Abstract
Immune checkpoint blockade (ICB) agents are prominent immunotherapies for the treatment of advanced melanoma. However, they fail to promote any durable clinical benefit in a large cohort of patients. This study assessed clinical and molecular predictors of ICB response and survival in advanced melanoma. A retrospective analysis was performed on 210 patients treated with PD-1 or CTLA-4 inhibitors at Barretos Cancer Hospital, Brazil. PD-L1 expression was assessed by immunohistochemistry using formalin-fixed paraffin-embedded tumor tissues collected prior to ICB therapy. Patients were divided into responders (complete and partial response and stable disease for more than 6 months) and non-responders (stable disease for less than 6 months and progressive disease). Among them, about 82% underwent anti-PD-1 immunotherapy, and 60.5% progressed after the ICB treatment. Patients that received ICB as first-line therapy showed higher response rates than previously treated patients. Higher response rates were further associated with superficial spreading melanomas and positive PD-L1 expression (>1%). Likewise, PD-L1 positive expression and BRAF V600 mutations were associated with a higher overall survival after ICB therapy. Since ICBs are expensive therapies, evaluation of PD-L1 tumor expression in melanoma patients should be routinely assessed to select patients that are most likely to respond.
1. Introduction
Cancer is considered a worldwide public health concern that affects a significant and growing number of individuals, especially among developing countries [1]. The recent success of immunomodulatory agents in patients with refractory solid tumors demonstrates that activating the immune system is effective as a therapeutic modality [2]. However, tumor cells develop mechanisms to evade recognition and activation of the immune response in a dynamic process called immunoediting [2].
Melanoma is the most aggressive type of skin cancer [3,4], responsible for 325,000 new cases and 57,000 deaths worldwide in 2020 [5]. The treatment for advanced melanomas may involve complex surgery, radiation therapy, and various systemic therapeutic approaches [6]. When the tumor is diagnosed at stage III, the 5-year overall survival rate is 66.5%, while for cases at stage IV, it is around 25.0% [7]. In the last decade, the use of immune checkpoint blockade (ICB) agents has significantly improved melanoma patients’ survival outcomes [8,9,10]. Nevertheless, about 30% of these patients benefit from the treatment as monotherapy [11,12], and the cellular and molecular aspects associated with this response need to be further elucidated [13].
Previous studies have identified an increased expression of programmed death-ligand 1 (PD-L1) on tumor cells as the most common biomarker to predict response to ICB [14,15]. However, the use of this marker is controversial due to the overall survival benefit of ICB regardless of PD-L1 tumor expression levels [16,17].
As in the wider world, ICB has been gaining ground in Brazil [18]. Thus, this study aimed to investigate the PD-L1 tumor expression profile as a predictive biomarker of response to ICB therapy in Brazilian patients with advanced melanoma in a real-world scenario. Here, we demonstrated that the use of PD-L1, together with clinical information, could be implemented in the clinical setting to identify patients that might benefit from ICB treatments.
2. Materials and Methods
2.1. Patients
We selected 210 consecutive patients with advanced melanoma who underwent immunotherapy treatment with antibodies against programmed cell death protein 1(PD-1) and/or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) at Barretos Cancer Hospital (BCH) between January 2011 and December 2021.
Epidemiological, clinical, and anatomopathological data were collected from medical records and internal data management systems. Clinical response was assessed by clinical oncologists based on the routine radiological evaluation. Patients were subjected to positron emission tomography with computed tomography (PET/CT) every 3 months for disease follow-up and treatment response evaluation. In addition, when brain metastasis was suspected, the patients underwent magnetic resonance imaging (MRI). Inclusion criteria included every advanced melanoma patient with irresectable (stage III) or metastatic (stage IV) disease treated with ICB, with biological tumor samples available. Patients without measurable disease, treated with ICB in the adjuvant setting, were excluded from this study.
2.2. PD-L1 Tumor Expression
The most representative areas of the formalin-fixed paraffin-embedded (FFPE) tumor tissues were selected for PD-L1 expression analysis by immunohistochemistry (IHC). Samples from all patients were collected prior to ICB treatment. The reaction was performed using the Benchmark® ULTRA platform using the rabbit anti-PD-L1 monoclonal antibody (clone E1L3N, 1:200 dilution, Cell Signaling Technology). The Optiview DAB visualization system was used for PD-L1 protein detection in 5 μm sections, according to the manufacturer’s specifications. A minimum of 100 viable tumor cells on the slide was required for an adequate evaluation of PD-L1.
PD-L1 expression was determined by a specialized pathologist using two scores: the tumor proportion score (TPS) and the combined positive score (CPS). The TPS evaluates the percentage of viable tumor cells that show partial or complete membrane staining at any intensity. The CPS evaluates the total number of PD-L1-positive stained cells (tumor and immune system cells) among the total number of viable tumor cells on the slide. Both indexes range from 0 to 100% and were considered positive when greater than or equal to 1%.
2.3. BRAF and NRAS Mutation Status
BRAF and NRAS mutation status were evaluated by next-generation sequencing using the TruSight Tumor 15 panel on MiSeq instrument (Illumina, San Diego, CA, USA), as previously described [19]. When DNA quality did not allow reliable sequencing results, BRAF mutation was evaluated by real-time PCR using the Cobas4800 BRAF V600 Mutation Test assay in the Cobas4800 system (Roche Molecular Diagnostics, Switzerland), following the manufacturer’s instructions.
2.4. Statistical Analysis
The statistical software SPSS 23.0 was used for data entry and statistical analysis. Patients with partial (PR), complete response (CR), and stable disease (SD) for more than 6 months were considered as responders to ICB treatment, whereas patients with progressive (PD) and SD for less than 6 months were considered non-responders. Clinical and molecular predictors of ICB response and survival in advanced melanoma were investigated. Survival rates were assessed using the Kaplan–Meier method, and curves were compared using the log-rank test. Multivariate analyses were performed by the logistic regression method, with treatment response as the dependent variable. p-values ≤ 0.05 were considered significant.
3. Results
3.1. Clinicopathological Data
About 59% of the patients were male and 86.2% were Caucasian. The median age at diagnosis was 53 (ranging from 19–91) years old. The main primary tumor site was the lower limbs (31.4%) followed by the trunk (21.4%). Most patients (81.9%) were treated with anti-PD-1 agents in monotherapy, and 60.5% of them showed disease progression after ICB treatment. Patients were divided into two groups, responders vs. non-responders, comprising 83 and 127 patients, respectively. Additional patient information is presented in Table 1.

Table 1.
Clinicopathological and treatment characteristics of the 210 advanced melanoma patients undergoing immune checkpoint blockade, by patient response groups.
We observed an increase in indications for ICB at BCH from 2014 until 2019 (Figure 1). However, there was a drop in the number of patients starting treatment with ICB in 2020 due to the impact of the SARS-CoV-2 pandemic on the Brazilian National Health System [20], which made access to this high-cost medication more challenging.
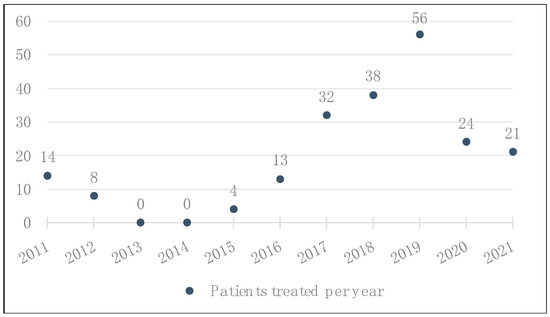
Figure 1.
Total number of patients with advanced or metastatic melanoma who received treatment with ICB at BCH, by year.
3.2. PD-L1 Expression Analysis
All 210 patients were subjected to PD-L1 expression analysis. The source of samples depended on the availability of FFPE blocks in the Department of Pathology. Approximately fifty-four percent of the patients had samples from their primary tumor, while for the remaining patients, only metastatic tumor tissue samples were available (Table 2).

Table 2.
Origin of tumor tissue samples from patients.
The evaluation of PD-L1 expression was performed using two scores, TPS and CPS: 44 (21.0%) samples were considered positive by both scoring methods, while 166 (79.0%) samples were considered negative for PD-L1 expression (IHC represented by Figure 2), with a positive correlation between the two scores (Pearson correlation = 0.987, p< 0.001, Figure 3).
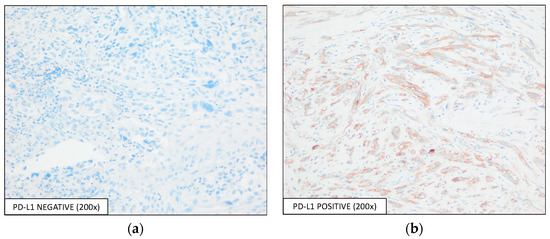
Figure 2.
Detection of PD-L1 expression by IHC using the E1L3N antibody, with an optical magnification of 200×. Representative images of PD-L1-(a) negative and -(b) positive expressions are shown.
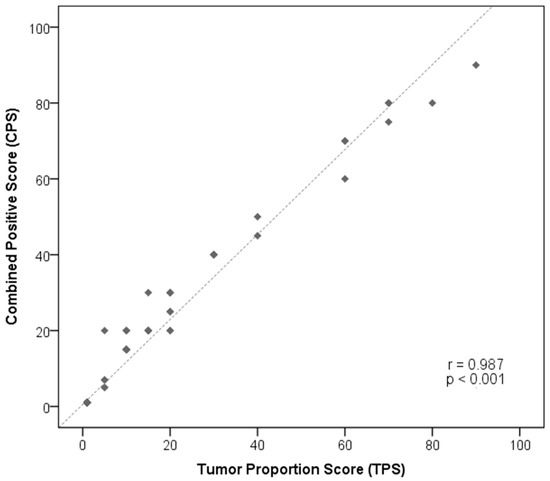
Figure 3.
Pearson correlation analysis between TPS and CPS scores to evaluate PD-L1 expression in advanced melanoma patients’ samples.
The PD-L1 expression was associated with BRAF V600 mutation status, where 31.2% (24/77) of the patients with mutations in this gene presented PD-L1 positive samples, compared to 15.1% (16/106) of BRAF WT tumors (p = 0.011, Figure 4a). PD-L1 expression was also associated with the FFPE sample source, where positive PD-L1 expression was detected in 30.9% (30/97) of the metastatic samples, against 12.4% (14/113) positivity in the primary tumor samples (p = 0.001, Figure 4b). The other clinical characteristics evaluated were not significantly associated with the PD-L1 expression profile (data not shown).

Figure 4.
Association of (a) BRAF V600 mutation status and (b) source of FFPE tumor samples with PD-L1 expression profile.
3.3. Biomarkers of Therapeutical Response and Survival
The clinical characteristics of the patients and the molecular characteristics of the tumor samples were evaluated as predictors of response to ICB treatment. We found that PD-L1 expression lower than 1% in tumor and immune cells, a line of immunotherapeutic treatment other than the first line, an acral lentiginous histological melanoma subtype, and an anti-CTLA-4 treatment as monotherapy were associated with poor responses (Table 1).
Moreover, the variables of BRAF mutation status, ICB treatment agent, ICB treatment line, and PD-L1 expression were included in the multivariate analysis. PD-L1 expression and ICB treatment line remained significant in the model (Table 3). Regarding PD-L1 expression analysis, 85.8% (109/127) of the non-responder patients presented less than 1% of PD-L1 tumor and immune cells’ expression, whereas 31.3% (26/83) of the responder patients presented higher expression (>1%) of this protein with both TPS and CPS methods (p = 0.005). In addition, patients treated with ICB therapy as the first line of systemic treatment had higher response rates when compared with patients treated in subsequent lines (49.6% vs. 34.3% in the second line and 13.3% in the third line; p = 0.001).

Table 3.
Univariate and multivariate analyses evaluating the effect of clinical and molecular features on the therapeutic response of advanced melanoma patients treated with ICB.
Kaplan–Meier survival curves were used to estimate the overall survival of the patients after ICB treatment initiation, according to the characteristics described above. Lower survival was associated with the line of the ICB treatment (log-rank p< 0.001; Figure 5a), the type of ICB used (log-rank p = 0.013; Figure 5b), the histological melanoma subtype (log-rank p = 0.009; Figure 5c), BRAF V600 mutation status (log-rank p = 0.009; Figure 5d) and PD-L1 expression (log-rank p = 0.049; Figure 5e).
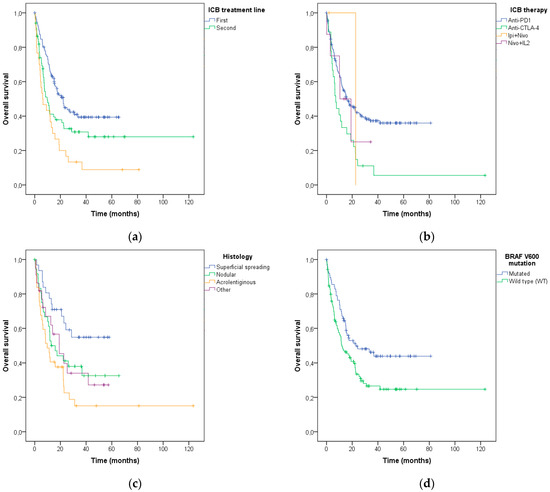
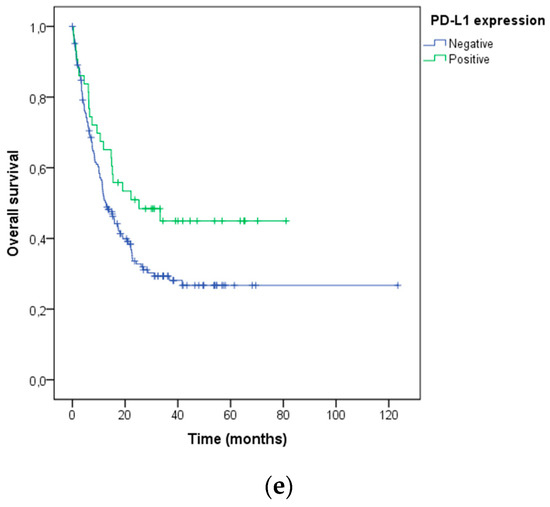
Figure 5.
Association of (a) systemic ICB treatment line, (b) type of ICB, (c) histological melanoma subtype, (d) BRAF V600 mutation status, and (e) PD-L1 expression with overall survival after the ICB treatment in advanced melanoma patients.
Since melanoma patients are treated with ICB therapies in the metastatic setting, we performed the same association analyses using only the metastatic samples (n = 97). The ICB treatment line and PD-L1 expression were also associated with the patient’s treatment response (p = 0.03 and p = 0.004, respectively) and overall survival (p = 0.153 and p = 0.026, respectively), showing that irrespective of the tumor sample type, PD-L1 expression maintains its relevance.
4. Discussion
This study aimed to characterize the clinical and molecular profile of advanced melanoma patients in a Brazilian population and to identify predictive biomarkers of response to ICB therapies. We demonstrated that PD-L1 expression by either TPS or CPS, the line and agent of immunotherapeutic treatment, the histological melanoma subtype, and BRAF V600 mutation status were associated with response and/or overall survival after ICB treatment.
Our immune system uses checkpoint receptors to regulate the duration and extent of immune responses to prevent damage to healthy tissues [21]. To avoid being killed by leukocytes, tumor cells exploit this immunosuppressive mechanism by upregulating checkpoint ligands that engage corresponding receptors expressed on immune cells [22]. An example of the mechanism of immune evasion is the ligation of PD-1 expressed on immune cells to PD-L1 expressed on tumor and tumor-infiltrating immune cells, which leads to effector cell inactivation. Therefore, the blockade of immune checkpoints is an approach through which anti-tumor immunity can be re-activated in the tumor microenvironment [21,22]. These agents have been responsible for the induction of long-lasting objective responses in about 40% of melanoma patients, and for the increase in overall survival in advanced-stage melanoma patients [12,23], as well as other tumor types such as non-small cell lung cancer [24], renal cancer [25] and head and neck squamous cell carcinoma [26]. Despite the higher response rates to ICB in comparison with chemotherapy, a large cohort of patients are refractory or acquire resistance to these therapies. Characterization of ICB resistance mechanisms would allow for superior selection of patients that would benefit from these therapies, and for the generation of novel ICB combinations that would benefit a wider population of patients. These developments would lead to more cost- and therapeutically effective ICB strategies [13], which is especially important in Brazil, where most of the healthcare system is public.
There is a growing need for viable biomarkers to be used in the day-to-day life of an oncologic center, wherein the cost must be feasible for expansion to the entire population. Ayers et al. analyzed the expression of a panel of genes using tumor cells from patients treated with pembrolizumab, and identified signatures related to the immune system which correlated to the clinical benefit of the treatment [13]. Another study demonstrated that global mutational load, neoantigen load, and expression of cytolytic markers in the tumor microenvironment were significantly associated with response in patients with advanced melanoma treated with ipilimumab [27]. Goodman et al. found that tumors with a high mutational load, such as melanoma, are more likely to respond to treatment [28]. Additionally, body mass index (BMI) can be used as a predictor of response to immunotherapy, where obese patients with advanced melanoma respond better, showing greater overall and progression-free survival after treatment with ICBs when compared to non-obese patients [29,30,31]. Unfortunately, all of the aforementioned, except for BMI, are limited by cost for the entire population.
In clinical trials, PD-L1 tumor expression is considered a poor predictor of objective response to checkpoint inhibition, where patients benefit from the treatment independently of PD-L1 expression levels detected in tumor samples [12,23,32]. This can be explained by its dynamic and heterogeneous expression [33,34], which varies upon disease progression [35] and different treatment protocols, including ICB [36]. Moreover, there are several reasons that could explain the heterogeneity in levels of PD-L1 expression as a predictable biomarker of response: differences in tissue samples, PD-L1 expression cut-off, the use of TPS or CPS, as well as the detection technique used. In this study, 34.3% of patients with negative PD-L1 expression presented objective responses after the ICB treatment, suggesting its use should be combined with other relevant clinical techniques in the practice of the specialty, thus allowing for better prognostic outlining as well as the development of new therapeutic schemes to contribute to improvements in survival rates and the quality of life of these patients. Patient stratification is particularly important to prevent patients who will not benefit from certain treatments from undergoing them, thereby avoiding toxicity, allowing time for other treatments to be considered, and saving on the cost of treatments.
To the best of our knowledge, this is the first study evaluating the PD-L1 tumor expression and clinicopathological data of the Brazilian advanced-stage melanoma population, and their association with ICB response, in a real-world scenario. Here, we validated some previously reported biomarkers such as PD-L1 tumor and immune cells’ expression [37], and BRAF V600 mutations [38], in a very heterogeneous population. Our results are also in accordance with other studies [10,38] in which patients receiving anti-PD1 monotherapy or a combination of anti-PD1 and anti-CTLA-4 responded better than those patients treated with anti-CTLA-4 alone. Moreover, a recent clinical trial has demonstrated that using ICB as first line is beneficial for advanced melanoma patients [39], and new ICB combinations show greater treatment outcomes [40].
One limitation of this study is the fact that most of the samples for PD-L1 analysis were from the primary tumors and not from the metastatic sites. This is due to the study’s retrospective nature and the difficulty of obtaining samples from visceral organs in which most melanoma metastases are located. Prospective studies are needed to validate our findings.
In conclusion, due to high ICB cost constraints, biomarker-driven selection of advanced melanoma patients should be implemented as a routine practice to predict treatment response as well as overall survival. This would reduce costs and improve the therapeutic efficacy of ICB therapies.
Author Contributions
Conceptualization, B.P.S. and L.M.R.B.A.; methodology, B.P.S., I.V.V.S. and C.A.D.P.; software, B.P.S. and R.d.J.T.; validation, B.P.S.; formal analysis, I.V.V.S.; investigation, B.P.S., R.d.J.T., C.A.D.P. and L.V.; resources, V.d.L.V. and L.M.R.B.A.; data curation, L.M.R.B.A.; writing—original draft preparation, B.P.S. and R.d.J.T.; writing—review and editing, C.A.D.P., I.V.V.S., L.V., V.d.L.V. and L.M.R.B.A.; supervision, V.d.L.V. and L.M.R.B.A.; project administration, L.M.R.B.A.; funding acquisition, L.M.R.B.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant number 2019/07111-9. B.S. was a recipient of two scholarships from FAPESP (2019/03570-9 and 2021/10922-9). L.A. was a recipient of a scholarship from FAPESP (2021/04100-6). L.A. and V.d.L.V. were recipients of the National Council for Scientific and Technological Development (CNPq) productivity.
Institutional Review Board Statement
The study was conducted according to the guidelines of the National Health Council’s Resolution 466/2012 and approved by the Institutional Ethics Committee of Barretos Cancer Hospital (1772/2019).
Informed Consent Statement
Patient consent was waived due to the retrospective aspect of the study, the minimal risk to the participants, and considering that its development does not result in an impact on clinical management or the need for genetic counseling.
Data Availability Statement
The data that support the findings of this study are available upon request from the authors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bray, F.; Soerjomataram, I. The Changing Global Burden of Cancer: Transitions in Human Development and Implications for Cancer Prevention and Control. In Disease Control Priorities: Cancer, 3rd ed.; The World Bank: Washington, DC, USA, 2015; Volume 3. [Google Scholar]
- Moskovitz, J.M.; Ferris, R.L. Tumor Immunology and Immunotherapy for Head and Neck Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. About Basal and Squamous Cell Skin Cancer; American Cancer Society: Atlanta, GA, USA, 2016. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- NCCN. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology—Melanoma: Cutaneous (Version 1.2023). 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf (accessed on 19 February 2023).
- Surveillance, Epidemiology, and End Results (SEER) 17 Registries. Cancer Statistics Factsheets: Melanoma of the Skin: National Cancer Institute. 2022. Available online: seer.cancer.gov/statfacts/html/melan.html (accessed on 18 February 2023).
- Olszanski, A.J. Current and future roles of targeted therapy and immunotherapy in advanced melanoma. J. Manag. Care Spec. Pharm. 2014, 20, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, W.; Gotlieb, V. The rapidly evolving therapies for advanced melanoma—Towards immunotherapy, molecular targeted therapy, and beyond. Crit. Rev. Oncol. Hematol. 2016, 99, 91–99. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Ribas, A.; Hamid, O.; Daud, A.; Hodi, F.S.; Wolchok, J.D.; Kefford, R.; Joshua, A.M.; Patnaik, A.; Hwu, W.-J.; Weber, J.S.; et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA 2016, 315, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, P.; Shankaran, V.; et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Xu-Monette, Z.Y.; Zhang, M.; Li, J.; Young, K.H. PD-1/PD-L1 Blockade: Have We Found the Key to Unleash the Antitumor Immune Response? Front. Immunol. 2017, 8, 1597. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.; Patel, S.P.; Kurzrock, R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat. Rev. Clin. Oncol. 2017, 14, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Grigg, C.; Rizvi, N.A. PD-L1 biomarker testing for non-small cell lung cancer: Truth or fiction? J. Immunother. Cancer 2016, 4, 48. [Google Scholar] [CrossRef]
- Da Costa, L.M.M.; Crovador, C.S.; de Carvalho, C.E.B.; Vazquez, V.L. Characteristics of Brazilian melanomas: Real-world results before and after the introduction of new therapies. BMC Res. Notes 2019, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Campanella, N.C.; Silva, E.C.; Dix, G.; de Lima Vazquez, F.; Escremim de Paula, F.; Berardinelli, G.N.; Balancin, M.; Chammas, R.; Lopez, R.V.M.; Silveira, H.C.S.; et al. Mutational Profiling of Driver Tumor Suppressor and Oncogenic Genes in Brazilian Malignant Pleural Mesotheliomas. Pathobiology 2020, 87, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.M.; Correa, F.M.; Migowski, A. Short-term effects of the COVID-19 pandemic on cancer screening, diagnosis and treatment procedures in Brazil: A descriptive study, 2019–2020. Epidemiol. Serv. Saude. 2022, 31, e2021405. [Google Scholar] [CrossRef]
- Ling, D.C.; Bakkenist, C.J.; Ferris, R.L.; Clump, D.A. Role of Immunotherapy in Head and Neck Cancer. Semin. Radiat. Oncol. 2018, 28, 12–16. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Redman, B.G.; Kuzel, T.M.; Harrison, M.R.; Vaishampayan, U.N.; Drabkin, H.A.; George, S.; Logan, T.F.; et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.; Seiwert, T.Y.; Pfister, D.G.; Worden, F.; Liu, S.V.; Gilbert, J.; Saba, N.F.; Weiss, J.; Wirth, L.; Sukari, A.; et al. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Van Allen, E.M.; Miao, D.; Schilling, B.; Shukla, S.A.; Blank, C.; Zimmer, L.; Sucker, A.; Hillen, U.; Foppen, M.H.G.; Goldinger, S.M.; et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015, 350, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef]
- Richtig, G.; Hoeller, C.; Wolf, M.; Wolf, I.; Rainer, B.M.; Schulter, G.; Richtig, M.; Grübler, M.R.; Gappmayer, A.; Haidn, T.; et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: An observational multi-centre study. PLoS ONE 2018, 13, e0204729. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Grossman, J.E.; Vasudevan, D.; Joyce, C.E.; Hildago, M. Is PD-L1 a consistent biomarker for anti-PD-1 therapy? The model of balstilimab in a virally-driven tumor. Oncogene 2021, 40, 1393–1395. [Google Scholar] [CrossRef]
- Zhao, X.; Bao, Y.; Meng, B.; Xu, Z.; Li, S.; Wang, X.; Hao, R.; Ma, W.; Liu, D.; Zheng, J.; et al. From rough to precise: PD-L1 evaluation for predicting the efficacy of PD-1/PD-L1 blockades. Front. Immunol. 2022, 13, 920021. [Google Scholar] [CrossRef]
- Boothman, A.M.; Scott, M.; Ratcliffe, M.; Whiteley, J.; Dennis, P.A.; Wadsworth, C.; Sharpe, A.; Rizvi, N.A.; Garassino, M.C.; Walker, J. Impact of Patient Characteristics, Prior Therapy, and Sample Type on Tumor Cell Programmed Cell Death Ligand 1 Expression in Patients with Advanced NSCLC Screened for the ATLANTIC Study. J. Thorac. Oncol. 2019, 14, 1390–1399. [Google Scholar] [CrossRef]
- Riaz, N.; Havel, J.J.; Makarov, V.; Desrichard, A.; Urba, W.J.; Sims, J.S.; Hodi, F.S.; Martín-Algarra, S.; Mandal, R.; Sharfman, W.H.; et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 2017, 171, 934–949.e16. [Google Scholar] [CrossRef]
- Tarhini, A.; Kudchadkar, R.R. Predictive and on-treatment monitoring biomarkers in advanced melanoma: Moving toward personalized medicine. Cancer Treat. Rev. 2018, 71, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Asher, N.; Ben-Betzalel, G.; Lev-Ari, S.; Shapira-Frommer, R.; Steinberg-Silman, Y.; Gochman, N.; Schachter, J.; Meirson, T.; Markel, G. Real World Outcomes of Ipilimumab and Nivolumab in Patients with Metastatic Melanoma. Cancers 2020, 12, 2329. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Ribas, A.; Tarhini, A.A.; Truong, T.-G.; Davar, D.; O’Rourke, M.A.; Curti, B.D.; Brell, J.M.; et al. DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing): A phase III trial—ECOG-ACRIN EA6134. J. Clin. Oncol. 2021, 39, 356154. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutierrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).