Abstract
(1) Background: Among new anti-angiogenesis agents being developed and ever-changing guidelines indications, the question of the benefits/safety ratio remains unclear. (2) Methods: We performed a systematic review combined with a meta-analysis of 23 randomized controlled trials (12,081 patients), evaluating overall survival (OS), progression free survival (PFS) and toxicity (grade ≥ 3 toxic effects, type, and number of all adverse effects. (3) Results: The analysis showed improvement of pooled-PFS (HR, 0.71; 95% CI, 0.64–0.78; I2 = 77%; p < 0.00001) in first-line (HR, 0.85; 95% CI, 0.78–0.93; p = 0.0003) or recurrent cancer (HR, 0.62; 95% CI, 0.56–0.70; p < 0.00001) and regardless of the type of anti-angiogenesis drug used (Vascular endothelial growth factor (VEGF) inhibitors, VEGF-receptors (VEGF-R) inhibitors or angiopoietin inhibitors). Improved OS was also observed (HR, 0.95; 95% CI, 0.90–0.99; p = 0.03). OS benefits were only observed in recurrent neoplasms, both platinum-sensitive and platinum-resistant neoplasms. Grade ≥ 3 adverse effects were increased across all trials. Anti-angiogenetic therapy increased the risk of hypertension, infection, thromboembolic/hemorrhagic events, and gastro-intestinal perforations but not the risk of wound-related issues, anemia or posterior leukoencephalopathy syndrome. (4) Conclusions: Although angiogenesis inhibitors improve PFS, there are little-to-no OS benefits. Given the high risk of severe adverse reactions, a careful selection of patients is required for obtaining the best results possible.
1. Introduction
Significant improvements are continuously made in the treatment of ovarian carcinoma, but this disease continues to place a tremendous burden on healthcare systems, especially in countries with a low index of human development. GLOBOCAN 2020 statistics indicate that ovarian cancer remains third in incidence and second as cause of death between the genital neoplasms. In Central and Eastern Europe, ovarian cancer has the highest incidence rate in the world (10.7/100,000) and a mortality rate of 5.6/100,000 [1]. At present, the guidelines promote optimal cytoreductive surgery and polychemotherapy consisting of platinum agents and taxanes as the standard of care. Although most patients will experience remission under therapy, 80% will relapse within 18 months [2]. The relapse is usually diagnosed in advanced stages, despite the encouraging response to platinum-based first-line therapy, and the recurrence usually shows increased chemo-resistance, leading in most cases to death [3,4]. Thus, new agents, such as angiogenesis inhibitors and poly-ADP-ribose polymerase (PARP) inhibitors, were introduced as treatment options. Regardless of the initial factors that lead to the neoplastic transformation of cells, due to the increased metabolic need and secondary tissular hypoxia, cancers require neo-angiogenesis for their progressive growth and metastasis, with cancer cells being able to stimulate surrounding normal cells to secret molecules with signaling properties in angiogenesis pathways [5,6]. Angiogenesis in solid tumors is a well-known fact, and targeting the pathways that regulate angiogenesis is suggested as a potential therapeutic approach [5,7]. After the initial paper by Folkman in 1971 [5] that shifted the therapeutic paradigm from targeting the tumor cell to an anti-angiogenetic approach, a new field of study in oncology emerged. Over the years, numerous discoveries have been made identifying several angiogenetic factors, understanding the regulation of pathological angiogenesis process in tumors and ultimately developing new drugs that block pathological tumoral vessel formation. After the establishment of VEGF as the principal mediator in tumoral angiogenetic pathways [8], targeting VEGF or its receptors VEGF-R has become the pivot in research for the development of antiangiogenetic agents. Clinical trials (number ranging in the thousand) demonstrated the benefits of anti-angiogenesis drugs. Numerous molecules have been identified, developed and approved for cancer therapy [9,10,11,12], as well as for secondary use as treatment for ocular neo-vascular diseases which share angiogenetic pathways and signaling molecules with carcinomas [11,13,14,15,16,17,18]. These therapies are credited with improved progression-free survival (PFS) and improved overall survival (OS), but those last results are inconstant. Due to the high costs, as well as the frequency and severity of therapy-specific adverse effects, in low- and middle-income countries, there is a tendency toward the careful consideration of the efficacy–safety ratio. In Romania, these therapies are fully refunded through national healthcare programs, and as a result, at the Oncology Institute of Bucharest, around 50 patients with ovarian cancers are treated each year with anti-angiogenesis drugs. Although we observed improved survival, some patients experience severe adverse reactions and extreme alterations of QoL, leading us to the conclusion that more in-depth analyses of security profiles are warranted. In Romania, we see no reduction in the incidence for ovarian cancer [19]; thus, the therapeutic option for these cases continues to be of concern.
The scope of our study is to provide an extensive review of the efficacy–safety ratio of angiogenesis-inhibitors-based therapies in ovarian cancer, thus providing a better evaluation and selection tool for clinicians.
2. Materials and Methods
2.1. Search Strategy
A systematic search of the existing literature, according to PRISMA guidelines [20], was performed in multiple international databases: PubMed, Embase, Web of Science, Cochrane Library and ASCO and ESMO abstract database, from establishment up to 1 May 2022. The first criterion used was that the publications contained a reference to ovarian cancer; thus, the following syntax was used: (ovar OR ovarian OR ovary) AND (neoplasm OR cancer OR carcinoma OR malignant OR tumor). The second criterion used was that the publications contained a reference to angiogenesis or antiangiogenetic therapy—(vascular endothelial growth factor OR angiogenesis inhibitor OR VEGF OR VEGFR OR VEGF-R OR anti-VEGF OR VEGF-target OR anti-angiogenic OR anti-angiogenesis OR antiangiogenetic). The third criterion was that the publications contained a reference to a specific anti-angiogenetic drug; thus, we used the following syntax: (bevacizumab OR avastin OR cediranib OR AZD2171 OR recentin OR aflibercept OR VEGF trap OR AVE0005 OR zaltrap OR sorafenib OR nexavar OR pazopanib OR Votrient OR trebananib OR AMG386 OR nintedanib OR BIBF 1120 OR tyrosine kinases inhibitors OR TIE OR AXL OR FLT OR sunitinib OR SU11248). We used a limitation in searching for articles written in English.
2.2. Study Selection
References were prepared by using Mendeley Reference Manager [21]. After removing duplicate records, an extensive title and abstract screening process was performed, and records with irrelevant focus were removed.
We included adult patients with histopathological confirmation of ovarian cancer regardless of histology and regardless of treatment settings (first-line, recurrence, or maintenance), receiving anti-angiogenetic drugs. The experimental arm needed to be compared to standard chemotherapy or placebo, with PFS and/or OS being reported by hazard ratio with 95% CI. PFS, OS and adverse effects were our outcomes of interest, but we did not limit the inclusion if a study did not report all three. The study design of trials included were Phase II/III randomized controlled. Non-randomized controlled studies or Phase I trials were also excluded due to the fact that they were not considered high-quality statistical data. Book chapters, reviews and case reports were also excluded for the same reason. Studies using cyclophosphamide as a control arm were excluded due to the fact that the superiority of carboplatin/paclitaxel over cyclophosphamide regimens was long-time established by prior trials [22].
The PICOS structure (Population-Intervention-Comparator-Outcomes-Study design) shown in Table 1 was used for including studies in our meta-analysis [23].

Table 1.
PICOS criteria for inclusion of trials.
The remaining articles were obtained as full text and were reviewed independently by two authors, and discrepancies were the subject of discussion between all authors. The review was not registered and has no external funding.
2.3. Data Extraction
From each trial, we selected data pertaining to name of primary author, year of publication, study phase, patient selection criteria, drug regimens on experimental and controlled arms of study, sample size, different bias factors and outcomes intended for use in meta-analysis (PFS, OS and adverse reactions—type of event, total number of events, number, and type of grade ≥ 3 adverse reactions).
2.4. Assessment of the Bias Risk
The bias risk was evaluated on six domains used by the Cochrane Collaboration’s tool [24]: bias of selection, performance, detection, attrition, reporting and other types of bias. Details regarding the risk of bias are presented in Figure 1a,b. Risk was categorized as being high, unclear, or low and was color coded.
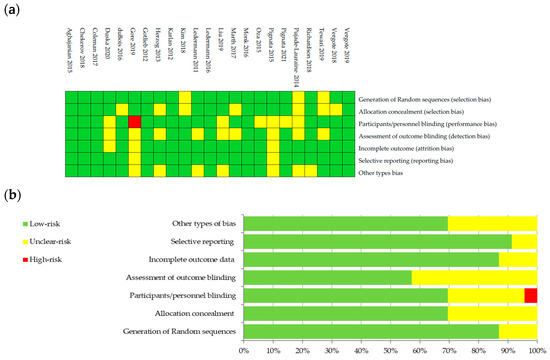
Figure 1.
Graphical representation of bias risk: (a) authors’ judgements about each risk item color coded as low risk (green), unclear risk (yellow) or high risk (red) for studies included; and (b) percentage summary of bias risk across all studies, using same color coding of risk categories.
2.5. Statistical Analysis
Pooled hazard ratios (HRs) for progression free and overall survival were calculated using a generic inverse variance in RevMan 5.4.1 software [25]. Risk ratios (RR) for adverse reactions were also calculated using 95% CI. I2 type statistic was used in order to see the statistical heterogeneity. When a high heterogeneity among the studies was observed, a random model was used for statistical analysis. For I2 < 40%, indicating low probability of heterogeneity, we used a fixed model. Random models were used when high heterogeneity was observed. A sensitivity analysis was performed by visually assessing the gross asymmetry of funnel plots, verifying lack of publication bias. Heterogeneity among studies was investigated further by producing Galbraith plots for each analysis with NCSS 2023 software, thus verifying the consistency of results.
3. Results
3.1. Literature Search
After the review of the literature, we identified n = 9754 records concerning the utilization of antiangiogenetic therapies for ovarian cancer. Another 54 additional records were found after reference review. Duplicate records (n = 2183) were removed, and after title and abstract screening, 7514 records were excluded: irrelevant focus (n = 5127), non-randomized controlled/Phase I studies (n = 443), reviews or case reports (n = 1908) and other reasons (n = 39). The remaining 119 records were obtained as full-text articles and assessed. Another 97 studies were excluded due to lacking outcomes of interest or being previous versions or exploratory outcomes of studies already included. In the end, 23 randomized controlled trials (RCTs) were included. The literature search flowchart is presented in Figure 2.
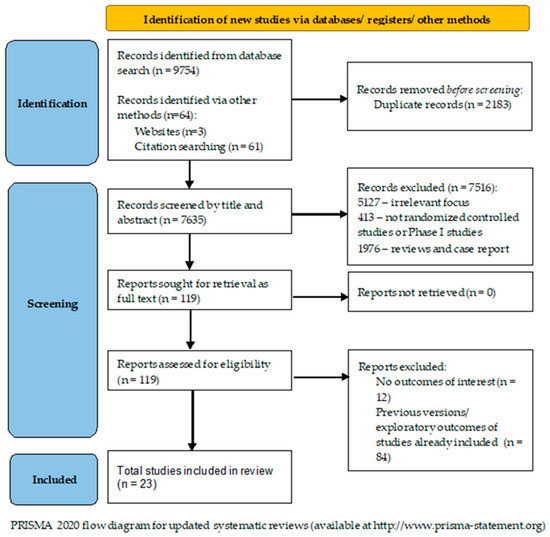
Figure 2.
The literature search flow diagram.
3.2. Studies Characteristics
A total of 12,082 patients were included (adults with confirmed ovarian cancer, having therapies including anti-angiogenetic agents and compared to other drug regimens without such agents or placebo). The 23 RCTs have a publication date between 2011 and 2022, and they evaluated seven inhibitors of angiogenesis (bevacizumab, six; pazopanib, five; trebananib, four; nintedanib, two; sorafenib, two; cediranib, two; and aflibercept, one). In the 2019 Tewari study, there were two experimental arms, which were both included in our study, the difference being in the maintenance therapy (one arm using bevacizumab—BEVm; and one arm using placebo—PLm). A similar situation was encountered in the Ledermann 2016 study, but we did not separately use the experimental arms due to the lack of HRs for OS and PFS between the control and PLm arms of the study. The general characteristics, the references and the summary of outcomes from the included trial are included in Table 2 and Table 3.

Table 2.
General characteristics of RCTs included.

Table 3.
Summary of outcomes of included trials.
3.3. Analysis of Survival: Overall and Progression-Free
In our study the anti-angiogenetic regimens were credited with an important improvement of PFS over the control arm (HR, 0.71; 95% CI, 0.64–0.78; I2 = 77%; p < 0.00001). Improved OS was also observed, but the results had a lower p-value (HR, 0.95; 95% CI, 0.91–0.99; I2 = 0; p = 0.02).
- Treatment settings analysis:
PFS: Five studies reported data about PFS in first-line therapy, 14 in recurrent disease and 3 in maintenance. Benefits of survival until progression were observed in all subjects in first line (HR, 0.85; 95% CI, 0.78–0.93; p = 0.0003) and recurrent cancer (HR, 0.62; 95% CI, 0.56–0.70; p < 0.00001) settings. No significant improvement of PFS was observed in maintenance settings (HR, 0.89; 95% CI, 0.65–1.22; p = 0.47). For the recurrent cancers, the PFS improvement did not vary according to platinum-sensitivity (platinum-sensitive recurrence—HR = 0.58, 95% = CI 0.49–0.69, and p < 0.00001; platinum-resistant recurrence—HR = 0.56, 95% CI = 0.42–0.75, and p < 0.00001, respectively)—Figure 3. Six studies were used for PFS analysis in P-S R disease, and four for P-R R.
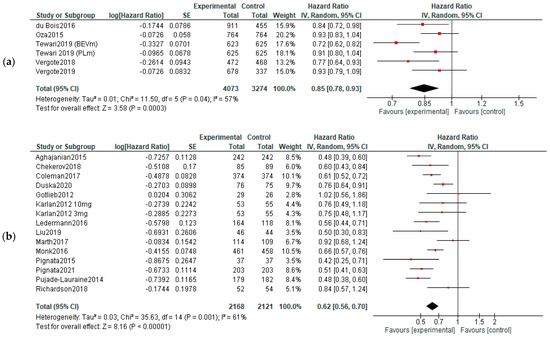
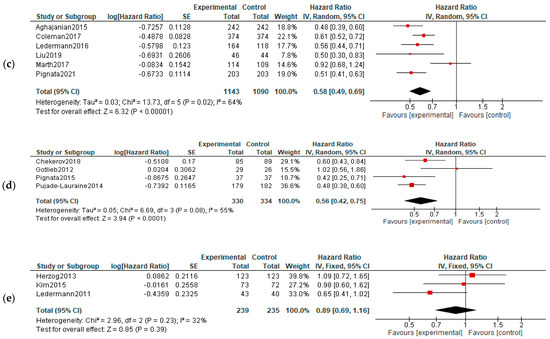
Figure 3.
Settings subgroup analysis—time to progression: (a) first line, (b) recurrent, (c) P-S R, (d) P-R R and (e) maintenance. The black diamonds are visual representations of pooled effect and its confidence intervals and are non-significant if they overlap 1. Red squares represent each study effect size and study weight (size of square). The lines represent each study confidence interval. PLm—placebo maintenance; BEVm—bevacizumab maintenance [26,27,28,29,30,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,50,52].
OS: When analyzing the OS in different treatment setting, the results were more heterogenic—significant improvements were observed in recurrent cancers (both in P-S R and P-R R—HR = 0.88, 95%CI = 0.79–0.99, and p = 0,03; HR = 0.78, 95% CI = 0.65–0.94, and p = 0.01, respectively). However, when employed as first-line or maintenance therapy, no improvement was observed (HR, 0.95; 95% CI, 0.66–1.37; p = 0.78)—Figure 4.
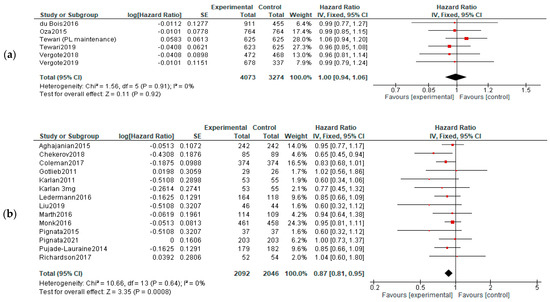
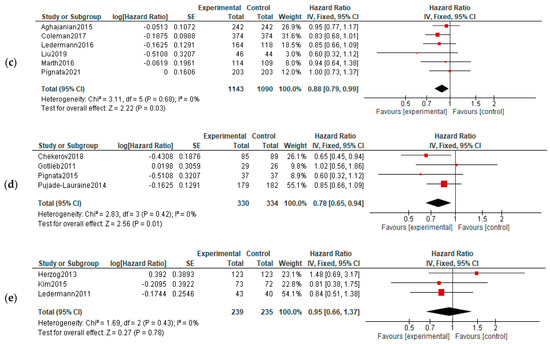
Figure 4.
Settings subgroup analysis of overall survival: (a) first line, (b) recurrent, (c) P-S R, (d) P-R R and (e) maintenance. The black diamonds are visual representations of the pooled effect and its confidence intervals and are non-significant if they overlap 1. Red squares represent each study effect size and study weight size of square). The lines represent each study confidence interval. PLm—placebo maintenance; BEVm—bevacizumab maintenance [26,27,28,29,30,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,50,52].
- Analysis by type of drugs used:
Different action mechanisms of the antiangiogenetic drugs may have different effects. We tried to establish that by evaluating PFS and OS for three distinct action mechanisms—vascular endothelial growth factor inhibitors (VEGF inhibitors such as bevacizumab and aflibercept), inhibitors of VEGF receptors (VEGF-R inhibitors such as pazopanib, cediranib, nintedanib and sorafenib) and angiopoietin inhibitors such as trebananib.
PFS: All three groups experienced improvements on PFS: VEGF inhibitors—HR = 0.66, 95% CI = 0.54–0.80, and p < 0.00001; VEGF-R inhibitors—HR = 0.72, 95% CI 0.63–0.82, and p < 0.0001; and angiopoietin inhibitors—HR = 0.82, 95% CI = 0.69–0.99, and p = 0.04 (Figure 5).
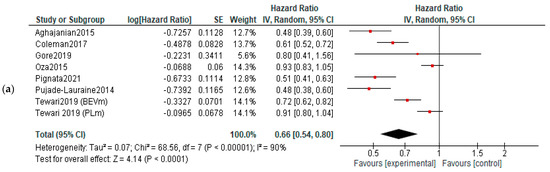
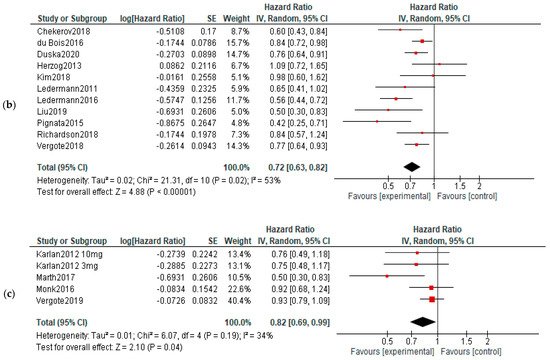
Figure 5.
Action-mechanisms subgroup analysis of progression: (a) VEGF inhibitors, (b) VEGF-R inhibitors and (c) angiopoietin inhibitors. The black diamonds are visual representations of pooled effect and its confidence intervals and are non-significant if they overlap 1. Red squares represent each study effect size and study weight (size of square). The lines represent each study confidence interval. PLm—placebo maintenance; BEVm—bevacizumab maintenance [26,27,28,29,30,32,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,50,52].
OS: No significant differences of overall survival were observed between experimental and control arms (Figure 6).
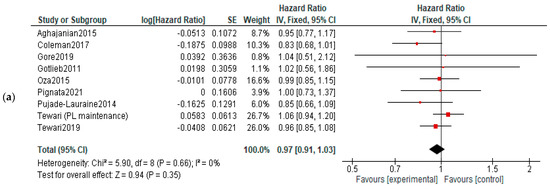
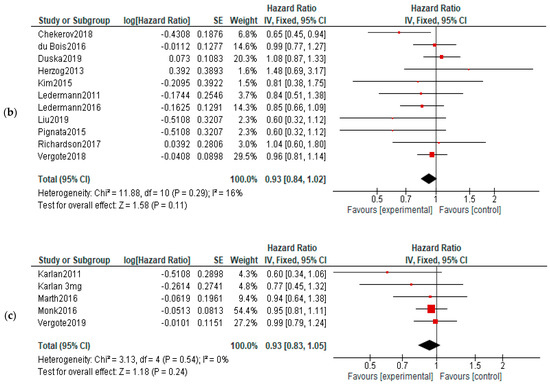
Figure 6.
Action-mechanisms subgroup analysis of overall survival: (a) VEGF inhibitors, (b) VEGF-R inhibitors and (c) angiopoietin inhibitors. The black diamonds are visual representations of pooled effect and its confidence intervals and are non-significant if they overlap 1. Red squares represent each study effect size and study weight (size of square). The lines represent each study confidence interval. PLm—placebo maintenance; BEVm—bevacizumab maintenance [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,50,52].
- Heterogeneity and publication bias:
We tried to evaluate if the heterogeneity of studies influenced the PFS/OS results and performed a sensitivity analysis by visual evaluation of asymmetry of the funnel plot for the 23 included trials and found no gross asymmetry, indicating absence of publication bias (Supplementary Materials 3). In addition, radial (Galbraith) plots were created for each analysis as a supplement to forest plots, thus demonstrating the consistency of the results (Supplementary Materials 3).
3.4. Adverse Events
We analyzed the adverse effects reported for the included studies. For the Karlan and Tewari studies, which included two experimental arms, the safety analyses were performed by comparing the two experimental arms together with the control arm. Our analysis included 31 adverse events that are considered to be associated with anti-angiogenetic therapy for which RR were calculated (Supplementary Materials Figures S1–S31). Hypertension was found to have a higher risk for the patients on angiogenesis inhibitors (RR, 4.26; 95% CI, 1.61–11.26; p = 0,0003; I2 = 99%). Similar results were found for hemorrhagic events (RR, 1.94; 95% CI, 1.00–3.76; p = 0.05; I2 = 77%) and for thromboembolic events (RR, 1.47; 95% CI, 1.12–1.92; p = 0.006; I2 = 2%). However, only arterial thromboembolism was statistically linked to the anti-angiogenetic therapy (RR, 2.38; 95% CI, 1.52–3.70; p = 0.0001; I2 = 4%). The same is not true for venous thromboembolism (RR, 1.13; 95% CI, 0.74–1.72; p = 0.58; I2 = 51%). A significantly increased risk was also seen for proteinuria (RR, 4.52; 95% CI, 1.93–10.55; p = 0.0005; I2 = 88%), gastro-intestinal perforations (RR, 2.86; 95% CI, 1.36–6.04; p = 0.006; I2 = 0%), infections (RR, 1.28; 95% CI, 1.07–1.53; p = 0.008; I2 = 0%), ascites (RR, 2.06; 95% CI, 1.65–2.57; p < 0.00001; I2 = 35%), anemia (RR, 0.86; 95% CI, 0.75–1.99; p = 0.03; I2 = 86%), neutropenia (RR, 1.20; 95% CI, 1.03–1.40; p = 0.02; I2 = 89%), leucopenia (RR, 1.23; 95% CI, 1.05–1.45; p = 0.01; I2 = 62%), thrombocytopenia (RR, 1.81; 95% CI, 1.43–2.29; p < 0.00001; I2 = 82%), nausea (RR, 1.28; 95% CI, 1.10–1.49; p = 0.002; I2 = 81%), vomiting (RR, 1.24; 95% CI, 1.04–1.47; p = 0.01; I2 = 51%), diarrhea (RR, 1.92; 95% CI, 1.46–2.53; p < 0.00001; I2 = 90%), dyspnea (RR, 1.25; 95% CI, 1.08–1.45; p = 0.003; I = 0%), hypokalemia (RR, 1.91; 95% CI, 1.45–2.51; p < 0.00001; I2 = 0%), pain (RR 1.12; 95% CI1.03–1.21; p = 0.0009; I2 = 0%) and headache (RR, 1.73; 95% CI, 1.34–2.22; p < 0.00001; I2 = 61%). No significant correlation was seen for the following symptoms and the anti-angiogenetic therapy: reversible posterior leukoencephalopathy syndrome (RR, 1.18; 95% CI, 0.18–7.94; p = 0.86; I2 = 0%), pyrexia (RR, 0.96; 95% CI, 0.75–1.23; p = 0.76; I2 = 2%), wound related issues (RR, 1.57; 95% CI, 0.96–2.56; p = 0.07; I2 = 24%), fatigue (RR, 1.12; 95% CI, 0.99–1.27; p = 0.06; I2 = 81%), hypomagnesemia (RR, 1.69; 95% CI, 0.72–3.93; p = 0.23; I2 = 46%), anorexia (RR, 1.53; 95% CI, 0.84–2.78; p = 0.16; I2 = 85%), constipation (RR, 1.08; 95% CI, 0.96–1.21; p = 0.19; I2 = 19%), alopecia (RR, 1.07; 95% CI, 0.97–1.19; p = 0.18; I2 = 0%), rash (RR, 1.34; 95% CI, 0.79–2.27; p = 0.287; I = 85%), abdominal pain (RR, 1.05; 95% CI, 0.95–1.17; p = 0.34; I2 = 0%) and back pain (RR, 0.92; 95% CI, 0.62–1.37; p = 0.70; I2 = 65%). Grade ≥ 3 adverse reaction risk was increased in therapeutic arms of our study (RR, 1.37; 95% CI, 1.17–1.60; p < 0.00001; I2 = 97%)—Figure 7.
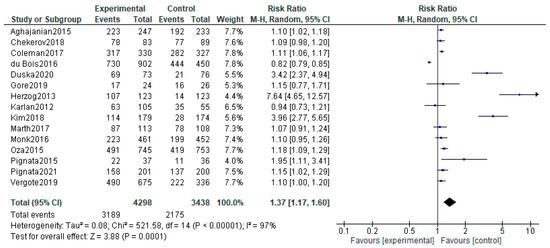
Figure 7.
Grade ≥ 3 adverse effects associated with antiangiogenetic therapy. The black diamonds are visual representations of pooled effect and its confidence intervals and are non-significant if they overlap 1. Blue squares represent each study effect size and study weight (size of square). The lines represent each study confidence interval [26,27,28,29,30,32,34,35,36,41,42,43,44,45,52].
4. Discussion
4.1. Interpretation of Results
Our study demonstrated that anti-angiogenetic drugs not only can improve progression-free survival in ovarian cancer in a significant way but also can increase the risk of common adverse reactions of all grades. The benefits regarding OS are more uncertain, being observed only in specific types of patients and tumors. We observed contradictory results for OS—the pooled OS for all trials showed a significant improvement of survival favoring the angiogenesis inhibitors, but we could not find a significant improvement when studying different treatment setting or types of anti-angiogenetic drugs separately. None of the therapeutic categories (VEGF blockade, VEGF-R inhibitors and angiopoietin inhibitors) were associated with improved OS. As first-line therapy, we could prove no significant correlation with improved OS. The possible reasons for this are as follows: (1) Most cases of ovarian cancer that benefit from first line chemotherapy are advanced (among the six trials included studying first-line therapy only one enrolled stage I/II cases [43]). (2) Three of the twenty-three RCTs [26,33,35] allowed patients in the control arm to receive anti-angiogenetic salvage therapy after disease progression, thus diminishing the OS differential between experimental and control arms. (3) High-risk ovarian cancer is more likely to have a maximal response when treated with anti-angiogenesis agents as first-line.
The ICON7 trial [43] considered as high-risk cancers FIGO IV, inoperable or sub-optimally resected (>1 cm residual disease) FIGO III disease. The AGO-OVAR 12 [31], TRINOVA3 [52] and GOG-0218 [48] trials all shared the same definition for high-risk tumors. However, the OS values between these trials were unconcordant: ICON7 showed that bevacizumab in high-risk tumors is associated with benefits of overall survival (HR, 0.78; 95% CI, 0.63–0.97; p = 0.01) [43]. On the contrary, AGO-OVAR 12 showed that an improved OS is obtained in standard chemotherapy rather than in the nintedanib-treated group (HR, 1.14; 95% CI, 0.89–1.45) [31]. The GOG-0218 study showed that, in advanced high-risk cancers, the concurrent use of bevacizumab with chemotherapy, followed by a bevacizumab maintenance arm, is optimal (HR, 0.72; 95% CI, 0.53–0.97) [48]. The 2022 network meta-analysis by Helali et al. also demonstrated that the use of bevacizumab concurrently with chemotherapy, followed by maintenance with bevacizumab until disease progression in chemo-naive disease, is associated with the highest probability of improvements of PFS and PFS [53]. Moreover, the same meta-analysis ranked the effects of different anti-angiogenetic drugs in high-risk chemo-naive disease (bevacizumab concurrent + maintenance > nintedanib concurrent + maintenance > trebananib concurrent + maintenance > standard-of-care carboplatin/paclitaxel). All mentioned trials showed no OS benefit favoring the usage of anti-angiogenetic drugs for chemo-naive non-high-risk cancers. The OS in chemotherapy-naive non-high-risk cancers can vary, thus making it difficult to propose any clinical recommendations for the usage of angiogenesis inhibitors in this disease setting, but most studies infer a probable lack of efficacy of angiogenesis inhibitors when used in the chemo-naive non-high-risk ovarian carcinoma [53].
In recurrent treatment settings, our study proved the benefits of anti-angiogenesis drugs regardless of the platinum sensitivity of disease. For both platinum-sensitive and platinum-resistant cancers, the PFS and OS were improved, so angiogenesis inhibitors can represent a valid treatment option. Helali et al. [53] ranked the angiogenesis inhibitors by probability of benefit in recurrent epithelial ovarian cancer. For the platinum-resistant group, they found that the concurrent chemotherapy and pazopanib has the best chance of improving OS, followed by the combination of chemotherapy with sorafenib. For the platinum-sensitive group, they demonstrated the lack of OS benefits when adding anti-angiogenetic drugs to standard chemotherapy, but the association of bevacizumab or cediranib to chemotherapy in the maintenance stage can improve PFS. Helali et al. also suggested that PARP inhibitors, in addition to chemotherapy, are the best option for platinum-sensitive recurrent disease [53]. Other studies suggested that PARP inhibitors in combination with antiangiogenetic agents may be a better therapeutic option than monotherapy with antiangiogenetic agents in platinum-sensitive cancers [54].
When analyzing the OS benefit of antiangiogenetic drugs used as maintenance therapy, we found no improvements.
The OS benefits are difficult to evaluate since most trials included did not report the OS based on PFI (platinum free interval), and the low sample size in some studies (MITO 11 [45] and TRIAS [27]) may influence results.
The results suggest that PFS is not a viable surrogate evaluation for OS response. The FDA approval process of various cancer drugs for solid tumors use surrogate endpoints (PFS) rather than clinical outcomes (OS). The definition of the FDA for clinical outcomes is “a direct measure of benefit of an intervention in a trial”. A predictive substitute for clinical outcomes is called a surrogate endpoint. PFS is frequently used as a surrogate endpoint for OS in solid tumors [55,56], and the usage is growing every day. Many cancer drugs received FDA approval based on surrogate endpoints such as PFS, rather than mature OS data. Among the cancer drugs that have received regulatory approval, 57% proved not to have an OS benefit after the data were available [57]. Given the results of our study, we consider that the usage of a surrogate endpoint for clinical outcomes (PFS) in the process of drug approval may need to be reconsidered, as PFS proved not to be a reliable substitute for OS. PFS is a suboptimal surrogate for OS, and this fact was highlighted by several different articles and studies [53,58,59,60].
The absence of reliable predictive biomarkers for response to angiogenesis inhibitor-based therapy makes it difficult to conduct a proper selection of patient categories that benefit most from anti-angiogenetic drugs. A series of markers have been suggested as response predictors: highAng1/lowTie2 values were associated with PFS benefits in experimental bevacizumab arm of ICON 7 patients [61]. The ICON 7 trial also validated the gene signature proposed by Gourley et al. to define the angiogenetic molecular subtypes of ovarian tumors [62]. In the serum samples from ICON 7 trial, Collinson et al. [63] identified several biomarkers that are predictive of therapeutic response: fms-like tyrosine kinase-4, mesothelin and acid-α1 glycoprotein. This resulted in identifying a patient subset likely to benefit from antiangiogenetic drugs. Patients exhibiting a positive signature profile had a median PFS improved by 5.5 months (significant p-value of 0.001). GOG-218 was the source of several articles identifying several potential predictors for response to anti-angiogenetic therapy: plasmatic concentrations of VEGF and VEGF-R2 [64], tumor VEGF-A expression [65], micro-vessel density (MVD) [66] and IL-6 levels [67]. Although the identification of these predictive biomarkers is encouraging, they need to be validated by more extensive trials in order to be incorporated into guideline recommendations for practicians. Oxidative-stress-related genes and factors have also been demonstrated to play a significant role in ovarian tumors as a predictive factor of response to angiogenesis inhibitor therapy in ovarian neoplasms [68,69,70]. It is known that OS affects tumor chemoresistance by specific mutations of important redox enzymes [71]. Oxidative stress factors have been identified in circulation among patients with malignant ovarian tumors [72]. However, the role of oxidative-stress-related prediction of response to therapy factors still needs to be investigated by trials. Other authors have suggested that non-coding RNAs play a role in angiogenesis in gynecological cancers [73].
Since for advanced ovarian cancer the treatment is mostly palliative in nature, and given the myriad of potentially severe adverse effects and dubitable OS benefits, the question of quality of life (QoL) of patients treated with anti-angiogenetic agents should be an important indicator of therapeutic success. At present, there are very few studies focusing on QoL in patients treated with angiogenesis inhibitors [74,75]. This aspect needs to be evaluated further by clinical trials. Our study demonstrated that some adverse effects that can significantly affect the QoL are more frequent when angiogenesis inhibitors are used. The anti-angiogenetic agents are associated with a higher incidence of gastro-intestinal perforations, infections, thromboembolic and hemorrhagic events.
When debating the efficacity–toxicity ratio of angiogenesis inhibitors, one more concern is the cross results when employed in conjunction with other another class of targeted therapies such as inhibitors of poly-ADP-ribose polymerase (PARP), especially since an ever-increasing number of trials have been published testing this therapeutic combination. This combination of drugs has a reported synergic effect, since angiogenesis inhibitors lead to hypoxia, which has been shown to induce homologous recombination repair deficiency by a downregulation of homologous recombination-repair genes. This effect results in an increased sensitivity to PARP inhibitors of tumoral cells [76,77,78]. Liu et al. [40] showed a significant improvement of PFS when treating the platinum sensitive ovarian recurrent cancer with cediranib plus olaparib, when compared to olaparib monotherapy. The combination also increases the OS in patients negative for a germline mutation of BRCA1/2. There are also data that suggested an improvement of PFS when adding olaparib maintenance in patients with advanced EOC who have received standard first-line chemotherapy plus bevacizumab—Phase II study conducted by Ray-Coquard in 2019 [54]. This combination of angiogenesis and PARP inhibitors can represent a future direction for research. Another small Phase I study (Lorusso et al., from 2020) focused on the association between bevacizumab and a different PARP inhibitor (rucaparib) and found no safety concerns about the combination [79]. The MITO 25 Phase II randomized study will investigate further rucaparib as maintenance in combination or without bevacizumab for patients with newly diagnosed FIGO III-IV ovarian cancer [80]. Mirza et al. [81] published a Phase I trial on combination niraparib and bevacizumab in platinum sensitive epithelial ovarian cancer which showed no safety concerns.
Anti-angiogenetic therapies, although constantly improving PFS, show suboptimal clinical effect [82,83]. This may be explained by the triggering of survival selection of hypoxic cells in the center of tumor [6]. Moreover, disruption of a specific angiogenic pathway may provoke compensatory reactions through compensatory production of alternative factors with roles in angiogenesis [84,85,86,87,88,89,90], leading to resistance to single-target therapeutic approaches. We therefore see an unmet need for the development and evaluation of novel strategies in order to mitigate the shortcomings of current therapeutic strategies by employing concurrent therapies that target multiple angiogenetic pathways.
4.2. Comparison with Other Studies
Our study consisted of 23 RCTs, thus making it the largest and a more recent meta-analysis combined with an extensive systematic review performed about anti-angiogenetic drugs and their usage in ovarian cancer, including 12,082 patients. Over time, several systematic reviews and meta-analyses demonstrating outcome variabilities were carried out on angiogenesis-inhibitor randomized controlled trials [53,91,92,93,94,95,96,97,98,99,100,101]. One of the latest (Guo) [92] included 22 RCTs published between 2011 and 2019, with 11,254 patients meeting the inclusion criteria. Our study included one more study (Duska et al., 2022). Although Helali et al. [53] included 23 RCTs, they focused on epithelial ovarian cancer, omitting studies such as Liu and Lederman’s (2016) [39,40], that were included in our study, in which we also included serous or endometrioid histology. They included the Matulonis 2019 (KEYNOTE 100) study [102] on the effects of pembrolizumab (a PD-1 inhibitor) in ovarian cancer, which we consider outside of the scope of this paper. The Hall et al., 2020 [103] study was not included due to the fact that cyclophosphamide was used as a treatment line rather than carboplatin/paclitaxel—the superiority of combination carboplatin–paclitaxel over the cisplatin–cyclophosphamide combination was demonstrated by a previous trial [22], and the usage of cyclophosphamide may falsely alter the PFS results in favor of anti-angiogenetic drugs. The most recent meta-analysis on the subject of inhibitors of angiogenesis in ovarian-tumor patients we came across is the one performed by Zhang et al. [104], referring only to recurrent ovarian tumors and containing 13 RTCs, which were all included in our study. The results of the Zhang et al. meta-analysis had similar results to ours, indicating both an OS and a PFS benefit of anti-angiogenetic drugs in recurrent neoplasm.
Angiogenesis inhibitors are associated with an increased PFS over all patient groups and all categories of drugs used, results similar to previous studies. In our study, the OS was only improved by the studied therapy for recurrent ovarian cancer, not influencing cases in first line or maintenance settings. The improved OS observed in other studies when using VEGF inhibitors or angiopoietin inhibitors was not observed in our study.
Most of previous meta-analyses carried out on angiogenesis inhibitors for patients with ovarian cancer focused on survival parameters, with toxicity being ignored. For a better understanding of the ratio of efficacy to safety of angiogenesis inhibitors, we also analyzed the therapy-specific adverse effects. By calculating the RR for 31 therapy-specific cases, we observed an increased risk for hypertension, hemorrhagic and thromboembolic events, proteinuria, gastro-intestinal perforations, infections, ascites, anemia, neutropenia, leucopenia, thrombocytopenia, nausea, vomiting, diarrhea, dyspnea, hypokalemia, headache and pain. For all grade-three-or-higher adverse reactions, there was an increased risk in the experimental arms of trials. There were no significant increased risks found in the following adverse events: venous thromboembolism, pyrexia, fatigue, hypomagnesemia, anorexia, constipation, alopecia, rash, abdominal pain and back pain. Two of the adverse effects specific to the angiogenesis inhibitors (wound-related issues and reversible posterior leukoencephalopathy syndrome) were proved not to be significantly linked to the therapy.
4.3. Strengths and Study Limitations
The strength of our study is the broad inclusion of 12,081 patients treated with seven different angiogenesis inhibitors, as this allowed us to perform a comprehensive analysis of survival parameters (PFS and OS) and therapy-specific toxicity. To the best of our knowledge, this is the largest meta-analysis on anti-angiogenetic drugs in ovarian cancer to this date. The RCTs included enrolled all stages of ovarian cancer and different treatment settings, thus allowing us to evaluate the role of the anti-angiogenetic therapy as first-line, as maintenance or as secondary therapy for recurrent disease. Furthermore, the subgroup analyses of different mechanism anti-angiogenetic drugs may allow us to make a prediction of the type of anti-angiogenetic therapy more likely to obtain the maximum benefit. As a side effect of the broad inclusion criteria, regardless of tumor type, stage of disease, drug regimens, number of previous lines of therapy, response to previous therapy, duration of follow-up and so on, the heterogeneity was increased, giving raise to the possibility of different results when focusing on more narrow patient types or more specific drug regimens (our study included VEGF blockade, VEGF-R inhibitors and angiopoietin inhibitors).
The high heterogeneity across studies is the most important limitation of our analysis. This high heterogeneity among trials may be explained by the inconsistency of characteristics of patients enrolled and also of disease particularities (stage of disease, pre- and postoperative tumoral burden, number of therapeutical lines used prior to inclusion in trial and histology of tumors). Different definitions for endpoints, different methodology and different sample size between studies and the fact that different anti-angiogenetic drugs were used may also account for heterogeneity. Another limitation of our study is the fact that our analysis is based on published data, rather than on actual patient records, thus making it prone to bias. Moreover, some of the studies included had an increased bias risk because of issues with randomization/blinding. The lack of registration of a Trial Sequential Analysis (TSA) protocol may also be considered a limitation of our study since some conclusions of our study may not have a sufficient effect size in order to be unlikely affected by further trials.
5. Conclusions
Our study showed that anti-angiogenetic agents can improve the PFS in ovarian cancer regardless of the treatment setting or type of drug used (VEGF inhibitors, VEGF-R inhibitors or angiopoietin inhibitors), thus being an option in the treatment of ovarian cancer. Secondly, due to the fact that OS improvements are only seen in high-risk chemo-naive cancers and in recurrent disease, we believe that angiogenesis inhibitors need to be administrated with prudence outside of these setting and after careful consideration of each case prognostic and response factors. Thirdly, we consider that further studies are required in order to better understand which categories benefit the most from angiogenesis inhibitors and which are only subjected to the risk of adverse effects and diminished QoL. The need to identify and validate the biomarkers for accurately predicting the response to therapy is a good future direction for study. Fourthly, for the platinum-sensitive recurrent disease, the role of concurrent therapy with angiogenetic and PARP inhibitors needs to be explored further. Lastly, we consider that the usage of a surrogate endpoint for clinical outcomes (PFS) in the process of drug approval may need to be reconsidered, as PFS proved not to be a viable substitute for OS.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/diagnostics13061040/s1. Supplementary Materials 1 containing Figures S1–S31: Figure S1: Hypertension; Figure S2: Hemorrhagic events; Figure S3: Thrombosis and embolism; Figure S4: Arterial thromboembolism; Figure S5: Venous thromboembolism; Figure S6: Proteinuria; Figure S7: Reversible posterior leukoencephalopathy syndrome; Figure S8: Gastrointestinal perforation; Figure S9: Infection; Figure S10: Pyrexia; Figure S11: Wound related issues; Figure S12: Ascites; Figure S13: Neutropenia; Figure S14: Anemia; Figure S15: Leucopenia; Figure S16: Thrombocytopenia; Figure S17: Nausea; Figure S18: Vomiting; Figure S19: Anorexia; Figure S20: Diarrhea; Figure S21: Constipation; Figure S22: Fatigue; Figure S23: Dyspnea; Figure S24: Alopecia; Figure S25: Rash; Figure S26: Hypomagnesemia; Figure S27: Hypokalemia; Figure S28: Pain; Figure S29: Headache; Figure S30: Abdominal pain; Figure S31: Back pain; Supplementary Materials 2—PRISMA checklist; Supplementary Materials 3—Graphical representation of statistical analysis containing Figures S32–S35: Figure S32—Sensitivity analysis (OS); Figure S33—Sensitivity analysis (PFS); Figure S34—Radial plots (OS); Figure S35—Radial plots (PFS).
Author Contributions
Conceptualization, E.C. and V.R.; methodology, E.C. and V.R.; software, D.C.L.; validation, M.G. and B.C.T.; formal analysis, C.C., S.I. and L.S.; data curation, S.I., C.C. and L.N.G.; writing—original draft preparation, E.C. and V.R.; writing—review and editing, E.C. and V.R.; visualization, E.C., B.C.T. and D.C.L.; supervision, L.S., D.-C.S. and L.N.G.; project administration, E.C. and V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Statement
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability Statement
The data is available online in the references we included. There is no outside storage of data since we did not use any data of our own and made a statistical analysis of data published by other authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ovary-Fact-Sheet. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/25-Ovary-fact-sheet.pdf (accessed on 2 January 2023).
- Luvero, D.; Milani, A.; Ledermann, J.A. Treatment Options in Recurrent Ovarian Cancer: Latest Evidence and Clinical Potential. Ther. Adv. Med. Oncol. 2014, 6, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian Cancer. Nat. Rev. Dis. Prim. 2016, 2, 16061. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian Cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Jászai, J.; Schmidt, M.H.H. Trends and Challenges in Tumor Anti-Angiogenic Therapies. Cells 2019, 8, 1102. [Google Scholar] [CrossRef] [PubMed]
- Akaza, H.; Nakagawa, M.; Tsuruo, T.; Saijo, N.; Sone, S.; Yamamoto, N.; Kakeji, Y.; Nakamura, S.; Kurebayashi, J.; Isonishi, S.; et al. Anti-Angiogenesis: New Concept for Therapy of Solid Tumors. Ann. Surg. 1972, 175, 409–416. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Dancey, J.; Sausville, E.A. Issues and Progress with Protein Kinase Inhibitors for Cancer Treatment. Nat. Rev. Drug Discov. 2003, 2, 296–313. [Google Scholar] [CrossRef]
- Ferrara, N. VEGF and the Quest for Tumour Angiogenesis Factors. Nat. Rev. Cancer 2002, 2, 795–803. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A.P. Ten Years of Anti-Vascular Endothelial Growth Factor Therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF Receptor Signalling ? In Control of Vascular Function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Elman, M.J.; Aiello, L.P.; Beck, R.W.; Bressler, N.M.; Bressler, S.B.; Edwards, A.R.; Ferris III, F.L.; Friedman, S.M.; Glassman, A.R.; Miller, K.M.; et al. Randomized Trial Evaluating Ranibizumab plus Prompt or Deferred Laser or Triamcinolone plus Prompt Laser for Diabetic Macular Edema. Ophthalmology 2010, 117, 1064–1077.e35. [Google Scholar] [CrossRef]
- CATT Research Group. Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.-F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-Related Macular Degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- Brown, D.M.; Campochiaro, P.A.; Bhisitkul, R.B.; Ho, A.C.; Gray, S.; Saroj, N.; Adamis, A.P.; Rubio, R.G.; Murahashi, W.Y. Sustained Benefits from Ranibizumab for Macular Edema Following Branch Retinal Vein Occlusion: 12-Month Outcomes of a Phase III Study. Ophthalmology 2011, 118, 1594–1602. [Google Scholar] [CrossRef]
- Miller, J.W. The Harvard Angiogenesis Story. Surv. Ophthalmol. 2014, 59, 361–364. [Google Scholar] [CrossRef]
- Turcan, N.; Baros, A.; Zugravu, C.; Mergeanu, M.; Sajin, M.; Andreescu, C.V.; Frincu, F.; Carp-Veliscu, A.; Edu, A.; Mehedintu, C.; et al. Trend of Incidence in the Last Five Years of Breast, Cervical, Ovarian and Uterine Cancer in the Main Hospital in Romania. Rom. J. Med. Pract. 2021, 16, 62–68. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Mendeley Reference Manager | Mendeley. Available online: https://www.mendeley.com/reference-management/reference-manager (accessed on 28 January 2023).
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. N. Engl. J. Med. 1996, 334, 1–6. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- RevMan | Cochrane Training. Available online: https://training.cochrane.org/online-learning/core-software/revman (accessed on 24 January 2023).
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard Chemotherapy with or without Bevacizumab for Women with Newly Diagnosed Ovarian Cancer (ICON7): Overall Survival Results of a Phase 3 Randomised Trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Aghajanian, C.; Goff, B.; Nycum, L.R.; Wang, Y.V.; Husain, A.; Blank, S.V. Final Overall Survival and Safety Analysis of OCEANS, a Phase 3 Trial of Chemotherapy with or without Bevacizumab in Patients with Platinum-Sensitive Recurrent Ovarian Cancer. Gynecol. Oncol. 2015, 139, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Gotlieb, W.H.; Amant, F.; Advani, S.; Goswami, C.; Hirte, H.; Provencher, D.; Somani, N.; Yamada, D.; Tamby, J.-F.; Vergote, I. Intravenous Afl Ibercept for Treatment of Recurrent Symptomatic Malignant Ascites in Patients with Advanced Ovarian Cancer: A Phase 2, Randomised, Double-Blind, Placebo-Controlled Study. Lancet Oncol. 2012, 13, 154–162. [Google Scholar] [CrossRef]
- Karlan, B.Y.; Oza, A.M.; Richardson, G.E.; Provencher, D.M.; Hansen, V.L.; Buck, M.; Chambers, S.K.; Ghatage, P.; Pippitt, C.H.; Brown, J.V.; et al. Randomized, Double-Blind, Placebo-Controlled Phase II Study of AMG 386 Combined with Weekly Paclitaxel in Patients with Recurrent Ovarian Cancer. J. Clin. Oncol. 2012, 30, 362–371. [Google Scholar] [CrossRef]
- Chekerov, R.; Hilpert, F.; Mahner, S.; El-Balat, A.; Harter, P.; de Gregorio, N.; Fridrich, C.; Markmann, S.; Potenberg, J.; Lorenz, R.; et al. Sorafenib plus Topotecan versus Placebo plus Topotecan for Platinum-Resistant Ovarian Cancer (TRIAS): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Oncol. 2018, 19, 1247–1258. [Google Scholar] [CrossRef]
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.G.; Fujiwara, K.; Tewari, K.S.; O’Malley, D.M.; et al. Bevacizumab and Paclitaxel–Carboplatin Chemotherapy and Secondary Cytoreduction in Recurrent, Platinum-Sensitive Ovarian Cancer (NRG Oncology/Gynecologic Oncology Group Study GOG-0213): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2017, 18, 779–791. [Google Scholar] [CrossRef]
- Duska, L.R.; Petroni, G.R.; Varhegyi, N.; Brown, J.; Jelovac, D.; Moore, K.N.; McGuire, W.P.; Darus, C.; Barroilhet, L.M.; Secord, A.A. A Randomized Phase II Evaluation of Weekly Gemcitabine plus Pazopanib versus Weekly Gemcitabine Alone in the Treatment of Persistent or Recurrent Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Carcinoma. Gynecol. Oncol. 2020, 157, 585–592. [Google Scholar] [CrossRef]
- du Bois, A.; Kristensen, G.; Ray-Coquard, I.; Reuss, A.; Pignata, S.; Colombo, N.; Denison, U.; Vergote, I.; del Campo, J.M.; Ottevanger, P.; et al. Standard First-Line Chemotherapy with or without Nintedanib for Advanced Ovarian Cancer (AGO-OVAR 12): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet Oncol. 2016, 17, 78–89. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cibula, D.; Mirza, M.R.; Reuss, A.; Ricci, C.; Colombo, N.; Koch, H.; Goffin, F.; González-Martin, A.; Ottevanger, P.B.; et al. Final Results from GCIG/ENGOT/AGO-OVAR 12, a Randomised Placebo-Controlled Phase III Trial of Nintedanib Combined with Chemotherapy for Newly Diagnosed Advanced Ovarian Cancer. Int. J. Cancer 2020, 146, 439–448. [Google Scholar] [CrossRef]
- Gore, M.; Hackshaw, A.; Brady, W.E.; Penson, R.T.; Zaino, R.; McCluggage, W.G.; Ganesan, R.; Wilkinson, N.; Perren, T.; Montes, A.; et al. An International, Phase III Randomized Trial in Patients with Mucinous Epithelial Ovarian Cancer (MEOC/GOG 0241) with Long-Term Follow-up: And Experience of Conducting a Clinical Trial in a Rare Gynecological Tumor. Gynecol. Oncol. 2019, 153, 541–548. [Google Scholar] [CrossRef]
- Herzog, T.J.; Scambia, G.; Kim, B.G.; Lhommé, C.; Markowska, J.; Ray-Coquard, I.; Sehouli, J.; Colombo, N.; Shan, M.; Petrenciuc, O.; et al. A Randomized Phase II Trial of Maintenance Therapy with Sorafenib in Front-Line Ovarian Carcinoma. Gynecol. Oncol. 2013, 130, 25–30. [Google Scholar] [CrossRef]
- Kim, J.W.; Mahner, S.; Wu, L.Y.; Shoji, T.; Kim, B.G.; Zhu, J.Q.; Takano, T.; Park, S.Y.; Kong, B.H.; Wu, Q.; et al. Pazopanib Maintenance Therapy in East Asian Women with Advanced Epithelial Ovarian Cancer: Results from AGO-OVAR16 and an East Asian Study. Int. J. Gynecol. Cancer 2018, 28, 2–10. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Hackshaw, A.; Kaye, S.; Jayson, G.; Gabra, H.; McNeish, I.; Earl, H.; Perren, T.; Gore, M.; Persic, M.; et al. Randomized Phase II Placebo-Controlled Trial of Maintenance Therapy Using the Oral Triple Angiokinase Inhibitor BIBF 1120 after Chemotherapy for Relapsed Ovarian Cancer. J. Clin. Oncol. 2011, 29, 3798–3804. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Embleton-Thirsk, A.C.; Perren, T.J.; Jayson, G.C.; Rustin, G.J.S.; Kaye, S.B.; Hirte, H.; Oza, A.; Vaughan, M.; Friedlander, M.; et al. Cediranib in Addition to Chemotherapy for Women with Relapsed Platinum-Sensitive Ovarian Cancer (ICON6): Overall Survival Results of a Phase III Randomised Trial. ESMO Open 2021, 6, 100043. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Embleton, A.C.; Raja, F.; Perren, T.J.; Jayson, G.C.; Rustin, G.J.S.; Kaye, S.B.; Hirte, H.; Eisenhauer, E.; Vaughan, M.; et al. Cediranib in Patients with Relapsed Platinum-Sensitive Ovarian Cancer (ICON6): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet 2016, 387, 1066–1074. [Google Scholar] [CrossRef]
- Liu, J.F.; Barry, W.T.; Birrer, M.; Lee, J.M.; Buckanovich, R.J.; Fleming, G.F.; Rimel, B.J.; Buss, M.K.; Nattam, S.R.; Hurteau, J.; et al. Overall Survival and Updated Progression-Free Survival Outcomes in a Randomized Phase II Study of Combination Cediranib and Olaparib versus Olaparib in Relapsed Platinum-Sensitive Ovarian Cancer. Ann. Oncol. 2019, 30, 551–557. [Google Scholar] [CrossRef]
- Marth, C.; Vergote, I.; Scambia, G.; Oberaigner, W.; Clamp, A.; Berger, R.; Kurzeder, C.; Colombo, N.; Vuylsteke, P.; Lorusso, D.; et al. ENGOT-Ov-6/TRINOVA-2: Randomised, Double-Blind, Phase 3 Study of Pegylated Liposomal Doxorubicin plus Trebananib or Placebo in Women with Recurrent Partially Platinum-Sensitive or Resistant Ovarian Cancer. Eur. J. Cancer 2017, 70, 111–121. [Google Scholar] [CrossRef]
- Monk, B.J.; Poveda, A.; Vergote, I.; Raspagliesi, F.; Fujiwara, K.; Bae, D.S.; Oaknin, A.; Ray-Coquard, I.; Provencher, D.M.; Karlan, B.Y.; et al. Final Results of a Phase 3 Study of Trebananib plus Weekly Paclitaxel in Recurrent Ovarian Cancer (TRINOVA-1): Long-Term Survival, Impact of Ascites, and Progression-Free Survival-2. Gynecol. Oncol. 2016, 143, 27–34. [Google Scholar] [CrossRef]
- Pignata, S.; Lorusso, D.; Joly, F.; Gallo, C.; Colombo, N.; Sessa, C.; Bamias, A.; Salutari, V.; Selle, F.; Frezzini, S.; et al. Carboplatin-Based Doublet plus Bevacizumab beyond Progression versus Carboplatin-Based Doublet Alone in Patients with Platinum-Sensitive Ovarian Cancer: A Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Lorusso, D.; Scambia, G.; Sambataro, D.; Tamberi, S.; Cinieri, S.; Mosconi, A.M.; Orditura, M.; Brandes, A.A.; Arcangeli, V.; et al. Pazopanib plus Weekly Paclitaxel versus Weekly Paclitaxel Alone for Platinum-Resistant or Platinum-Refractory Advanced Ovarian Cancer (MITO 11): A Randomised, Open-Label, Phase 2 Trial. Lancet Oncol. 2015, 16, 561–568. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab Combined with Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer: The AURELIA Open-Label Randomized Phase III Trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef]
- Richardson, D.L.; Sill, M.W.; Coleman, R.L.; Sood, A.K.; Pearl, M.L.; Kehoe, S.M.; Carney, M.E.; Hanjani, P.; van Le, L.; Zhou, X.C.; et al. Paclitaxel with and without Pazopanib for Persistent or Recurrent Ovarian Cancer: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 196–202. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Vergote, I.; du Bois, A.; Floquet, A.; Rau, J.; Kim, J.W.; del Campo, J.M.; Friedlander, M.; Pignata, S.; Fujiwara, K.; Colombo, N.; et al. Overall Survival Results of AGO-OVAR16: A Phase 3 Study of Maintenance Pazopanib versus Placebo in Women Who Have Not Progressed after First-Line Chemotherapy for Advanced Ovarian Cancer. Gynecol. Oncol. 2019, 155, 186–191. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Floquet, A.; Kim, J.W.; Rau, J.; del Campo, J.M.; Friedlander, M.; Pignata, S.; Fujiwara, K.; Vergote, I.; Colombo, N.; et al. Incorporation of Pazopanib in Maintenance Therapy of Ovarian Cancer. J. Clin. Oncol. 2014, 32, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Scambia, G.; O’Malley, D.; van Calster, B.; Park, S.Y.; del Campo, J.M.; Meier, W.; Bamias, A.; Colombo, N.; Wenham, R.M.; et al. Trebananib or Placebo plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Ovarian Cancer (TRINOVA-3/ENGOT-Ov2/GOG-3001): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2019, 20, 862–876. [Google Scholar] [CrossRef]
- El Helali, A.; Wong, C.H.L.; Choi, H.C.W.; Chan, W.W.L.; Dickson, N.; Siu, S.W.K.; Chan, K.K.; Ngan, H.Y.S.; Ngan, R.K.C.; Kennedy, R.D. A Comprehensive Systematic Review and Network Meta-Analysis: The Role of Anti-Angiogenic Agents in Advanced Epithelial Ovarian Cancer. Sci. Rep. 2022, 12, 3803. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.; Higgins, J.; Day, A.G.; Meyer, R.M.; Booth, C.M. Randomized Controlled Trials in the Era of Molecular Oncology: Methodology, Biomarkers, and End Points. Ann. Oncol. 2012, 23, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- A Review of Studies Examining the Relationship between Progression-Free Survival and Overall Survival in Advanced or Metastatic Cancer. Available online: https://pubmed.ncbi.nlm.nih.gov/28481488/ (accessed on 27 January 2023).
- Kim, C.; Prasad, V. Cancer Drugs Approved on the Basis of a Surrogate End Point and Subsequent Overall Survival: An Analysis of 5 Years of US Food and Drug Administration Approvals. JAMA Intern. Med. 2015, 175, 1992–1994. [Google Scholar] [CrossRef]
- Pasalic, D.; McGinnis, G.J.; Fuller, C.D.; Grossberg, A.J.; Verma, V.; Mainwaring, W.; Miller, A.B.; Lin, T.A.; Jethanandani, A.; Espinoza, A.F.; et al. Progression-Free Survival Is a Suboptimal Predictor for Overall Survival Among Metastatic Solid Tumor Clinical Trials. Eur. J. Cancer 2020, 136, 176. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Kim, C.; Burotto, M.; Vandross, A. The Strength of Association between Surrogate End Points and Survival in Oncology: A Systematic Review of Trial-Level Meta-Analyses. JAMA Intern. Med. 2015, 175, 1389–1398. [Google Scholar] [CrossRef]
- Paoletti, X.; Lewsley, L.A.; Daniele, G.; Cook, A.; Yanaihara, N.; Tinker, A.; Kristensen, G.; Ottevanger, P.B.; Aravantinos, G.; Miller, A.; et al. Assessment of Progression-Free Survival as a Surrogate End Point of Overall Survival in First-Line Treatment of Ovarian Cancer: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2020, 3, e1918939. [Google Scholar] [CrossRef]
- Backen, A.; Renehan, A.G.; Clamp, A.R.; Berzuini, C.; Zhou, C.; Oza, A.; Bannoo, S.; Scherer, S.J.; Banks, R.E.; Dive, C.; et al. The Combination of Circulating Ang1 and Tie2 Levels Predict Progression Free Survival Advantage in Bevacizumab-Treated Ovarian Cancer Patients. Clin. Cancer Res. 2014, 20, 4549. [Google Scholar] [CrossRef]
- Gourley, C.; McCavigan, A.; Perren, T.; Paul, J.; Michie, C.O.; Churchman, M.; Williams, A.; McCluggage, W.G.; Parmar, M.; Kaplan, R.S.; et al. Molecular Subgroup of High-Grade Serous Ovarian Cancer (HGSOC) as a Predictor of Outcome Following Bevacizumab. J. Clin. Oncol. 2014, 32, 5502. [Google Scholar] [CrossRef]
- Collinson, F.; Hutchinson, M.; Craven, R.A.; Cairns, D.A.; Zougman, A.; Wind, T.C.; Gahir, N.; Messenger, M.P.; Jackson, S.; Thompson, D.; et al. Predicting Response to Bevacizumab in Ovarian Cancer: A Panel of Potential Biomarkers Informing Treatment Selection. Clin. Cancer Res. 2013, 19, 5227. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kerbel, R.; Ellis, L.M.; Harris, A.L. Antiangiogenic Therapy in Oncology: Current Status and Future Directions. Lancet 2016, 388, 518–529. [Google Scholar] [CrossRef]
- Birrer, M.J.; Choi, Y.; Brady, M.F.; Mannel, R.S.; Burger, R.A.; WEI, W.; Husain, A.; Bais, C. Retrospective Analysis of Candidate Predictive Tumor Biomarkers (BMs) for Efficacy in the GOG-0218 Trial Evaluating Front-Line Carboplatin–Paclitaxel (CP) ± Bevacizumab (BEV) for Epithelial Ovarian Cancer (EOC). J. Clin. Oncol. 2015, 33, 5505. [Google Scholar] [CrossRef]
- Bais, C.; Mueller, B.; Brady, M.F.; Mannel, R.S.; Burger, R.A.; Wei, W.; Marien, K.M.; Kockx, M.M.; Husain, A.; Birrer, M.J. Tumor Microvessel Density as a Potential Predictive Marker for Bevacizumab Benefit: GOG-0218 Biomarker Analyses. JNCI J. Natl. Cancer Inst. 2017, 109, djx066. [Google Scholar] [CrossRef]
- Secord, A.A.; Burdett, K.B.; Owzar, K.; Tritchler, D.; Sibley, A.B.; Liu, Y.; Starr, M.D.; Chris Brady, J.; Lankes, H.A.; Hurwitz, H.I.; et al. Predictive Blood-Based Biomarkers in Patients with Epithelial Ovarian Cancer Treated with Carboplatin and Paclitaxel with or without Bevacizumab: Results from GOG-0218. Clin. Cancer Res. 2020, 26, 1288–1296. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, X.; Yin, Y.; Zhang, H.; Yin, F.; Guo, P.; Zhang, X.; Sun, C.; Li, S.; Han, Y.; et al. Identifying the Role of Oxidative Stress-Related Genes as Prognostic Biomarkers and Predicting the Response of Immunotherapy and Chemotherapy in Ovarian Cancer. Oxid. Med. Cell. Longev. 2022, 2022, 6575534. [Google Scholar] [CrossRef] [PubMed]
- Chaiswing, L.; Yarana, C.; Clair, W.S.; Tovmasyan, A.; Batinic-Haberle, I.; Spasojevic, I.; St Clair, D. A Redox-Active Mn Porphyrin, MnTnBuOE-2-PyP5+, Synergizes with Carboplatin in Treatment of Chemoresistant Ovarian Cell Line. Oxid. Med. Cell. Longev. 2022, 2022, 9664636. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryć, E.; Surowska, O.; Heryć, R.; Serwin, N.; Napiontek-Balińska, S.; Dołęgowska, B. Are Antioxidant Enzymes Essential Markers in the Diagnosis and Monitoring of Cancer Patients—A Review. Clin. Biochem 2021, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N.M.; Belotte, J.; Saed, M.G.; Memaj, I.; Diamond, M.P.; Morris, R.T.; Saed, G.M. Specific Point Mutations in Key Redox Enzymes Are Associated with Chemoresistance in Epithelial Ovarian Cancer. Free Radic. Biol. Med. 2017, 102, 122–132. [Google Scholar] [CrossRef]
- Senthil, K.; Aranganathan, S.; Nalini, N. Evidence of Oxidative Stress in the Circulation of Ovarian Cancer Patients. Clin. Chim. Acta 2004, 339, 27–32. [Google Scholar] [CrossRef]
- Rahimian, N.; Razavi, Z.S.; Aslanbeigi, F.; Mirkhabbaz, A.M.; Piroozmand, H.; Shahrzad, M.K.; Hamblin, M.R.; Mirzaei, H. Non-Coding RNAs Related to Angiogenesis in Gynecological Cancer. Gynecol. Oncol. 2021, 161, 896–912. [Google Scholar] [CrossRef]
- Friedlander, M.; Rau, J.; Lee, C.K.; Meier, W.; Lesoin, A.; Kim, J.W.; Poveda, A.; Buck, M.; Scambia, G.; Shimada, M.; et al. Quality of Life in Patients with Advanced Epithelial Ovarian Cancer (EOC) Randomized to Maintenance Pazopanib or Placebo after First-Line Chemotherapy in the AGO-OVAR 16 Trial. Measuring What Matters-Patient-Centered End Points in Trials of Maintenance Therapy. Ann. Oncol. 2018, 29, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Stark, D.; Nankivell, M.; Pujade-Lauraine, E.; Kristensen, G.; Elit, L.; Stockler, M.; Hilpert, F.; Cervantes, A.; Brown, J.; Lanceley, A.; et al. Standard Chemotherapy with or without Bevacizumab in Advanced Ovarian Cancer: Quality-of-Life Outcomes from the International Collaboration on Ovarian Neoplasms (ICON7) Phase 3 Randomised Trial. Lancet Oncol. 2013, 14, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Bindra, R.S.; Schaffer, P.J.; Meng, A.; Woo, J.; Måseide, K.; Roth, M.E.; Lizardi, P.; Hedley, D.W.; Bristow, R.G.; Glazer, P.M. Down-Regulation of Rad51 and Decreased Homologous Recombination in Hypoxic Cancer Cells. Mol. Cell. Biol. 2004, 24, 8504–8518. [Google Scholar] [CrossRef]
- Mirza, M.R.; Pignata, S.; Ledermann, J.A. Latest Clinical Evidence and Further Development of PARP Inhibitors in Ovarian Cancer. Ann. Oncol. 2018, 29, 1366–1376. [Google Scholar] [CrossRef]
- Bindra, R.S.; Gibson, S.L.; Meng, A.; Westermark, U.; Jasin, M.; Pierce, A.J.; Bristow, R.G.; Classon, M.K.; Glazer, P.M. Hypoxia-Induced down-Regulation of BRCA1 Expression by E2Fs. Cancer Res. 2005, 65, 11597–11604. [Google Scholar] [CrossRef]
- Lorusso, D.; Maltese, G.; Sabatucci, I.; Cresta, S.; Matteo, C.; Ceruti, T.; D’Incalci, M.; Zucchetti, M.; Raspagliesi, F.; Sonetto, C.; et al. Phase I Study of Rucaparib in Combination with Bevacizumab in Ovarian Cancer Patients: Maximum Tolerated Dose and Pharmacokinetic Profile. Target. Oncol. 2021, 16, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Carboplatin-Paclitaxel-Bevacizumab vs Carbo-Pacli-Beva-Rucaparib vs Carbo-Pacli-Ruca, Selected According to HRD Status, in Patients With Advanced Ovarian, Primary Peritoneal and Fallopian Tube Cancer, Preceded by a Phase I Dose Escalation Study on Ruca-Beva Combination—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03462212 (accessed on 27 January 2023).
- Mirza, M.R.; Bergmann, T.K.; Mau-Sørensen, M.; Christensen, R.d.P.; Åvall-Lundqvist, E.; Birrer, M.J.; Jørgensen, M.; Roed, H.; Malander, S.; Nielsen, F.; et al. A Phase I Study of the PARP Inhibitor Niraparib in Combination with Bevacizumab in Platinum-Sensitive Epithelial Ovarian Cancer: NSGO AVANOVA1/ENGOT-OV24. Cancer Chemother. Pharmacol. 2019, 84, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Stapor, P.; Wang, X.; Goveia, J.; Moens, S.; Carmeliet, P. Angiogenesis Revisited-Role and Therapeutic Potential of Targeting Endothelial Metabolism. J. Cell Sci. 2014, 127, 4331–4341. [Google Scholar] [CrossRef]
- de Bock, K.; Cauwenberghs, S.; Carmeliet, P. Vessel Abnormalization: Another Hallmark of Cancer?: Molecular Mechanisms and Therapeutic Implications. Curr. Opin. Genet. Dev. 2011, 21, 73–79. [Google Scholar] [CrossRef]
- Michaelsen, S.R.; Staberg, M.; Pedersen, H.; Jensen, K.E.; Majewski, W.; Broholm, H.; Nedergaard, M.K.; Meulengracht, C.; Urup, T.; Villingshøj, M.; et al. VEGF-C Sustains VEGFR2 Activation under Bevacizumab Therapy and Promotes Glioblastoma Maintenance. Neuro Oncol. 2018, 20, 1462–1474. [Google Scholar] [CrossRef]
- Taylor, A.P.; Rodriguez, M.; Adams, K.; Goldenberg, D.M.; Blumenthal, R.D. Altered Tumor Vessel Maturation and Proliferation in Placenta Growth Factor-Producing Tumors: Potential Relationship to Post-Therapy Tumor Angiogenesis and Recurrence. Int. J. Cancer 2003, 105, 158–164. [Google Scholar] [CrossRef]
- Shojaei, F.; Wu, X.; Zhong, C.; Yu, L.; Liang, X.H.; Yao, J.; Blanchard, D.; Bais, C.; Peale, F.V.; van Bruggen, N.; et al. Bv8 Regulates Myeloid-Cell-Dependent Tumour Angiogenesis. Nature 2007, 450, 825–831. [Google Scholar] [CrossRef]
- Shojaei, F.; Wu, X.; Malik, A.K.; Zhong, C.; Baldwin, M.E.; Schanz, S.; Fuh, G.; Gerber, H.-P.; Ferrara, N. Tumor Refractoriness to Anti-VEGF Treatment Is Mediated by CD11b+Gr1+ Myeloid Cells. Nat. Biotechnol. 2007, 25, 911–920. [Google Scholar] [CrossRef]
- Li, D.; Xie, K.; Ding, G.; Li, J.; Chen, K.; Li, H.; Qian, J.; Jiang, C.; Fang, J. Tumor Resistance to Anti-VEGF Therapy through up-Regulation of VEGF-C Expression. Cancer Lett. 2014, 346, 45–52. [Google Scholar] [CrossRef]
- Crawford, Y.; Kasman, I.; Yu, L.; Zhong, C.; Wu, X.; Modrusan, Z.; Kaminker, J.; Ferrara, N. PDGF-C Mediates the Angiogenic and Tumorigenic Properties of Fibroblasts Associated with Tumors Refractory to Anti-VEGF Treatment. Cancer Cell 2009, 15, 21–34. [Google Scholar] [CrossRef]
- Casanovas, O.; Hicklin, D.J.; Bergers, G.; Hanahan, D. Drug Resistance by Evasion of Antiangiogenic Targeting of VEGF Signaling in Late-Stage Pancreatic Islet Tumors. Cancer Cell 2005, 8, 299–309. [Google Scholar] [CrossRef]
- Wang, H.; Xu, T.; Zheng, L.; Li, G. Angiogenesis Inhibitors for the Treatment of Ovarian Cancer: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Gynecol. Cancer 2018, 28, 903–914. [Google Scholar] [CrossRef]
- Guo, C.; Yan, C.; Qu, L.; Du, R.; Lin, J. The Efficacy and Toxicity of Angiogenesis Inhibitors for Ovarian Cancer: A Meta-Analysis of Randomized Controlled Trials. Arch. Gynecol. Obstet. 2021, 303, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Chen, H.L. Bevacizumab in the Treatment of Ovarian Cancer: A Meta-Analysis from Four Phase III Randomized Controlled Trials. Arch. Gynecol. Obstet. 2013, 288, 655–666. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, P.; Qu, X.; Liu, Y.; Zhang, J. Phase III Trials of Standard Chemotherapy with or without Bevacizumab for Ovarian Cancer: A Meta-Analysis. PLoS ONE 2013, 8, e81858. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, S.; Hong, C.; Cai, H. Angiogenesis Inhibitors for Patients with Ovarian Cancer: A Meta-Analysis of 12 Randomized Controlled Trials. Curr. Med. Res. Opin. 2016, 32, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Shen Wu, Y.; Shui, L.; Shen, D.; Chen, X. Bevacizumab Combined with Chemotherapy for Ovarian Cancer: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Oncotarget 2017, 8, 10703–10713. [Google Scholar]
- Jiang, Y.; Sun, X.; Kong, B.; Jiang, J. Antiangiogenesis Therapy in Ovarian Cancer Patients: An Updated Meta-Analysis for 15 Randomized Controlled Trials. Medicine 2018, 97, e11920. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Chen, R.; Yu, H.; Lu, X. The Prognostic Significance of Anti-Angiogenesis Therapy in Ovarian Cancer: A Meta-Analysis. J. Ovarian Res. 2015, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; de Felice, F.; Palaia, I.; Musella, A.; di Donato, V.; Gasparri, M.L.; Musio, D.; Muzii, L.; Tombolini, V.; Panici, P.B. Efficacy and Toxicity of Bevacizumab in Recurrent Ovarian Disease: An Update Meta-Analysis on Phase III Trials. Oncotarget 2016, 7, 13221. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Chen, X.; Ba, Y. Addition of Bevacizumab to Chemotherapy in Patients with Ovarian Cancer: A Systematic Review and Meta-Analysis of Randomized Trials. Clin. Transl. Oncol. 2015, 17, 673–683. [Google Scholar] [CrossRef]
- Wang, T.S.; Lei, W.; Cui, W.; Wen, P.; Guo, H.F.; Ding, S.G.; Yang, Y.P.; Xu, Y.Q.; Lv, S.W.; Zhu, Y.L. A Meta-Analysis of Bevacizumab Combined with Chemotherapy in the Treatment of Ovarian Cancer. Indian J. Cancer 2014, 51 (Suppl. 3), e95–e98. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor Activity and Safety of Pembrolizumab in Patients with Advanced Recurrent Ovarian Cancer: Results from the Phase II KEYNOTE-100 Study. Ann. Oncol. 2019, 30, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.R.; Dehbi, H.M.; Banerjee, S.; Lord, R.; Clamp, A.; Ledermann, J.A.; Nicum, S.; Lilleywhite, R.; Bowen, R.; Michael, A.; et al. A Phase II Randomised, Placebo-Controlled Trial of Low Dose (Metronomic) Cyclophosphamide and Nintedanib (BIBF1120) in Advanced Ovarian, Fallopian Tube or Primary Peritoneal Cancer. Gynecol. Oncol. 2020, 159, 692–698. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, W. The Efficacy and Safety of Angiogenesis Inhibitors for Recurrent Ovarian Cancer: A Meta-analysis. J. Ovarian Res. 2022, 15, 99. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).