IgG N-glycan Signatures as Potential Diagnostic and Prognostic Biomarkers
Abstract
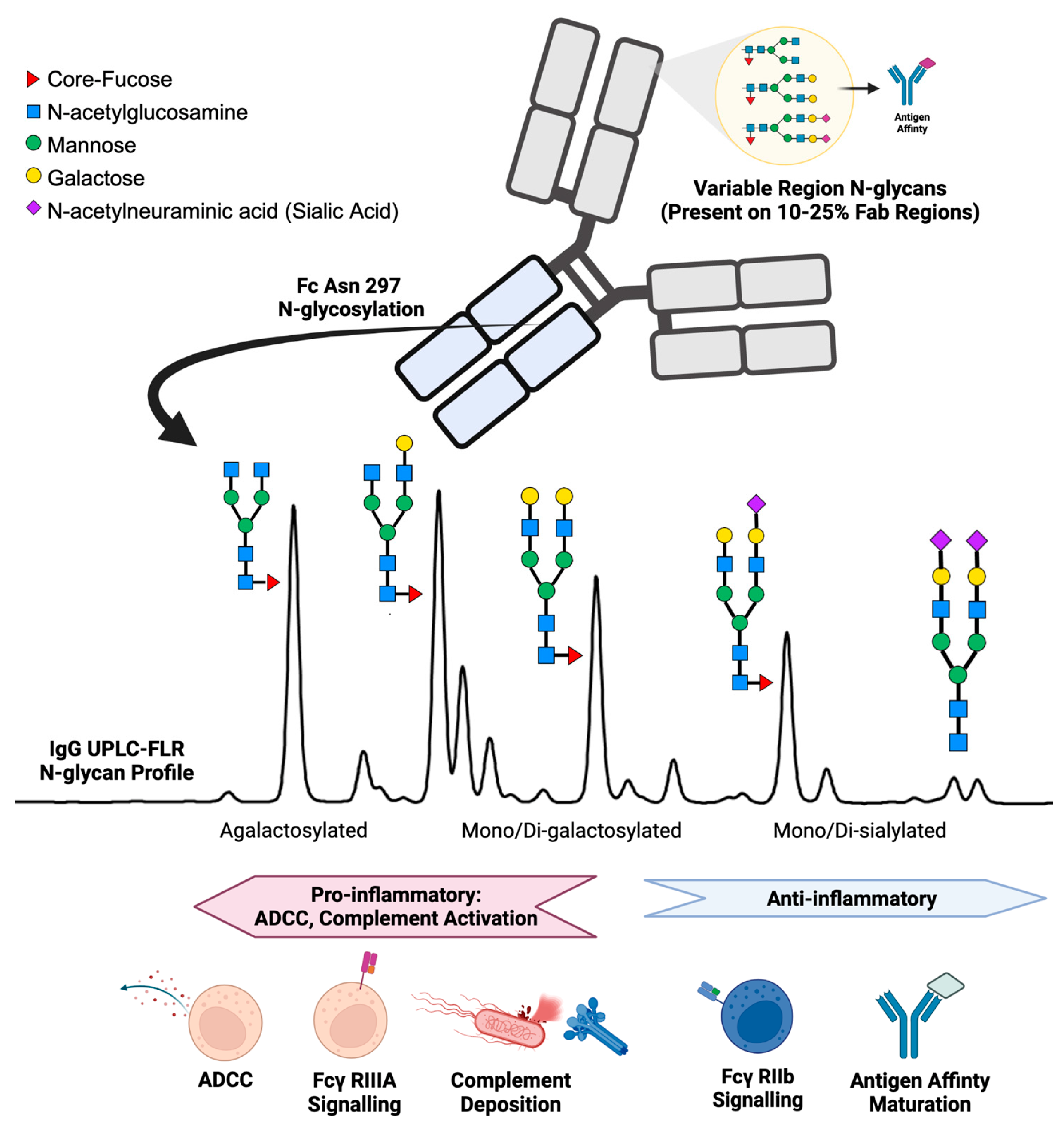
1. Introduction
2. IgG N-Glycans in Healthy Humans
3. IgG N-Glycans Altered during Infectious Disease
3.1. IgG N-Glycans Altered during Viral Infections
3.2. Dengue Fever
3.3. Influenza and RSV
3.4. SARS-CoV-2
3.5. HIV
3.6. HBV
4. IgG N-Glycans Altered during Bacterial and Parasitic Infections
4.1. Tuberculosis
4.2. Lyme Disease
4.3. Meningococcus
4.4. Malaria
5. IgG N-Glycans Altered during Autoimmune and Inflammatory Diseases
5.1. Guillain–Barré Syndrome
5.2. Systemic Erythematosus Lupus
5.3. Crohn's Disease and Ulcerative Colitis
5.4. Immune Thrombocytopenia
5.5. Idiopathic Membranous Nephropathy
5.6. Vasculitis
5.7. Chronic Inflammatory Airway Diseases
5.8. Amyotrophic Lateral Sclerosis
5.9. Parkinson’s Disease
6. IgG N-Glycans Altered in Cancer
6.1. Breast and Cervical Cancer
6.2. Thyroid Cancer
6.3. Colorectal Cancer
6.4. Pancreatic Cancer
6.5. B-Cell Cancer
7. Limitations
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cook, C.E.; Décary, S. Higher order thinking about differential diagnosis. Braz. J. Phys. Ther. 2020, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H. Principles of Diagnosis of Infectious Diseases. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 222–225. [Google Scholar] [CrossRef]
- Socarras, K.M.; Haslund-Gourley, B.S.; Cramer, N.A.; Comunale, M.A.; Marconi, R.T.; Ehrlich, G.D. Large-Scale Sequencing of Borreliaceae for the Construction of Pan-Genomic-Based Diagnostics. Genes 2022, 13, 1604. [Google Scholar] [CrossRef]
- Zwaan, L.; Singh, H. The challenges in defining and measuring diagnostic error. Diagnosis 2015, 2, 97–103. [Google Scholar] [CrossRef]
- Holtedahl, K. Challenges in early diagnosis of cancer: The fast track. Scand. J. Prim. Health Care 2020, 38, 251–252. [Google Scholar] [CrossRef]
- Duncan, C.F.; Youngstein, T.; Kirrane, M.D.; Lonsdale, D.O. Diagnostic Challenges in Sepsis. Curr. Infect. Dis. Rep. 2021, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Tufail, A.B.; Ma, Y.-K.; Kaabar, M.K.A.; Martínez, F.; Junejo, A.R.; Ullah, I.; Khan, R. Deep Learning in Cancer Diagnosis and Prognosis Prediction: A Minireview on Challenges, Recent Trends, and Future Directions. Comput. Math. Methods Med. 2021, 2021, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Thornblade, L.W.; Mulligan, M.S.; Odem-Davis, K.; Hwang, B.; Waworuntu, R.L.; Wolff, E.M.; Kessler, L.; Wood, D.E.; Farjah, F. Challenges in Predicting Recurrence After Resection of Node-Negative Non-Small Cell Lung Cancer. Ann. Thoracic Surgery 2018, 106, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Hires, M.; Jane, E.; Mego, M.; Chovanec, M.; Kasak, P.; Tkac, J. Glycan Analysis as Biomarkers for Testicular Cancer. Diagnostics 2019, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Comunale, M.A.; Wang, M.; Hafner, J.; Krakover, J.; Rodemich, L.; Kopenhaver, B.; Long, R.E.; Junaidi, O.; Bisceglie, A.M.D.; Block, T.M.; et al. Identification and Development of Fucosylated Glycoproteins as Biomarkers of Primary Hepatocellular Carcinoma. J. Proteome Res. 2009, 8, 595–602. [Google Scholar] [CrossRef]
- Wanyama, F.M.; Blanchard, V. Glycomic-Based Biomarkers for Ovarian Cancer: Advances and Challenges. Diagnostics 2021, 11, 643. [Google Scholar] [CrossRef]
- Alter, G.; Ottenhoff, T.H.M.; Joosten, S.A. Antibody glycosylation in inflammation, disease and vaccination. Seminars Immunol. 2018, 39, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. in Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Pucić, M.; Knezević, A.; Vidic, J.; Adamczyk, B.; Novokmet, M.; Polasek, O.; Gornik, O.; Supraha-Goreta, S.; Wormald, M.R.; Redzić, I.; et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell Proteom. 2011, 10, M111.010090. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The Impact of Glycosylation on the Biological Function and Structure of Human Immunoglobulins. Annu. Rev. Immunol. 2007, 25, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Van De Bovenkamp, F.S.; Hafkenscheid, L.; Rispens, T.; Rombouts, Y. The Emerging Importance of IgG Fab Glycosylation in Immunity. J. Immunol. 2016, 196, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Bondt, A.; Rombouts, Y.; Selman, M.H.; Hensbergen, P.J.; Reiding, K.R.; Hazes, J.M.; Dolhain, R.J.; Wuhrer, M. Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes. Mol. Cell Proteom. 2014, 13, 3029–3039. [Google Scholar] [CrossRef]
- Lu, L.L.; Das, J.; Grace, P.S.; Fortune, S.M.; Restrepo, B.I.; Alter, G. Antibody Fc Glycosylation Discriminates Between Latent and Active Tuberculosis. J. Infect. Dis. 2020, 222, 2093–2102. [Google Scholar] [CrossRef]
- Cobb, B.A. The history of IgG glycosylation and where we are now. Glycobiology 2020, 30, 202–213. [Google Scholar] [CrossRef]
- Jennewein, M.F.; Alter, G. The Immunoregulatory Roles of Antibody Glycosylation. Trends Immunol. 2017, 38, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, D.I.C. Improvement of interferon-γ sialylation in Chinese hamster ovary cell culture by feeding of N-acetylmannosamine. Biotechnol. Bioeng. 1998, 58, 642–648. [Google Scholar] [CrossRef]
- Kemna, M.J.; Plomp, R.; Van Paassen, P.; Koeleman, C.A.M.; Jansen, B.C.; Damoiseaux, J.G.M.C.; Cohen Tervaert, J.W.; Wuhrer, M. Galactosylation and Sialylation Levels of IgG Predict Relapse in Patients With PR3-ANCA Associated Vasculitis. EBioMedicine 2017, 17, 108–118. [Google Scholar] [CrossRef]
- Irvine, E.B.; Alter, G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology 2020, 30, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Jassal, R.; Jenkins, N.; Charlwood, J.; Camilleri, P.; Jefferis, R.; Lund, J. Sialylation of human IgG-Fc carbohydrate by transfected rat alpha2,6-sialyltransferase. Biochem. Biophys. Res. Commun. 2001, 286, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Boune, S.; Hu, P.; Epstein, A.L.; Khawli, L.A. Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T. IgG Fc Glycosylation in Human Immunity. Curr. Top Microbiol. Immunol. 2019, 423, 63–75. [Google Scholar] [CrossRef]
- Subedi, G.P.; Barb, A.W. The immunoglobulin G1 N-glycan composition affects binding to each low affinity Fc γ receptor. mAbs 2016, 8, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Yang, H.; Liu, P.; Huang, C.; Wang, M.; Li, Y.; Zhu, M.; Wang, J.; Xu, Y.; Wang, Y.; et al. Profile of Immunoglobulin G N-Glycome in COVID-19 Patients: A Case-Control Study. Front. Immunol. 2021, 12, 748566. [Google Scholar] [CrossRef]
- Pincetic, A.; Bournazos, S.; Dilillo, D.J.; Maamary, J.; Wang, T.T.; Dahan, R.; Fiebiger, B.-M.; Ravetch, J.V. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat. Immunol. 2014, 15, 707–716. [Google Scholar] [CrossRef]
- Quast, I.; Peschke, B.; Lünemann, J.D. Regulation of antibody effector functions through IgG Fc N-glycosylation. Cell. Mol. Life Sci. 2017, 74, 837–847. [Google Scholar] [CrossRef]
- Bournazos, S.; Ravetch, J.V. Fcγ Receptor Function and the Design of Vaccination Strategies. Immunity 2017, 47, 224–233. [Google Scholar] [CrossRef]
- Giuntini, S.; Reason, D.C.; Granoff, D.M. Combined Roles of Human IgG Subclass, Alternative Complement Pathway Activation, and Epitope Density in the Bactericidal Activity of Antibodies to Meningococcal Factor H Binding Protein. Infect. Immun. 2012, 80, 187–194. [Google Scholar] [CrossRef]
- Lofano, G.; Gorman, M.J.; Yousif, A.S.; Yu, W.-H.; Fox, J.M.; Dugast, A.-S.; Ackerman, M.E.; Suscovich, T.J.; Weiner, J.; Barouch, D.; et al. Antigen-specific antibody Fc glycosylation enhances humoral immunity via the recruitment of complement. Sci. Immunol. 2018, 3, eaat7796. [Google Scholar] [CrossRef] [PubMed]
- Quast, I.; Keller, C.W.; Maurer, M.A.; Giddens, J.P.; Tackenberg, B.; Wang, L.-X.; Münz, C.; Nimmerjahn, F.; Dalakas, M.C.; Lünemann, J.D. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Investig. 2015, 125, 4160–4170. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; McCraw, A.J.; Gardner, R.A.; Spencer, D.I.R.; Karagiannis, S.N.; Wagner, G.K. Glycoengineering of Therapeutic Antibodies with Small Molecule Inhibitors. Antibodies 2021, 10, 44. [Google Scholar] [CrossRef]
- Pereira, N.A.; Chan, K.F.; Lin, P.C.; Song, Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. mAbs 2018, 10, 693–711. [Google Scholar] [CrossRef]
- Nandakumar, K.S.; Collin, M.; Olsén, A.; Nimmerjahn, F.; Blom, A.M.; Ravetch, J.V.; Holmdahl, R. Endoglycosidase treatment abrogates IgG arthritogenicity: Importance of IgG glycosylation in arthritis. Eur. J. Immunol. 2007, 37, 2973–2982. [Google Scholar] [CrossRef]
- Albert, H.; Collin, M.; Dudziak, D.; Ravetch, J.V.; Nimmerjahn, F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc. Natl. Acad. Sci. USA 2008, 105, 15005–15009. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Ratelade, J.; Zhang, H.; Verkman, A.S. Enzymatic deglycosylation converts pathogenic neuromyelitis optica anti-aquaporin-4 immunoglobulin G into therapeutic antibody. Ann. Neurol. 2013, 73, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wessels, H.J.; Kulkarni, P.; Van Dael, M.; Suppers, A.; Willems, E.; Zijlstra, F.; Kragt, E.; Gloerich, J.; Schmit, P.-O.; Pengelley, S.; et al. Plasma Glycoproteomics Delivers High-Specificity Disease Biomarkers by Detecting Site-Specific Glycosylation Abnormalities; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2022. [Google Scholar] [CrossRef]
- Nagae, M.; Yamaguchi, Y.; Taniguchi, N.; Kizuka, Y. 3D Structure and Function of Glycosyltransferases Involved in N-glycan Maturation. Int. J. Mol. Sci. 2020, 21, 437. [Google Scholar] [CrossRef]
- Pfeifle, R.; Rothe, T.; Ipseiz, N.; Scherer, H.U.; Culemann, S.; Harre, U.; Ackermann, J.A.; Seefried, M.; Kleyer, A.; Uderhardt, S.; et al. Regulation of autoantibody activity by the IL-23–TH17 axis determines the onset of autoimmune disease. Nat. Immunol. 2017, 18, 104–113. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Z.; Guo, Z.; Zhao, X.; Gong, Y.; Zhao, K.; Qu, C.; Huang, Y.; Li, Y.; Gao, Y.; et al. Cytokines in the Immune Microenvironment Change the Glycosylation of IgG by Regulating Intracellular Glycosyltransferases. Front. Immunol. 2022, 12, 724379. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Balog, C.I.; Stavenhagen, K.; Koeleman, C.A.; Scherer, H.U.; Selman, M.H.; Deelder, A.M.; Huizinga, T.W.; Toes, R.E.; Wuhrer, M. Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol. Cell Proteom. 2011, 10, M110.004655. [Google Scholar] [CrossRef]
- Novokmet, M.; Lukić, E.; Vučković, F.; Durić, Ž.; Keser, T.; Rajšl, K.; Remondini, D.; Castellani, G.; Gašparović, H.; Gornik, O.; et al. Changes in IgG and total plasma protein glycomes in acute systemic inflammation. Sci. Rep. 2015, 4, 4347. [Google Scholar] [CrossRef] [PubMed]
- Schaffert, A.; Hanić, M.; Novokmet, M.; Zaytseva, O.; Krištić, J.; Lux, A.; Nitschke, L.; Peipp, M.; Pezer, M.; Hennig, R.; et al. Minimal B Cell Extrinsic IgG Glycan Modifications of Pro- and Anti-Inflammatory IgG Preparations in vivo. Front. Immunol. 2020, 10, 3024. [Google Scholar] [CrossRef] [PubMed]
- Oswald, D.M.; Lehoux, S.D.; Zhou, J.Y.; Glendenning, L.M.; Cummings, R.D.; Cobb, B.A. ST6Gal1 in plasma is dispensable for IgG sialylation. Glycobiology 2022, 32, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Gornik, O.; Pavić, T.; Lauc, G. Alternative glycosylation modulates function of IgG and other proteins—mplications on evolution and disease. Biochim. Biophys. Acta 2012, 1820, 1318–1326. [Google Scholar] [CrossRef]
- Pučić, M.; Mužinić, A.; Novokmet, M.; Škledar, M.; Pivac, N.; Lauc, G.; Gornik, O. Changes in plasma and IgG N-glycome during childhood and adolescence. Glycobiology 2012, 22, 975–982. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Kristic, J.; Dong, J.; Chu, X.; Ge, S.; Wang, H.; Fang, H.; Gao, Q.; Liu, D.; et al. Profiling IgG N-glycans as potential biomarker of chronological and biological ages: A community-based study in a Han Chinese population. Medicine 2016, 95, e4112. [Google Scholar] [CrossRef]
- Shikata, K.; Yasuda, T.; Takeuchi, F.; Konishi, T.; Nakata, M.; Mizuochi, T. Structural changes in the oligosaccharide moiety of human IgG with aging. Glycoconj. J. 1998, 15, 683–689. [Google Scholar] [CrossRef]
- Haan, N.d.; Boeddha, N.P.; Ekinci, E.; Reiding, K.R.; Emonts, M.; Hazelzet, J.A.; Wuhrer, M.; Driessen, G.J. Differences in IgG Fc Glycosylation Are Associated with Outcome of Pediatric Meningococcal Sepsis. mBio 2018, 9, e00546-18. [Google Scholar] [CrossRef]
- Kronimus, Y.; Dodel, R.; Galuska, S.P.; Neumann, S. IgG Fc N-glycosylation: Alterations in neurologic diseases and potential therapeutic target? J. Autoimmun. 2019, 96, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Nie, H.; Sun, X.; Sun, W.; Qu, Y.; Liu, X.; Yao, Y.; Liang, X.; Chen, C.C.; Li, Y. Human serum N-glycan profiles are age and sex dependent. Age Ageing 2011, 40, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Deriš, H.; Tominac, P.; Vučković, F.; Briški, N.; Astrup, A.; Blaak, E.E.; Lauc, G.; Gudelj, I. Effects of low-calorie and different weight-maintenance diets on IgG glycome composition. Front. Immunol. 2022, 13, 995186. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, C.; Bondt, A.; Harre, U.; Raufer, J.; Pfeifle, R.; Camponeschi, A.; Wuhrer, M.; Seeling, M.; Mårtensson, I.-L.; Nimmerjahn, F.; et al. Estrogen induces St6gal1 expression and increases IgG sialylation in mice and patients with rheumatoid arthritis: A potential explanation for the increased risk of rheumatoid arthritis in postmenopausal women. Arthritis Res. Ther. 2018, 20, 84. [Google Scholar] [CrossRef]
- Deriš, H.; Kifer, D.; Cindrić, A.; Petrović, T.; Cvetko, A.; Trbojević-Akmačić, I.; Kolčić, I.; Polašek, O.; Newson, L.; Spector, T.; et al. Immunoglobulin G glycome composition in transition from premenopause to postmenopause. iScience 2022, 25, 103897. [Google Scholar] [CrossRef]
- Tijardović, M.; Marijančević, D.; Bok, D.; Kifer, D.; Lauc, G.; Gornik, O.; Keser, T. Intense Physical Exercise Induces an Anti-inflammatory Change in IgG N-Glycosylation Profile. Front. Physiol. 2019, 10, 1522. [Google Scholar] [CrossRef]
- Štambuk, J.; Nakić, N.; Vučković, F.; Pučić-Baković, M.; Razdorov, G.; Trbojević-Akmačić, I.; Novokmet, M.; Keser, T.; Vilaj, M.; Štambuk, T.; et al. Global variability of the human IgG glycome. Aging 2020, 12, 15222–15259. [Google Scholar] [CrossRef]
- Greto, V.L.; Cvetko, A.; Štambuk, T.; Dempster, N.J.; Kifer, D.; Deriš, H.; Cindrić, A.; Vučković, F.; Falchi, M.; Gillies, R.S.; et al. Extensive weight loss reduces glycan age by altering IgG N-glycosylation. Int. J. Obes. 2021, 45, 1521–1531. [Google Scholar] [CrossRef]
- Nikolac Perkovic, M.; Pucic Bakovic, M.; Kristic, J.; Novokmet, M.; Huffman, J.E.; Vitart, V.; Hayward, C.; Rudan, I.; Wilson, J.F.; Campbell, H.; et al. The association between galactosylation of immunoglobulin G and body mass index. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 20–25. [Google Scholar] [CrossRef]
- Russell, A.C.; Kepka, A.; Trbojević-Akmačić, I.; Ugrina, I.; Song, M.; Hui, J.; Hunter, M.; Laws, S.M.; Lauc, G.; Wang, W. Increased central adiposity is associated with pro-inflammatory immunoglobulin G N-glycans. Immunobiology 2019, 224, 110–115. [Google Scholar] [CrossRef]
- Liu, D.; Li, Q.; Dong, J.; Li, D.; Xu, X.; Xing, W.; Zhang, X.; Cao, W.; Hou, H.; Wang, H.; et al. The Association Between Normal BMI With Central Adiposity And Proinflammatory Potential Immunoglobulin G N-Glycosylation. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2373–2385. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Huang, Z.; Fu, Y.; Fei, J.; Liu, X.; He, Z. Associations between the serum levels of PFOS/PFOA and IgG N-glycosylation in adult or children. Environ. Pollut. 2020, 265, 114285. [Google Scholar] [CrossRef] [PubMed]
- Gudelj, I.; Lauc, G.; Pezer, M. Immunoglobulin G glycosylation in aging and diseases. Cell. Immunol. 2018, 333, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Flevaris, K.; Kontoravdi, C. Immunoglobulin G N-glycan Biomarkers for Autoimmune Diseases: Current State and a Glycoinformatics Perspective. Int. J. Mol. Sci. 2022, 23, 5180. [Google Scholar] [CrossRef]
- Haslund-Gourley, B.S.; Grauzam, S.; Mehta, A.S.; Wigdahl, B.; Comunale, M.A. Acute lyme disease IgG N-linked glycans contrast the canonical inflammatory signature. Front. Immunol. 2022, 13, 949118. [Google Scholar] [CrossRef]
- Kljaković-Gašpić Batinjan, M.; Petrović, T.; Vučković, F.; Hadžibegović, I.; Radovani, B.; Jurin, I.; Đerek, L.; Huljev, E.; Markotić, A.; Lukšić, I.; et al. Differences in Immunoglobulin G Glycosylation Between Influenza and COVID-19 Patients. Engineering 2022, in press. [Google Scholar] [CrossRef]
- van Erp, E.A.; Lakerveld, A.J.; de Graaf, E.; Larsen, M.D.; Schepp, R.M.; Hipgrave Ederveen, A.L.; Ahout, I.M.; de Haan, C.A.; Wuhrer, M.; Luytjes, W.; et al. Natural killer cell activation by respiratory syncytial virus-specific antibodies is decreased in infants with severe respiratory infections and correlates with Fc-glycosylation. Clin. Transl. Immunol. 2020, 9, e1112. [Google Scholar]
- Ash, M.K.; Bhimalli, P.P.; Cho, B.-K.; Mattamana, B.B.; Gambut, S.; Tarhoni, I.; Fhied, C.L.; Reyes, A.F.; Welninski, S.J.; Arivalagan, J.; et al. Bulk IgG Glycosylation Predicts COVID-19 Severity and Vaccine Antibody Response. Cell Rep. 2022, 41, 111799. [Google Scholar] [CrossRef]
- Pongracz, T.; Nouta, J.; Wang, W.; van Meijgaarden, K.E.; Linty, F.; Vidarsson, G.; Joosten, S.A.; Ottenhoff, T.H.M.; Hokke, C.H.; de Vries, J.J.C.; et al. Immunoglobulin G1 Fc glycosylation as an early hallmark of severe COVID-19. EBioMedicine 2022, 78, 103957. [Google Scholar] [CrossRef]
- Hoepel, W.; Chen, H.-J.; Geyer, C.E.; Allahverdiyeva, S.; Manz, X.D.; De Taeye, S.W.; Aman, J.; Mes, L.; Steenhuis, M.; Griffith, G.R.; et al. High titers and low fucosylation of early human anti–SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci. Transl. Med. 2021, 13, eabf8654. [Google Scholar] [CrossRef] [PubMed]
- Petrović, T.; Vijay, A.; Vučković, F.; Trbojević-Akmačić, I.; Ollivere, B.J.; Marjanović, D.; Bego, T.; Prnjavorac, B.; Đerek, L.; Markotić, A.; et al. IgG N-glycome changes during the course of severe COVID-19: An observational study. eBioMedicine 2022, 81, 104101. [Google Scholar] [CrossRef]
- Vicente, M.M.; Alves, I.; Gaifem, J.; Rodrigues, C.S.; Fernandes, Â.; Dias, A.M.; Štambuk, J.; Petrović, T.; Oliveira, P.; Ferreira-Da-Silva, F.; et al. Altered IgG glycosylation at COVID-19 diagnosis predicts disease severity. Eur. J. Immunol. 2022, 52, 946–957. [Google Scholar] [CrossRef]
- Wang, T.T.; Sewatanon, J.; Memoli, M.J.; Wrammert, J.; Bournazos, S.; Bhaumik, S.K.; Pinsky, B.A.; Chokephaibulkit, K.; Onlamoon, N.; Pattanapanyasat, K.; et al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 2017, 355, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Thulin, N.K.; Brewer, R.C.; Sherwood, R.; Bournazos, S.; Edwards, K.G.; Ramadoss, N.S.; Taubenberger, J.K.; Memoli, M.; Gentles, A.J.; Jagannathan, P.; et al. Maternal Anti-Dengue IgG Fucosylation Predicts Susceptibility to Dengue Disease in Infants. Cell Rep. 2020, 31, 107642. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Vo, H.T.M.; Duong, V.; Auerswald, H.; Ly, S.; Sakuntabhai, A.; Dussart, P.; Cantaert, T.; Ravetch, J.V. Antibody fucosylation predicts disease severity in secondary dengue infection. Science 2021, 372, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Fokkink, W.J.; Selman, M.H.; Dortland, J.R.; Durmuş, B.; Kuitwaard, K.; Huizinga, R.; van Rijs, W.; Tio-Gillen, A.P.; van Doorn, P.A.; Deelder, A.M.; et al. IgG Fc N-glycosylation in Guillain-Barré syndrome treated with immunoglobulins. J. Proteome Res. 2014, 13, 1722–1730. [Google Scholar] [CrossRef]
- Lauc, G.; Huffman, J.E.; Pučić, M.; Zgaga, L.; Adamczyk, B.; Mužinić, A.; Novokmet, M.; Polašek, O.; Gornik, O.; Krištić, J.; et al. Loci Associated with N-Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers. PLoS Genet. 2013, 9, e1003225. [Google Scholar] [CrossRef]
- Šimurina, M.; de Haan, N.; Vučković, F.; Kennedy, N.A.; Štambuk, J.; Falck, D.; Trbojević-Akmačić, I.; Clerc, F.; Razdorov, G.; Khon, A.; et al. Glycosylation of Immunoglobulin G Associates With Clinical Features of Inflammatory Bowel Diseases. Gastroenterology 2018, 154, 1320–1333.e1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, X.; Huang, C.; Gao, C. N-glycan profiling alterations of serum and immunoglobulin G in immune thrombocytopenia. J. Clin. Lab. Anal. 2022, 36, e24201. [Google Scholar] [CrossRef]
- Heyder, T.; Wiklundh, E.; Eklund, A.; James, A.; Dahlén, S.-E.; Grunewald, J.; Zubarev, R.A.; Lundström, S.L. Altered Fc galactosylation in IgG4 is a potential serum marker for chronic lung disease. ERJ Open Res. 2018, 4, 00033–02018. [Google Scholar] [CrossRef]
- Chinello, C.; de Haan, N.; Capitoli, G.; Trezzi, B.; Radice, A.; Pagani, L.; Criscuolo, L.; Signorini, S.; Galimberti, S.; Sinico, R.A.; et al. Definition of IgG Subclass-Specific Glycopatterns in Idiopathic Membranous Nephropathy: Aberrant IgG Glycoforms in Blood. Int. J. Mol. Sci. 2022, 23, 4664. [Google Scholar] [CrossRef]
- Haddad, G.; Lorenzen, J.M.; Ma, H.; De Haan, N.; Seeger, H.; Zaghrini, C.; Brandt, S.; Kölling, M.; Wegmann, U.; Kiss, B.; et al. Altered glycosylation of IgG4 promotes lectin complement pathway activation in anti-PLA2R1–associated membranous nephropathy. J. Clin. Investig. 2021, 131, e140453. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-C.; Chang, M.-C.; Chen, C.-H.; Tsai, I.L.; Wang, S.-Y.; Kuo, Y.-P.; Chen, C.-H.; Chang, Y.-T. High accuracy differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma by immunoglobulin G glycosylation. Clin. Proteom. 2019, 16, 1. [Google Scholar] [CrossRef]
- Iwamura, H.; Mizuno, K.; Akamatsu, S.; Hatakeyama, S.; Tobisawa, Y.; Narita, S.; Narita, T.; Yamashita, S.; Kawamura, S.; Sakurai, T.; et al. Machine learning diagnosis by immunoglobulin N-glycan signatures for precision diagnosis of urological diseases. Cancer Sci. 2022, 113, 2434–2445. [Google Scholar] [CrossRef]
- Gebrehiwot, A.G.; Melka, D.S.; Kassaye, Y.M.; Gemechu, T.; Lako, W.; Hinou, H.; Nishimura, S.-I. Exploring serum and immunoglobulin G N-glycome as diagnostic biomarkers for early detection of breast cancer in Ethiopian women. BMC Cancer 2019, 19, 588. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Y.; Wang, X.; Yan, B.; Lou, W.; Di, W. Serum immunoglobulin G N-glycome: A potential biomarker in endometrial cancer. Ann. Transl. Med. 2020, 8, 748. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, S.C.; Kim, H.J.; Ju, W.; Kim, Y.H.; Kim, H.-J. A lectin-based diagnostic system using circulating antibodies to detect cervical intraepithelial neoplasia and cervical cancer. Glycobiology 2015, 26, 100–107. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.; Liu, P.; Kang, L.; Xu, X. Diagnostic Potential of Plasma IgG N-glycans in Discriminating Thyroid Cancer from Benign Thyroid Nodules and Healthy Controls. Front. Oncol. 2021, 11, 658223. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Duan, B.; Sha, J.; Zhang, R.; Fan, J.; Xu, X.; Zhao, H.; Niu, X.; Geng, Z.; Gu, J.; et al. Serum IgG N-glycans enable early detection and early relapse prediction of colorectal cancer. Int. J. Cancer 2022, 152, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Mittermayr, S.; Lê, G.N.; Clarke, C.; Millán Martín, S.; Larkin, A.-M.; O’Gorman, P.; Bones, J. Polyclonal Immunoglobulin G N-Glycosylation in the Pathogenesis of Plasma Cell Disorders. J. Proteome Res. 2017, 16, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Zhong, A.; Qin, R.; Qin, W.; Han, J.; Gu, Y.; Zhou, L.; Zhang, H.; Ren, S.; Lu, R.; Guo, L.; et al. Diagnostic Significance of Serum IgG Galactosylation in CA19-9-Negative Pancreatic Carcinoma Patients. Front. Oncol. 2019, 9, 114. [Google Scholar] [CrossRef]
- Lu, L.L.; Chung, A.W.; Rosebrock, T.R.; Ghebremichael, M.; Yu, W.H.; Grace, P.S.; Schoen, M.K.; Tafesse, F.; Martin, C.; Leung, V.; et al. A Functional Role for Antibodies in Tuberculosis. Cell 2016, 167, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.S.; Dolatshahi, S.; Lu, L.L.; Cain, A.; Palmieri, F.; Petrone, L.; Fortune, S.M.; Ottenhoff, T.H.M.; Lauffenburger, D.A.; Goletti, D.; et al. Antibody Subclass and Glycosylation Shift Following Effective TB Treatment. Front. Immunol. 2021, 12, 679973. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ren, S.; Xie, Y.; Liu, C.; Qin, W.; Zhou, Y.; Zhang, M.; Yang, Q.; Chen, X.-C.; Liu, T.; et al. Quantitative analysis of serum-based IgG agalactosylation for tuberculosis auxiliary diagnosis. Glycobiology 2020, 30, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Vadrevu, S.K.; Trbojevic-Akmacic, I.; Kossenkov, A.V.; Colomb, F.; Giron, L.B.; Anzurez, A.; Lynn, K.; Mounzer, K.; Landay, A.L.; Kaplan, R.C.; et al. Frontline Science: Plasma and immunoglobulin G galactosylation associate with HIV persistence during antiretroviral therapy. J. Leukoc. Biol. 2018, 104, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Giron, L.B.; Papasavvas, E.; Azzoni, L.; Yin, X.; Anzurez, A.; Damra, M.; Mounzer, K.; Kostman, J.R.; Sanne, I.; Firnhaber, C.S.; et al. Plasma and antibody glycomic biomarkers of time to HIV rebound and viral setpoint. Aids 2020, 34, 681–686. [Google Scholar] [CrossRef]
- Muenchhoff, M.; Chung, A.W.; Roider, J.; Dugast, A.-S.; Richardson, S.; Kløverpris, H.; Leslie, A.; Ndung’U, T.; Moore, P.; Alter, G.; et al. Distinct Immunoglobulin Fc Glycosylation Patterns Are Associated with Disease Nonprogression and Broadly Neutralizing Antibody Responses in Children with HIV Infection. mSphere 2020, 5, e00880-20. [Google Scholar] [CrossRef]
- Ho, C.-H.; Chien, R.-N.; Cheng, P.-N.; Liu, J.-H.; Liu, C.-K.; Su, C.-S.; Wu, I.C.; Li, I.C.; Tsai, H.-W.; Wu, S.-L.; et al. Aberrant Serum Immunoglobulin G Glycosylation in Chronic Hepatitis B Is Associated With Histological Liver Damage and Reversible by Antiviral Therapy. J. Infect. Dis. 2015, 211, 115–124. [Google Scholar] [CrossRef]
- Edri-Brami, M.; Rosental, B.; Hayoun, D.; Welt, M.; Rosen, H.; Wirguin, I.; Nefussy, B.; Drory, V.E.; Porgador, A.; Lichtenstein, R.G. Glycans in Sera of Amyotrophic Lateral Sclerosis Patients and Their Role in Killing Neuronal Cells. PLoS ONE 2012, 7, e35772. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Streich, L.; Pinto, S.; Pronto-Laborinho, A.; Nimtz, M.; Conradt, H.S.; De Carvalho, M. Exploring Cerebrospinal Fluid IgG N-Glycosylation as Potential Biomarker for Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2019, 56, 5729–5739. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.C.; Šimurina, M.; Garcia, M.T.; Novokmet, M.; Wang, Y.; Rudan, I.; Campbell, H.; Lauc, G.; Thomas, M.G.; Wang, W. The N-glycosylation of immunoglobulin G as a novel biomarker of Parkinson's disease. Glycobiology 2017, 27, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Wang, M.; Grauzam, S.; Pippin, S.; Black, A.; Angel, P.M.; Drake, R.R.; Castellino, S.; Kono, Y.; Rockey, D.C.; et al. GlycoFibroTyper: A Novel Method for the Glycan Analysis of IgG and the Development of a Biomarker Signature of Liver Fibrosis. Front. Immunol. 2022, 13, 797460. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.D.; Lopez-Perez, M.; Dickson, E.K.; Ampomah, P.; Tuikue Ndam, N.; Nouta, J.; Koeleman, C.A.M.; Ederveen, A.L.H.; Mordmüller, B.; Salanti, A.; et al. Afucosylated Plasmodium falciparum-specific IgG is induced by infection but not by subunit vaccination. Nat. Commun. 2021, 12, 5838. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Strauss, E.G. CHAPTER 1—Overview of Viruses and Virus Infection. In Viruses and Human Disease, 2nd ed.; Strauss, J.H., Strauss, E.G., Eds.; Academic Press: London, UK, 2008; pp. 1–33. [Google Scholar]
- Louten, J. Chapter 7—Detection and Diagnosis of Viral Infections. In Essential Human Virology; Louten, J., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 111–132. [Google Scholar]
- Yam-Puc, J.C.; Cedillo-Barrón, L.; Aguilar-Medina, E.M.; Ramos-Payán, R.; Escobar-Gutiérrez, A.; Flores-Romo, L. The Cellular Bases of Antibody Responses during Dengue Virus Infection. Front. Immunol. 2016, 7, 218. [Google Scholar] [CrossRef]
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 020421. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O'Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Jha, P.; Brown, P.E.; Ansumana, R. Counting the global COVID-19 dead. Lancet 2022, 399, 1937–1938. [Google Scholar] [CrossRef]
- Peeling, R.W.; Heymann, D.L.; Teo, Y.Y.; Garcia, P.J. Diagnostics for COVID-19: Moving from pandemic response to control. Lancet 2022, 399, 757–768. [Google Scholar] [CrossRef]
- Ackerman, M.E.; Crispin, M.; Yu, X.; Baruah, K.; Boesch, A.W.; Harvey, D.J.; Dugast, A.-S.; Heizen, E.L.; Ercan, A.; Choi, I.; et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Investig. 2013, 123, 2183–2192. [Google Scholar] [CrossRef]
- Freeland, C.; Racho, R.; Kamischke, M.; Moraras, K.; Wang, E.; Cohen, C.; Kendrick, S. Health-related quality of life for adults living with hepatitis B in the United States: A qualitative assessment. J. Patient Rep. Outcomes 2021, 5, 121. [Google Scholar] [CrossRef]
- Callewaert, N.; Vlierberghe, H.V.; Hecke, A.V.; Laroy, W.; Delanghe, J.; Contreras, R. Noninvasive diagnosis of liver cirrhosis using DNA sequencer–based total serum protein glycomics. Nat. Med. 2004, 10, 429–434. [Google Scholar] [CrossRef]
- Mehta, A.S.; Long, R.E.; Comunale, M.A.; Wang, M.; Rodemich, L.; Krakover, J.; Philip, R.; Marrero, J.A.; Dwek, R.A.; Block, T.M. Increased Levels of Galactose-Deficient Anti-Gal Immunoglobulin G in the Sera of Hepatitis C Virus-Infected Individuals with Fibrosis and Cirrhosis. J. Virol. 2008, 82, 1259–1270. [Google Scholar] [CrossRef]
- Gerace, E.; Mancuso, G.; Midiri, A.; Poidomani, S.; Zummo, S.; Biondo, C. Recent Advances in the Use of Molecular Methods for the Diagnosis of Bacterial Infections. Pathogens 2022, 11, 663. [Google Scholar] [CrossRef]
- Zumla, A.; Raviglione, M.; Hafner, R.; Fordham von Reyn, C. Tuberculosis. N. Engl. J. Med. 2013, 368, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Montero-Martín, M.; Inwald, D.P.; Carrol, E.D.; Martinón-Torres, F. Prognostic markers of meningococcal disease in children: Recent advances and future challenges. Expert Rev. Anti-Infect. Ther. 2014, 12, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- El-Houderi, A.; Constantin, J.; Castelnuovo, E.; Sauboin, C. Economic and Resource Use Associated With Management of Malaria in Children Aged <5 Years in Sub-Saharan Africa: A Systematic Literature Review. MDM Policy Pract. 2019, 4, 2381468319893986. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Motta, F.; Selmi, C.; Ridgway, W.M.; Gershwin, M.E.; Zhang, W. Antibody glycosylation in autoimmune diseases. Autoimmun. Rev. 2021, 20, 102804. [Google Scholar] [CrossRef]
- Leonhard, S.E.; Mandarakas, M.R.; Gondim, F.A.A.; Bateman, K.; Ferreira, M.L.B.; Cornblath, D.R.; van Doorn, P.A.; Dourado, M.E.; Hughes, R.A.C.; Islam, B.; et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat. Rev. Neurol. 2019, 15, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Mimura, Y.; Mimura-Kimura, Y.; Saldova, R.; Rudd, P.M.; Jefferis, R. Enhanced Immunomodulatory Effect of Intravenous Immunoglobulin by Fc Galactosylation and Nonfucosylation. Front. Immunol. 2022, 13, 818382. [Google Scholar] [CrossRef]
- Ameer, M.A.; Chaudhry, H.; Mushtaq, J.; Khan, O.S.; Babar, M.; Hashim, T.; Zeb, S.; Tariq, M.A.; Patlolla, S.R.; Ali, J.; et al. An Overview of Systemic Lupus Erythematosus (SLE) Pathogenesis, Classification, and Management. Cureus 2022, 14, e30330. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Samson, M.; Fraser, W.; Lebowitz, D. Treatments for Primary Immune Thrombocytopenia: A Review. Cureus 2019, 11, e5849. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.E.; De Haan, N.; Sonneveld, M.E.; Porcelijn, L.; Van Der Schoot, C.E.; De Haas, M.; Zwaginga, J.-J.; Wuhrer, M.; Vidarsson, G. IgG-Fc glycosylation before and after rituximab treatment in immune thrombocytopenia. Sci. Rep. 2020, 10, 3051. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Yamamoto, H.; Sutoh Yoneyama, M.; Tobisawa, Y.; Hatakeyama, S.; Narita, T.; Kodama, H.; Momota, M.; Ito, H.; Narita, S.; et al. Characteristics of α2,3-sialyl N-glycosylated PSA as a biomarker for clinically significant prostate cancer in men with elevated PSA level. Prostate 2021, 81, 1411–1427. [Google Scholar] [CrossRef]
- Wasserstein, A.G. Membranous glomerulonephritis. J. Am. Soc. Nephrol. 1997, 8, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Wilde, B.; Van Paassen, P.; Witzke, O.; Tervaert, J.W.C. New pathophysiological insights and treatment of ANCA-associated vasculitis. Kidney Int. 2011, 79, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease. JAMA 2020, 323, 548. [Google Scholar] [CrossRef]
- Cloutier, J.M.; Charville, G.W. Diagnostic classification of soft tissue malignancies: A review and update from a surgical pathology perspective. Curr. Probl. Cancer 2019, 43, 250–272. [Google Scholar] [CrossRef]
- Ginsburg, O.; Yip, C.H.; Brooks, A.; Cabanes, A.; Caleffi, M.; Dunstan Yataco, J.A.; Gyawali, B.; McCormack, V.; McLaughlin de Anderson, M.; Mehrotra, R.; et al. Breast cancer early detection: A phased approach to implementation. Cancer 2020, 126 (Suppl. 10), 2379–2393. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Lee, E.J.; Huang, M.G.; Park, Y.I.; Khullar, A.; Plodkowski, R.A. Diagnosis and treatment of patients with thyroid cancer. Am. Health Drug Benefits 2015, 8, 30–40. [Google Scholar] [PubMed]
- Michor, F.; Iwasa, Y.; Lengauer, C.; Nowak, M.A. Dynamics of colorectal cancer. Semin. Cancer Biol. 2005, 15, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Church, T.R.; Wandell, M.; Lofton-Day, C.; Mongin, S.J.; Burger, M.; Payne, S.R.; Castaños-Vélez, E.; Blumenstein, B.A.; Rösch, T.; Osborn, N.; et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut 2014, 63, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Cai, M.; Feng, Z.; Liu, R.; Liang, L.; Zhou, P.; Zhu, B.; Mo, S.; Wang, H.; Lan, X.; et al. A Multilocus Blood-Based Assay Targeting Circulating Tumor DNA Methylation Enables Early Detection and Early Relapse Prediction of Colorectal Cancer. Gastroenterology 2021, 161, 2053–2056. [Google Scholar] [CrossRef]
- Kim, E.; Voaklander, R.; Kasmin, F.E.; Brown, W.H.; Mannan, R.; Siegel, J.H. Autoimmune Pancreatitis: A Multiorgan Disease Presenting a Conundrum for Clinicians in the West. Gastroenterol. Hepatol. 2015, 11, 606–611. [Google Scholar]
- Li, D.; Lou, Y.; Zhang, Y.; Liu, S.; Li, J.; Tao, J. Sialylated immunoglobulin G: A promising diagnostic and therapeutic strategy for autoimmune diseases. Theranostics 2021, 11, 5430–5446. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, H.; Wu, S.; Yang, X.; Cao, C.; Wang, F.; Jia, J.; Yan, T. Plasma ST6GAL1 regulates IgG sialylation to control IgA nephropathy progression. Ther. Adv. Chronic. Dis. 2021, 12, 1–11. [Google Scholar] [CrossRef]
| General Immune State | Condition, IgG Source | Diagnostic/Prognostic Performance | F | Aga | G | S | B | M/H | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Acute Bacterial Infection | Pediatric meningococcal sepsis | - | ↓ | - | - | ↓ | - | ↑ | [52] |
| Acute Bacterial Infection | Acute Lyme disease (LD) | LD vs healthy control (HC): Sen 75%, Spec 100% | - | ↓ | ↑ | - | - | - | [67] |
| Acute Viral Infection | Influenza | - | ↓ | - | - | ↑ | - | - | [68] |
| Acute Viral Infection | Pediatric RSV infection, ag-specific IgG | - | ↓ | - | - | - | - | - | [69] |
| Acute Viral Infection | Severe COVID-19 | Severe vs mild COVID-19 prognosis: AUC 0.72 | ↓ | ↑ | ↓ | ↓ | ↑/↓ | - | [28,68,70,71,72,73,74] |
| Acute Viral Infection | Dengue virus (DENV) | Fucose predicts infant DENV susceptibility | ↓ | ↑ | - | ↓ | - | - | [75,76,77] |
| Autoimmune | Vasculitis IgG1 patients likely to relapse | Vasculitis relapse vs non-relapse: AUC 0.65 | - | ↑ | ↓ | ↓ | - | - | [22] |
| Autoimmune | Guillain-Barré syndrome | - | - | - | ↓ | ↓ | - | - | [78] |
| Autoimmune | Systemic erythematosus lupus (SLE) | SLE vs HC: Sen 80%, Spec 80%, AUC 0.84 | ↓ | ↑ | ↓ | - | ↑ | - | [79] |
| Autoimmune | Crohn's disease (CD) | CD vs UC: Sen 60%, Spec 80%, AUC 0.75 | ↑ | ↑ | - | - | - | - | [80] |
| Autoimmune | Ulcerative colitis (UC) | UC vs CD: Sen 60%, Spec 80%, AUC 0.75 | ↓ | ↑ | - | - | - | - | [80] |
| Autoimmune | Immune thrombocytopenia (ITP) IgG4 | IMN vs HC: Sen 80%, Spec 100%, AUC 0.96 | ↓ | - | ↓ | - | - | - | [81] |
| Autoimmune | Chronic inflammatory airway disease | Severe asthma/sarcoidosis vs HC: AUC 0.83 | - | ↑ | ↓ | - | - | - | [82] |
| Autoimmune | Idiopathic membranous nephropathy (IMN) | - | ↑ | - | ↓ | - | - | - | [83,84] |
| Autoimmune | Autoimmune pancreatitis (AIP) | AIP vs PDAC: Sen 94% Spec 93% AUC: 0.93 | - | ↓ | - | - | - | - | [85] |
| Cancer | Renal cell carcinoma (RCC) | RCC vs HC, UTI, CRPC: Sen 100%, Spec 100%, AUC 0.99 | - | - | - | ↑ | ↑ | - | [86] |
| Cancer | Castration resistant prostate cancer (CRPC) | CRPC vs HC, UTI, RCC: Sen 90%, Spec 95%, AUC 0.96 | - | - | - | ↑ | - | - | [86] |
| Cancer | Stage II breast cancer | Stage II breast cancer vs HC: AUC 0.92 | - | ↑ | - | - | - | - | [87] |
| Cancer | Endometrial cancer (EC) | EC vs HC: AUC 0.87 | - | - | ↓ | ↓ | - | - | [88] |
| Cancer | Cervical intraepithelial neoplasia I (CIN I) | CIN I vs HC: Sen 73%, Spec 62% | ↓ | - | ↑ | - | - | - | [89] |
| Cancer | Early thyroid cancer (ETC) | ETC vs HC: AUC 0.81 | - | ↑ | ↑ | - | ↑ | - | [90] |
| Cancer | Precancerous advanced colonic adenomas (PACA) | PACA vs HC: Sen 61%, Spec 85%, AUC 0.84 | - | - | ↑ | ↑ | ↑ | - | [91] |
| Cancer | Colorectal cancer (CRC) | CRC vs HC: Sen 72%, Spec 87%, AUC: 0.84 | - | ↑ | ↓ | ↓ | - | - | [91] |
| Cancer | Multiple myeloma (MM) | MM trends to healthy normal in remission | ↓ | ↑ | - | - | - | - | [92] |
| Cancer | Pancreatic ductal adenocarcinoma (PDAC) | PDAC vs HC: AUC 0.91 | - | ↑ | - | - | - | - | [85,93] |
| Cancer | Cervical cancer (CC) | CC vs HC: Sens 87%, Spec 72% | ↓ | - | ↓ | - | - | - | [89] |
| Chronic Bact. Infection | Active tuberculosis (ATB) | ATB vs HC: Sen 76%, Spec 71% | - | ↑ | ↓ | - | - | - | [94,95,96] |
| Chronic Viral Infection | Adult HIV shorter viral rebound | - | - | ↓ | ↑ | - | ↑ | - | [97] |
| Chronic Viral Infection | HIV unsuppressed | - | ↑ | ↑ | - | ↓ | - | - | [98] |
| Chronic Viral Infection | HIV + ART suppression | - | ↑/- | - | ↑ | ↓ | - | - | [98] |
| Chronic Viral Infection | Pediatric HIV + ART | - | ↑/- | ↑ | ↓ | ↑ | ↓ | - | [99] |
| Chronic Viral Infection | Hepatitis B chronic untreated | - | - | ↑ | ↓ | ↓ | - | - | [100] |
| Inflammatory | Urinary tract infection (UTI) | UTI vs HC, RCC, CRPC: Sen 95%, Spec 95%, AUC 0.95 | - | ↑ | ↓ | - | - | - | [86] |
| Inflammatory/Neurodegenerative | Amyotrophic lateral sclerosis (ALS) | - | ↓ | - | - | - | - | - | [101] |
| Inflammatory/Neurodegenerative | Amyotrophic lateral sclerosis (ALS) | ALS vs HC: AUC 0.79 | - | - | ↑ | - | - | - | [102] |
| Inflammatory/Neurodegenerative | Parkinson's disease | Parkinson's disease vs HC: Sen 87%, Spec 92% | - | - | ↑ | - | - | ↓ | [103] |
| Liver Cirrhosis | Severe liver fibrosis | Severe liver fibrosis vs HC: Sen 94%, Spec 90% | - | ↑ | - | - | ↑ | - | [104] |
| Parasitic Infection | Naturally acquired malaria, Ag-specific IgG | - | ↓ | - | - | - | - | - | [105] |
| Pre-Cancer | Smoldering myeloma (SMM) | - | - | - | ↑ | ↑ | - | - | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haslund-Gourley, B.S.; Wigdahl, B.; Comunale, M.A. IgG N-glycan Signatures as Potential Diagnostic and Prognostic Biomarkers. Diagnostics 2023, 13, 1016. https://doi.org/10.3390/diagnostics13061016
Haslund-Gourley BS, Wigdahl B, Comunale MA. IgG N-glycan Signatures as Potential Diagnostic and Prognostic Biomarkers. Diagnostics. 2023; 13(6):1016. https://doi.org/10.3390/diagnostics13061016
Chicago/Turabian StyleHaslund-Gourley, Benjamin S., Brian Wigdahl, and Mary Ann Comunale. 2023. "IgG N-glycan Signatures as Potential Diagnostic and Prognostic Biomarkers" Diagnostics 13, no. 6: 1016. https://doi.org/10.3390/diagnostics13061016
APA StyleHaslund-Gourley, B. S., Wigdahl, B., & Comunale, M. A. (2023). IgG N-glycan Signatures as Potential Diagnostic and Prognostic Biomarkers. Diagnostics, 13(6), 1016. https://doi.org/10.3390/diagnostics13061016








