122. [Google Scholar] [CrossRef]
- Harmon, B.; Wells, M.; Park, D.; Gao, J. Musculoskeletal and Emergency Imaging Ultrasound Elastography in Neuromuscular and Movement Disorders. Clin. Imaging 2018, 53, 35–42. [Google Scholar] [CrossRef]
- Kronlage, C.; Grimm, A.; Romano, A.; Stahl, J.H.; Martin, P.; Winter, N.; Marquetand, J. Muscle Ultrasound Shear Wave Elastography as a Non-Invasive Biomarker in Myotonia. Diagnostics 2021, 11, 163. [Google Scholar] [CrossRef]
- Du, L.J.; He, W.; Cheng, L.G.; Li, S.; Pan, Y.S.; Gao, J. Ultrasound Shear Wave Elastography in Assessment of Muscle Stiffness in Patients with Parkinson’s Disease: A Primary Observation. Clin. Imaging 2016, 40, 1075–1080. [Google Scholar] [CrossRef]
- Ding, C.W.; Song, X.; Fu, X.Y.; Zhang, Y.C.; Mao, P.; Sheng, Y.J.; Yang, M.; Wang, C.S.; Zhang, Y.; Chen, X.F.; et al. Shear Wave Elastography Characteristics of Upper Limb Muscle in Rigidity-Dominant Parkinson’s Disease. Neurol. Sci. 2021, 42, 4155–4162. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Hansen, E.A.; Lee, H.D.; Barrett, K.; Herzog, W. The Shape of the Force-Elbow Angle Relationship for Maximal Voluntary Contractions and Sub-Maximal Electrically Induced Contractions in Human Elbow Flexors. J. Biomech. 2003, 36, 1713–1718. [Google Scholar] [CrossRef]
- Leedham, J.S.; Dowling, J.J. Force-Length, Torque-Angle and EMG-Joint Angle Relationships of the Human in Vivo Biceps Brachii. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 421–426. [Google Scholar] [CrossRef]
- van Zuylen, E.J.; van Velzen, A.; van der Gon, J.J.D. A Biomechanical Model for Flexion Torques of Human Arm Muscles as a Function of Elbow Angle. J. Biomech. 1988, 21, 183–190. [Google Scholar] [CrossRef]
- Gordon, A.M.; Huxley, A.F.; Julian, F.J. The Variation in Isometric Tension with Sarcomere Length in Vertebrate Muscle Fibres. J. Physiol. 1966, 184, 170. [Google Scholar] [CrossRef]
- Ateş, F.; Yucesoy, C.A. Botulinum Toxin Type-A Affects Mechanics of Non-Injected Antagonistic Rat Muscles. J. Mech. Behav. Biomed. Mater. 2018, 84, 208–216. [Google Scholar] [CrossRef]
- Ateş, F.; Özdeşlik, R.N.; Huijing, P.A.; Yucesoy, C.A. Muscle Lengthening Surgery Causes Differential Acute Mechanical Effects in Both Targeted and Non-Targeted Synergistic Muscles. J. Electromyogr. Kinesiol. 2013, 23, 1199–1205. [Google Scholar] [CrossRef]
- Ateş, F.; Temelli, Y.; Yucesoy, C.A. Intraoperative Experiments Show Relevance of Inter-Antagonistic Mechanical Interaction for Spastic Muscle’s Contribution to Joint Movement Disorder. Clin. Biomech. 2014, 29, 943–949. [Google Scholar] [CrossRef]
- Yucesoy, C.A.; Temelli, Y.; Ateş, F. Intra-Operatively Measured Spastic Semimembranosus Forces of Children with Cerebral Palsy. J. Electromyogr. Kinesiol. 2017, 36, 49–55. [Google Scholar] [CrossRef]
- Chino, K.; Takahashi, H. Measurement of Gastrocnemius Muscle Elasticity by Shear Wave Elastography: Association with Passive Ankle Joint Stiffness and Sex Differences. Eur. J. Appl. Physiol. 2016, 116, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Bernabei, X.M.; Lee, S.S.M.; Perreault, E.J.; Sandercock, T.G. Shear Wave Velocity Is Sensitive to Changes in Muscle Stiffness That Occur Independently from Changes in Force. J. Appl. Physiol. 2020, 128, 8–16. [Google Scholar] [CrossRef]
- Gajdosik, R.L. Passive Extensibility of Skeletal Muscle: Review of the Literature with Clinical Implications. Clin. Biomech. 2001, 16, 87–101. [Google Scholar] [CrossRef]
- le Sant, G.; Gross, R.; Hug, F.; Nordez, A. Influence of Low Muscle Activation Levels on the Ankle Torque and Muscle Shear Modulus during Plantar Flexor Stretching. J. Biomech. 2019, 93, 111–117. [Google Scholar] [CrossRef]
- Meriggioli, M.N.; Sanders, D.B. Advances in the Diagnosis of Neuromuscular Junction Disorders. Am. J. Phys. Med. Rehabil. 2005, 84, 627–638. [Google Scholar] [CrossRef]
- Merletti, R.; Cerone, G.L. Tutorial. Surface EMG Detection, Conditioning and Pre-Processing: Best Practices. J. Electromyogr. Kinesiol. 2020, 54, 102440. [Google Scholar] [CrossRef]
- Eby, S.F.; Cloud, B.A.; Brandenburg, J.E.; Giambini, H.; Song, P.; Chen, S.; Lebrasseur, N.K.; An, K.N. Shear Wave Elastography of Passive Skeletal Muscle Stiffness: Influences of Sex and Age throughout Adulthood. Clin. Biomech. 2015, 30, 22–27. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.-K.; Sheng, S.-Y.; Liang, S.-M.; Lu, H.; Chen, R.-Y.; Pan, M.; Wen, Z.-B. Quantitative Evaluation of Passive Muscle Stiffness by Shear Wave Elastography in Healthy Individuals of Different Ages. Eur. Radiol. 2021, 31, 3187–3194. [Google Scholar] [CrossRef]
- Alfuraih, A.M.; Tan, A.L.; O’Connor, P.; Emery, P.; Wakefield, R.J. The Effect of Ageing on Shear Wave Elastography Muscle Stiffness in Adults. Aging Clin. Exp. Res. 2019, 31, 1755–1763. [Google Scholar] [CrossRef]
- Do, Y.; Lall, P.S.; Lee, H. Assessing the Effects of Aging on Muscle Stiffness Using Shear Wave Elastography and Myotonometer. Healthcare 2021, 9, 1733. [Google Scholar] [CrossRef]

| ID | Sex | Age | BMI (kg/m2) | Handedness | Antibody Specificity | Medicaments * | Regular Sports | B.S., Total | B.S., Arms |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 64 | 23.88 | Left | AChR | Mycophenolatmofetil 1500 mg | - | 2 | 0 |
| 2 | M | 42 | 29.83 | Right | AChR | Azathioprin 150 mg, Pyridostigmin ret. 720 mg | Running, volleyball | 0 | 0 |
| 3 | M | 23 | 25.08 | Right | MuSK | Pyridostigmin 90 mg | Bouldering | 1 | 0 |
| 4 | F | 57 | 33.62 | Right | AChR | Rituximab | Walks | 2 | 0 |
| 5 | F | 35 | 24.68 | Right | AChR | Prednisolon 3 mg, Immunoglobulins, Pyridostigmin ret. 270 mg | Aqua jogging | 5 | 1 |
| 6 | M | 24 | 23.45 | Right | CMS | Pyridostigmin 180 mg + ret. 90 mg | Walks | 7 | 2 |
| 7 | F | 60 | 30.00 | Right | AChR | Prednisolon 7.5 mg, Pyridostigmin 180 mg | - | 5 | 1 |
| 8 | F | 65 | 32.32 | Right | AChR | Mycophenolatmofetil 750 mg, Pyridostigmin 60 mg + ret. 90 mg | - | 6 | 2 |
| 9 | M | 53 | 22.13 | Right | MuSK | Rituximab | Biking, walks, gym | 5 | 1 |
| 10 | F | 62 | 22.43 | Right | MuSK | Rituximab | Yoga, Walks | 9 | 1 |
| 11 | F | 39 | 23.23 | Right | AChR, Anti-Titin | Azathioprin 175 mg, Pyridostigmin 180 mg + ret. 90 mg | Walks | 7 | 0 |
| Step | Trial Type | Duration | Elbow Angles |
|---|---|---|---|
| 1 | Extension MVC | 5 s | 120° |
| Break | 2 min | ||
| 2 | Rest | 5 s | 60–180° |
| 3 | Flexion MVC | 5 s | 60–180° |
| Break | 2 min | ||
| 4 | Ramp contraction up to 25% MVC torque | 19 s | 60–180° |
| Break | 1 min | ||
| 5 | Ramp contraction up to 50% MVC torque | 27 s | 60–180° |
| Break | 1 min | ||
| 6 | Ramp contraction up to 75% MVC torque | 35 s | 60–180° |
| Break | 1 min |
| MVC Torque (Nm) | ||||||
|---|---|---|---|---|---|---|
| Patient ID | Elbow Angles: Flexion | Extension | ||||
| 60° | 90° | 120° | 150° | 180° | 120° | |
| 1 | 27.91 | 21.32 | 17.92 | 4.60 | 8.63 | 13.61 |
| 2 | 51.15 | 54.35 | 47.63 | 36.24 | 27.95 | 38.64 |
| 3 | 76.02 | 72.47 | 74.32 | 54.65 | 43.08 | 29.21 |
| 4 | 18.90 | 20.53 | 22.11 | 16.51 | 8.84 | 14.14 |
| 5 | 25.04 | 26.21 | 28.77 | 23.29 | 15.70 | 18.36 |
| 6 | 44.49 | 36.06 | 36.25 | 31.53 | 37.90 | 24.53 |
| 7 | 24.72 | 29.83 | 19.12 | 19.04 | 19.12 | 16.33 |
| 8 | 8.90 | 10.68 | 14.10 | 9.05 | 6.94 | 7.97 |
| 9 | 57.19 | 55.64 | 48.26 | 37.81 | 41.12 | 26.61 |
| 10 | 14.77 | 21.53 | 17.12 | 12.33 | 9.06 | 16.33 |
| 11 | 25.99 | 19.35 | 18.86 | 21.60 | 8.50 | 13.38 |
| Mean | 35.35 | 33.45 | 31.32 | 24.24 | 20.62 | 20.17 |
| STD | 18.67 | 18.33 | 17.84 | 14.01 | 13.66 | 8.29 |
| BB CSA | SWE at MVC | sEMG at Rest | SWE at Rest | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R Value | p Value | R Value | p Value | R Value | p Value | R Value | p Value | ||
| MVC elbow flexion torque | 60° | 0.642 | 0.033 | 0.914 | <0.001 | −0.625 | 0.040 | −0.670 | 0.024 |
| 90° | 0.583 | 0.060 | 0.869 | 0.001 | −0.335 | 0.314 | −0.574 | 0.065 | |
| 120° | 0.515 | 0.105 | 0.285 | 0.395 | −0.427 | 0.181 | −0.458 | 0.157 | |
| 150° | 0.599 | 0.052 | 0.017 | 0.959 | −0.726 | 0.155 | −0.444 | 0.172 | |
| 180° | 0.778 | 0.005 | 0.223 | 0.510 | −0.601 | 0.022 | −0.682 | 0.021 | |
| SWE at Rest | Age | BMI | BB CSA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R Value | p Value | R Value | p Value | R Value | p Value | R Value | p Value | ||
| sEMG at rest | 60° | 0.941 | <0.001 | 0.465 | 0.150 | 0.647 | 0.031 | −0.276 | 0.411 |
| 90° | 0.763 | 0.006 | 0.481 | 0.134 | 0.572 | 0.066 | −0.243 | 0.472 | |
| 120° | 0.516 | 0.105 | 0.693 | 0.018 | 0.389 | 0.237 | −0.580 | 0.061 | |
| 150° | 0.808 | 0.003 | 0.567 | 0.069 | 0.479 | 0.136 | −0.444 | 0.171 | |
| 180° | 0.432 | 0.184 | 0.354 | 0.285 | 0.250 | 0.458 | −0.355 | 0.285 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmer, M.; Kleiser, B.; Marquetand, J.; Ates, F. Characterization of Muscle Weakness Due to Myasthenia Gravis Using Shear Wave Elastography. Diagnostics 2023, 13, 1108. https://doi.org/10.3390/diagnostics13061108
Zimmer M, Kleiser B, Marquetand J, Ates F. Characterization of Muscle Weakness Due to Myasthenia Gravis Using Shear Wave Elastography. Diagnostics. 2023; 13(6):1108. https://doi.org/10.3390/diagnostics13061108
Chicago/Turabian StyleZimmer, Manuela, Benedict Kleiser, Justus Marquetand, and Filiz Ates. 2023. "Characterization of Muscle Weakness Due to Myasthenia Gravis Using Shear Wave Elastography" Diagnostics 13, no. 6: 1108. https://doi.org/10.3390/diagnostics13061108
APA StyleZimmer, M., Kleiser, B., Marquetand, J., & Ates, F. (2023). Characterization of Muscle Weakness Due to Myasthenia Gravis Using Shear Wave Elastography. Diagnostics, 13(6), 1108. https://doi.org/10.3390/diagnostics13061108






