Precision Medicine for Chronic Endometritis: Computer-Aided Diagnosis Using Deep Learning Model
Abstract
1. Introduction
2. Unsolved Questions on Histopathologic CE
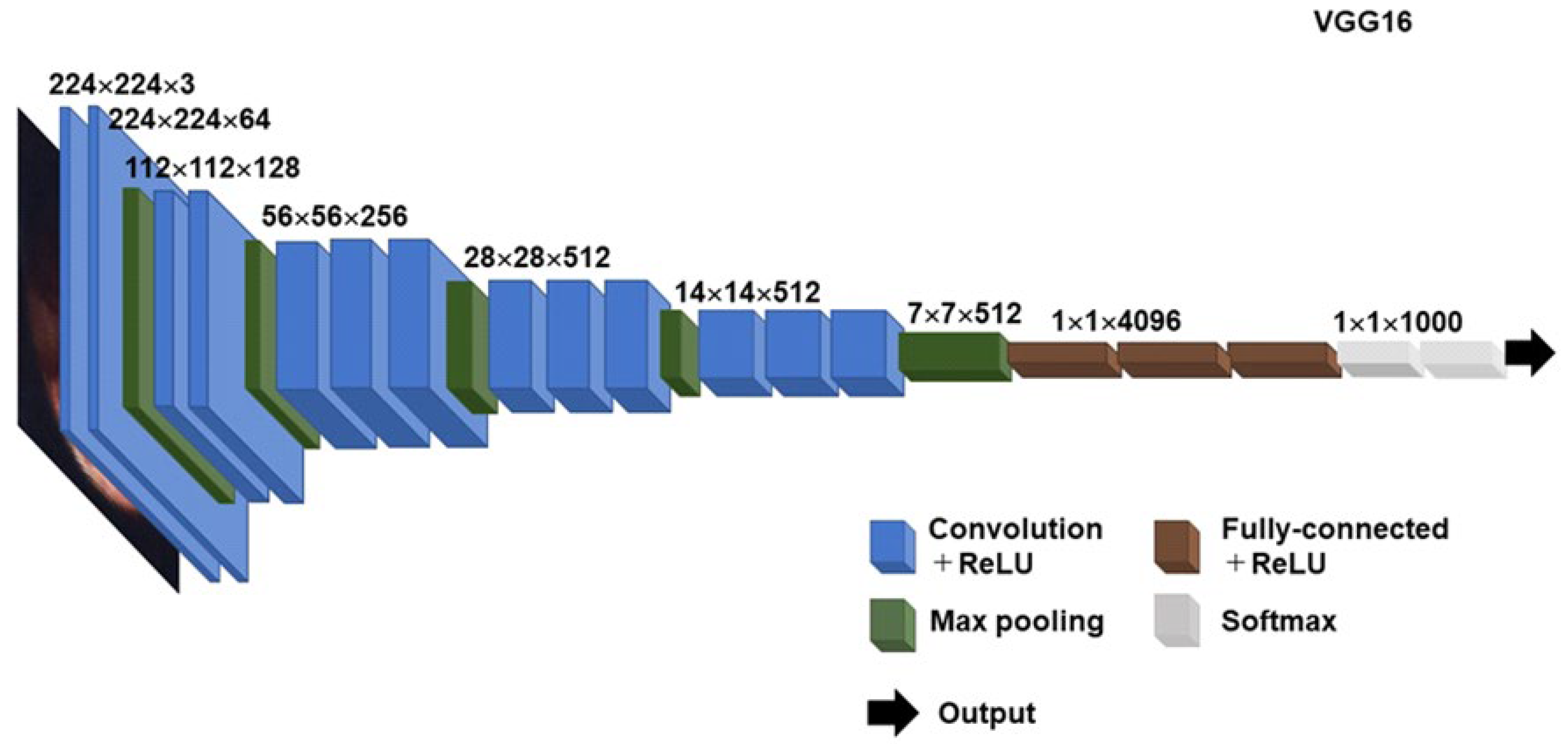
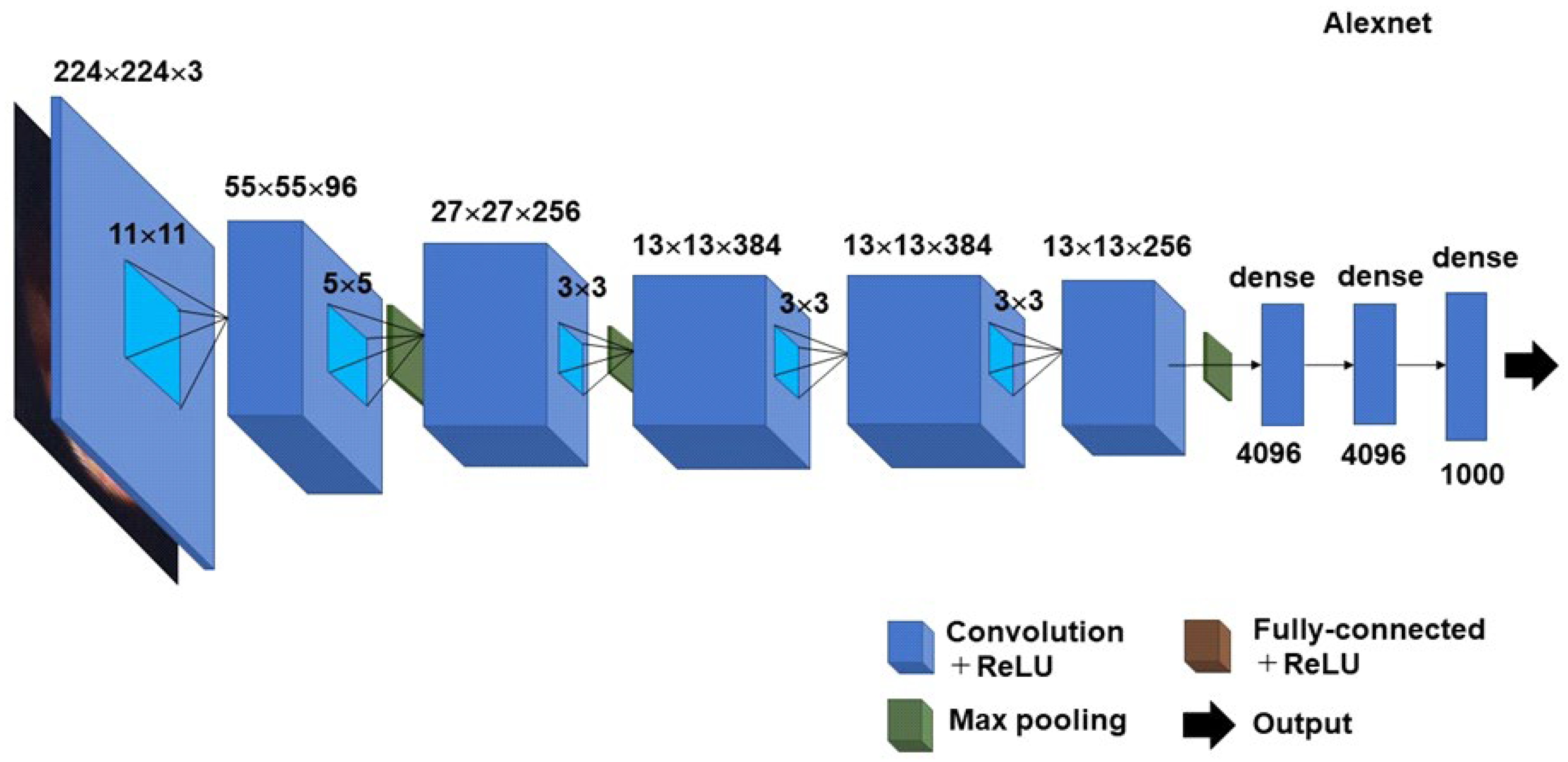
3. CADx Using Deep Learning Model for Unsolved Questions on Histopathologic CE
4. Unsolved Questions on Hysteroscopic CE
- (1)
- strawberry aspect: localized/scattered large hyperemic endometrial areas flushed with white central points [48],
- (2)
- focal hyperemia,
- (3)
- hemorrhagic spots: focal lurid endometrium with sharp and irregular borders possibly in continuity with capillary,
- (4)
- micropolyposis: a cluster of typically less than 1 mm-sized protrusions on the focal or entire surface with a distinct connective vascular axis [49],
- (5)
- stromal edema: the thick and pale appearance of the endometrium in the proliferative phase originating from the stroma (a nonpathologic finding that is observed during the secretory phase).
5. CADx Using Deep Learning Model for Unsolved Questions on Hysteroscopic CE
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cicinelli, E.; De Ziegler, D.; Nicoletti, R.; Colafiglio, G.; Saliani, N.; Resta, L.; Rizzi, D.; De Vito, D. Chronic Endometritis: Correlation among Hysteroscopic, Histologic, and Bacteriologic Findings in a Prospective Trial with 2190 Consecutive Office Hysteroscopies. Fertil. Steril. 2008, 89, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Cicinelli, E.; Garcia-Grau, I.; Gonzalez-Monfort, M.; Bau, D.; Vilella, F.; De Ziegler, D.; Resta, L.; Valbuena, D.; Simon, C. The Diagnosis of Chronic Endometritis in Infertile Asymptomatic Women: A Comparative Study of Histology, Microbial Cultures, Hysteroscopy, and Molecular Microbiology. Am. J. Obstet. Gynecol. 2018, 218, 602.e1–602.e16. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Takeuchi, T.; Mizuta, S.; Matsubayashi, H.; Ishikawa, T. Endometritis: New Time, New Concepts. Fertil. Steril. 2018, 110, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Johnston-MacAnanny, E.B.; Hartnett, J.; Engmann, L.L.; Nulsen, J.C.; Sanders, M.M.; Benadiva, C.A. Chronic Endometritis Is a Frequent Finding in Women with Recurrent Implantation Failure after in vitro Fertilization. Fertil. Steril. 2010, 93, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Yasuo, T. Aberrant Expression of Selectin E, CXCL1, and CXCL13 in Chronic Endometritis. Mod. Pathol. 2010, 23, 1136–1146. [Google Scholar] [CrossRef]
- Takebayashi, A.; Kimura, F.; Kishi, Y.; Ishida, M.; Takahashi, A.; Yamanaka, A.; Takahashi, K.; Suginami, H.; Murakami, T. The Association between Endometriosis and Chronic Endometritis. PLoS One 2014, 9, e88354. [Google Scholar] [CrossRef]
- Kitaya, K. Prevalence of Chronic Endometritis in Recurrent Miscarriages. Fertil. Steril. 2011, 95, 1156–1158. [Google Scholar] [CrossRef]
- Morimune, A.; Kimura, F.; Moritani, S.; Tsuji, S.; Katusra, D.; Hoshiyama, T.; Nakamura, A.; Kitazawa, J.; Hanada, T.; Amano, T.; et al. The Association between Chronic Deciduitis and Preeclampsia. J. Reprod. Immunol. 2022, 150, 103474. [Google Scholar] [CrossRef]
- Salafia, C.; Ernst, L.; Pezzullo, J.; Wolf, E.; Rosenkrantz, T.; Vintzileos, A. The Very Low Birthweight Infant: Maternal Complications Leading to Preterm Birth, Placental Lesions, and Intrauterine Growth. Am. J. Perinatol. 1995, 12, 106–110. [Google Scholar] [CrossRef]
- Maleki, Z.; Bailis, A.J.; Argani, C.H.; Askin, F.B.; Graham, E.M. Periventricular Leukomalacia and Placental Histopathologic Abnormalities. Obstet. Gynecol. 2009, 114, 1115–1120. [Google Scholar] [CrossRef]
- Ghidini, A.; Salafia, C.M. Histologic Placental Lesions in Women with Recurrent Preterm Delivery. Acta Obstet. Gynecol. Scand. 2005, 84, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R. Syndecan-1 (CD138), Carcinomas and EMT. Int. J. Mol. Sci. 2021, 22, 4227. [Google Scholar] [CrossRef] [PubMed]
- Toson, B.; Simon, C.; Moreno, I. The Endometrial Microbiome and Its Impact on Human Conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, A.; Laganà, A.S.; De Ziegler, D.; Cicinelli, R.; Santarsiero, C.M.; Buzzaccarini, G.; Chiantera, V.; Cicinelli, E.; Marinaccio, M. Chronic Endometritis in Infertile Women: Impact of Untreated Disease, Plasma Cell Count and Antibiotic Therapy on IVF Outcome-A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2250. [Google Scholar] [CrossRef]
- Barberis, E.; Khoso, S.; Sica, A.; Falasca, M.; Gennari, A.; Dondero, F.; Afantitis, A.; Manfredi, M. Precision Medicine Approaches with Metabolomics and Artificial Intelligence. Int. J. Mol. Sci. 2022, 23, 11269. [Google Scholar] [CrossRef]
- Blatter, T.U.; Witte, H.; Nakas, C.T.; Leichtle, A.B. Big Data in Laboratory Medicine—FAIR Quality for AI? Diagnostics 2022, 12, 1923. [Google Scholar] [CrossRef]
- Niazi, S.K. Molecular Biosimilarity-an AI-Driven Paradigm Shift. Int. J. Mol. Sci. 2022, 23, 10690. [Google Scholar] [CrossRef]
- Greenwood, S.M.; Moran, J.J. Chronic Endometritis: Morphologic and Clinical Observations. Obstet. Gynecol. 1981, 58, 176–184. [Google Scholar]
- Crum, C.P.; Egawa, K.; Fenoglio, C.M.; Richart, R.M. Chronic Endometritis: The Role of Immunohistochemistry in the Detection of Plasma Cells. Am. J. Obstet. Gynecol. 1983, 147, 812–815. [Google Scholar] [CrossRef]
- Bayer-Garner, I.B.; Nickell, J.A.; Korourian, S. Routine Syndecan-1 Immunohistochemistry Aids in the Diagnosis of Chronic Endometritis. Arch. Pathol. Lab. Med. 2004, 128, 1000–1003. [Google Scholar] [CrossRef]
- Vicetti Miguel, R.D.; Chivukula, M.; Krishnamurti, U.; Amortegui, A.J.; Kant, J.A.; Sweet, R.L.; Wiesenfeld, H.C.; Phillips, J.M.; Cherpes, T.L. Limitations of the Criteria Used to Diagnose Histologic Endometritis in Epidemiologic Pelvic Inflammatory Disease Research. Pathol. Res. Pract. 2011, 207, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Yasuo, T. Inter-observer and Intra-observer Variability in Immunohistochemical Detection of Endometrial Stromal Plasmacytes in Chronic Endometritis. Exp. Ther. Med. 2013, 5, 485–488. [Google Scholar] [CrossRef] [PubMed]
- McQueen, D.B.; Bernardi, L.A.; Stephenson, M.D. Chronic Endometritis in Women with Recurrent Early Pregnancy Loss and/or Fetal Demise. Fertil. Steril. 2014, 101, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Resta, L.; Loizzi, V.; Pinto, V.; Santarsiero, C.; Cicinelli, R.; Greco, P.; Vitagliano, A. Antibiotic Therapy versus No Treatment for Chronic Endometritis: A Case-control Study. Fertil. Steril. 2021, 115, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, Q.; Chen, C.; Tan, J.; Wang, Z.; Gu, F.; Xu, Y. Impact of Oral Antibiotic Treatment for Chronic Endometritis on Pregnancy Outcomes in the Following Frozen-thawed Embryo Transfer Cycles of Infertile Women: A Cohort Study of 640 Embryo Transfer Cycles. Fertil. Steril. 2021, 116, 413–442. [Google Scholar] [CrossRef]
- Song, D.; He, Y.; Wang, Y.; Liu, Z.; Xia, E.; Huang, X.; Xiao, Y.; Li, T.-C. Impact of Antibiotic Therapy on the Rate of Negative Test Results for Chronic Endometritis: A Prospective Randomized Control Trial. Fertil. Steril. 2021, 115, 1549–1556. [Google Scholar] [CrossRef]
- Kitaya, K.; Matsubayashi, H.; Takaya, Y.; Nishiyama, R.; Yamaguchi, K.; Takeuchi, T.; Ishikawa, T. Live Birth Rate Following Oral Antibiotic Treatment for Chronic Endometritis in Infertile Women with Repeated Implantation Failure. Am. J. Reprod. Immunol. 2017, 78, e12719. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, Z.; Xiao, Z.; Bai, Y. Does Antibiotic Therapy for Chronic Endometritis Improve Clinical Outcomes of Patients with Recurrent Implantation Failure in Subsequent IVF Cycles? A Systematic Review and Meta-analysis. J. Assist. Reprod. Genet. 2022, 39, 1797–1813. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Lepera, A.; Alfonso, R.; Indraccolo, U.; Marrocchella, S.; Greco, P.; Resta, L. Prevalence of Chronic Endometritis in Repeated Unexplained Implantation Failure and the IVF Success Rate after Antibiotic Therapy. Hum. Reprod. 2015, 30, 323–330. [Google Scholar] [CrossRef]
- Kitaya, K.; Tanaka, S.E.; Sakuraba, Y.; Ishikawa, T. Multi-drug-resistant Chronic Endometritis in Infertile Women with Repeated Implantation Failure: Trend over the Decade and Pilot Study for Third-line Oral Antibiotic Treatment. J. Assist. Reprod. Genet. 2022, 39, 1839–1848. [Google Scholar] [CrossRef]
- Kitaya, K.; Ishikawa, T. Lincomycin Administration against Persistent Multi-Drug Resistant Chronic Endometritis in Infertile Women with a History of Repeated Implantation Failure. Appl. Microbiol. 2022, 2, 554–560. [Google Scholar] [CrossRef]
- Kasius, J.C.; Fatemi, H.M.; Bourgain, C.; Sie-Go, D.M.D.S.; Eijkemans, R.J.C.; Fauser, B.C.; Devroey, P.; Broekmans, F.J.M. The Impact of Chronic Endometritis on Reproductive Outcome. Fertil. Steril. 2011, 96, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Yasuo, T. Immunohistochemistrical and Clinicopathological Characterization of Chronic Endometritis. Am. J. Reprod. Immunol. 2011, 66, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wu, M.; He, R.; Ye, Y.; Sun, X. Administration of Growth Hormone Improves Endometrial Function in Women Undergoing in Vitro Fertilization: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2022, 28, 838–857. [Google Scholar] [CrossRef]
- Xue, X.; Li, X.; Yao, J.; Zhang, X.; Ren, X.; Xu, S. Transient and Prolonged Activation of Wnt Signaling Contribute Oppositely to the Pathogenesis of Asherman’s Syndrome. Int. J. Mol. Sci. 2022, 23, 8808. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Liu, N.; Li, Y.; Yao, Z.; Tian, F.; Xu, A.; Li, Y. An Endometrial Thickness < 8 mm Was Associated with a Significantly Increased Risk of EP after Freeze-Thaw Transfer: An Analysis of 5960 Pregnancy Cycles. Front. Endocrinol. 2022, 13, 884553. [Google Scholar]
- Hessami, K.; Salmanian, B.; Einerson, B.D.; Carusi, D.A.; Shamshirsaz, A.A.; Shainker, S.A.; Subramaniam, A.; Shrivastava, V.K.; Nieto-Calvache, A.J.; Gilner, J.B.; et al. Clinical Correlates of Placenta Accreta Spectrum Disorder Depending on the Presence or Absence of Placenta Previa: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2022. [CrossRef]
- Zhang, Q.; Meng, Z.; Zhao, A.; Su, F. GSLD: A Global Scanner with Local Discriminator Network for Fast Detection of Sparse Plasma Cell in Immunohistochemistry. IEEE Conf. Proc. 2021, 2021, 86–90. [Google Scholar]
- Li, Q.; Li, J.; Yang, K.; Peng, Y.; Xiang, Y.; Sun, S.; Zeng, J.; Zhang, X.; Wang, J. EBV-Positive Intravascular Large B-Cell Lymphoma of the Liver: A Case Report and Literature Review. Diagn. Pathol. 2020, 15, 72. [Google Scholar] [CrossRef]
- Parks, R.N.; Kim, C.J.; Al-Safi, Z.A.; Armstrong, A.A.; Zore, T.; Noatamed, N.A. Multiple Myeloma 1 Transcription Factor Is Superior to CD138 as a Marker of Plasma Cells in Endometrium. Int. J. Surg. Pathol. 2019, 27, 372–379. [Google Scholar] [CrossRef]
- Cicinelli, E.; Haimovich, S.; De Ziegler, D.; Raz, N.; Ben-Tzur, D.; Andrisani, A.; Ambrosini, G.; Picardi, N.; Cataldo, V.; Balzani, M.; et al. MUM-1 Immunohistochemistry Has High Accuracy and Reliability in the Diagnosis of Chronic Endometritis: A Multi-Centre Comparative Study with CD-138 Immunostaining. J. Assist. Reprod. Genet. 2022, 39, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Xiong, Z.; Zhang, W.; Liu, S.; Liu, K.; Wang, J.; Qin, P.; Liu, Y. The Combination of CD138/MUM1 Dual-staining and Artificial Intelligence for Plasma Cell Counting in the Diagnosis of Chronic Endometritis. Am. J. Reprod. Immunol. 2023, 89, e13671. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-level Histograms. IEEE. Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost A Scalable Tree Boosting System. In Proceedings of the 22nd ACM Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Zheng, G.; Liu, S.; Feng, W.; Li, Z.; Sun, J. YOLOX: Exceeding YOLO Series in 2021. arXiv 2021, arXiv:2107.08430. [Google Scholar]
- Cicinelli, E.; Vitagliano, A.; Kumar, A.; Lasmar, R.B.; Bettocchi, S.; Haimovich, S.; International Working Group for Standardization of Chronic Endometritis Diagnosis. Unified Diagnostic Criteria for Chronic Endometritis at Fluid Hysteroscopy: Proposal and Reliability Evaluation through an International Randomized-Controlled Observer Study. Fertil. Steril. 2019, 112, 162–173.e2. [Google Scholar] [CrossRef] [PubMed]
- Cravello, L.; Porcu, G.; D’Ercole, C.; Roger, V.; Blanc, B. Identification and Treatment of Endometritis. Contracept. Fertil. Sex. 1997, 25, 585–586. [Google Scholar]
- Cicinelli, E.; Resta, L.; Nicoletti, R.; Zappimbulso, V.; Tartagni, M.; Saliani, N. Endometrial Micropolyps at Fluid Hysteroscopy Suggest the Existence of Chronic Endometritis. Hum. Reprod. 2005, 20, 1386–1389. [Google Scholar] [CrossRef]
- Cicinelli, E.; Resta, L.; Nicoletti, R.; Tartagni, M.; Marinaccio, M.; Bulletti, C.; Colafiglio, G. Detection of Chronic Endometritis at Fluid Hysteroscopy. J. Minim. Invasive Gynecol. 2005, 12, 514–518. [Google Scholar] [CrossRef]
- Zolghadri, J.; Momtahan, M.; Aminian, K.; Ghaffarpasand, F.; Tavana, Z. The Value of Hysteroscopy in Diagnosis of Chronic Endometritis in Patients with Unexplained Recurrent Spontaneous Abortion. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 217–220. [Google Scholar] [CrossRef]
- Kitaya, K.; Tada, Y.; Taguchi, S.; Funabiki, M.; Hayashi, T.; Nakamura, Y. Local Mononuclear Cell Infiltrates in Infertile Patients with Endometrial Macropolyps versus Micropolyps. Hum. Reprod. 2012, 27, 3474–3480. [Google Scholar] [CrossRef]
- Song, D.; Li, T.C.; Zhang, Y.; Feng, X.; Xia, E.; Huang, X.; Xiao, Y. Correlation between Hysteroscopy Findings and Chronic Endometritis. Fertil. Steril. 2019, 11, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Bouet, P.E.; Hachem, H.E.; Monceau, E.; Gariépy, G.; Kadoch, I.J.; Sylvestre, C. Chronic Endometritis in Women with Recurrent Pregnancy Loss and Recurrent Implantation Failure: Prevalence and Role of Office Hysteroscopy and Immunohistochemistry in Diagnosis. Fertil. Steril. 2016, 105, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Tsonis, O.; Gkrozou, F.; Dimitriou, E.; Paschopoulos, M. Hysteroscopic Detection of Chronic Endometritis: Evaluating Proposed Hysteroscopic Features Suggestive of Chronic Endometritis. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102182. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, J.; Zhang, F.; Li, J.; Lv, S.; Zhang, L.; Yan, L. A New Hysteroscopic Scoring System for Diagnosing Chronic Endometritis. J. Minim. Invasive Gynecol. 2020, 27, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Gkrozou, F.; Tsonis, O.; Dimitriou, E.; Paschopoulos, M. In Women with Chronic or Subclinical Endometritis Is Hysteroscopy Suitable for Setting the Diagnosis? A Systematic Review. J. Obstet. Gynaecol. Res. 2020, 46, 1639–1650. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Zhang, W.; Sui, X.; Hao, C. Investigation of the Relationship between Chronic Endometritis Manifestations under Hysteroscope and CD138 Expression. Appl. Bionics. Biomech. 2022, 2022, 8323017. [Google Scholar] [CrossRef]
- Yasuo, T.; Kitaya, K. Challenges in Clinical Diagnosis and Management of Chronic Endometritis. Diagnostics 2022, 12, 2711. [Google Scholar] [CrossRef]
- Moneim, M.E.A.; Latif, A.A.A.; Shehata, M.S.; Ghanem, I.B.L. Accuracy of Office Hysteroscopy in the Diagnosis of Chronic Endometritis. Clin. Exp. Obstet. Gynecol. 2022, 49, 44. [Google Scholar] [CrossRef]
- Sun, H.; Zeng, X.; Xu, T.; Peng, G.; Ma, Y. Computer-Aided Diagnosis in Histopathological Images of the Endometrium Using a Convolutional Neural Network and Attention Mechanisms. IEEE J. Biomed. Health. Inform. 2020, 24, 1664–1676. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Zhang, J.; Wang, C.; Wang, Y.; Chen, H.; Shan, L.; Huo, J.; Gu, J.; Ma, X. Deep Learning Model for Classifying Endometrial Lesions. J. Transl. Med. 2021, 19, 10. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sone, K.; Noda, K.; Yoshida, K.; Toyohara, Y.; Kato, K.; Inoue, F.; Kukita, A.; Taguchi, A.; Nishida, H.; et al. Automated System for Diagnosing Endometrial Cancer by Adopting Deep-Learning Technology in Hysteroscopy. PLoS ONE 2021, 16, e0248526. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. ImageNet Large Scale Visual Recognition Challenge. Int. J. Comput. Vis. 2015, 115, 211–252. [Google Scholar] [CrossRef]
- Buzzaccarini, G.; Vitagliano, A.; Andrisani, A.; Santarsiero, C.M.; Cicinelli, R.; Nardelli, C.; Ambrosini, G.; Cicinelli, E. Chronic Endometritis and Altered Embryo Implantation: A Unified Pathophysiological Theory from a Literature Systematic Review. J. Assist. Reprod. Genet. 2020, 37, 2897–2911. [Google Scholar] [CrossRef] [PubMed]
- Prapas, Y.; Petousis, S.; Panagiotidis, Y.; Gullo, G.; Kasapi, L.; Papadeothodorou, A.; Prapas, N. Injection of Embryo Culture Supernatant to the Endometrial Cavity Does Not Affect Outcomes in IVF/ICSI or Oocyte Donation Cycles: A Randomized Clinical Trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 162, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Medenica, S.; Zivanovic, D.; Batkoska, L.; Marinelli, S.; Basile, G.; Perino, A.; Cucinella, G.; Gullo, G.; Zaami, S. The Future Is Coming: Artificial Intelligence in the Treatment of Infertility Could Improve Assisted Reproduction Outcomes—The Value of Regulatory Frameworks. Diagnostics 2022, 12, 2979. [Google Scholar] [CrossRef]
- Habib, N.; Buzzaccarini, G.; Centini, G.; Moawad, G.; Ceccaldi, P.-F.; Gitas, G.; Alkatout, I.; Gullo, G.; Terzic, S.; Sleiman, Z. Impact of Lifestyle and Diet on Endometriosis: A Fresh Look to a Busy Corner. Prz. Menopauzalny 2022, 21, 124–132. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Franchi, M.; Casarin, J.; Gullo, G.; Ghezzi, F. Translational Animal Models for Endometriosis Research: A Long and Windy Road. Ann. Transl. Med. 2018, 6, 431. [Google Scholar] [CrossRef]
- Kitaya, K.; Yasuo, T. Commonalities and Disparities between Endometriosis and Chronic Endometritis: Therapeutic Potential of Novel Antibiotic Treatment Strategy against Ectopic Endometrium. Int. J. Mol. Sci. 2023, 24, 2059. [Google Scholar] [CrossRef]
- Ochiai, K.; Ozawa, T.; Shibata, J.; Ishihara, S.; Tada, T. Current Status of Artificial Intelligence-Based Computer-Assisted Diagnosis Systems for Gastric Cancer in Endoscopy. Diagnostics 2022, 12, 3153. [Google Scholar] [CrossRef]
- Souaidi, M.; El Ansari, M. Multi-Scale Hybrid Network for Polyp Detection in Wireless Capsule Endoscopy and Colonoscopy Images. Diagnostics 2022, 12, 2030. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, J.; Gong, C.; Wang, T.D.; Seibel, E.J. Deep-Learning-Based Real-Time and Automatic Target-to-Background Ratio Calculation in Fluorescence Endoscopy for Cancer Detection and Localization. Diagnostics 2022, 12, 2031. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihara, M.; Yasuo, T.; Kitaya, K. Precision Medicine for Chronic Endometritis: Computer-Aided Diagnosis Using Deep Learning Model. Diagnostics 2023, 13, 936. https://doi.org/10.3390/diagnostics13050936
Mihara M, Yasuo T, Kitaya K. Precision Medicine for Chronic Endometritis: Computer-Aided Diagnosis Using Deep Learning Model. Diagnostics. 2023; 13(5):936. https://doi.org/10.3390/diagnostics13050936
Chicago/Turabian StyleMihara, Masaya, Tadahiro Yasuo, and Kotaro Kitaya. 2023. "Precision Medicine for Chronic Endometritis: Computer-Aided Diagnosis Using Deep Learning Model" Diagnostics 13, no. 5: 936. https://doi.org/10.3390/diagnostics13050936
APA StyleMihara, M., Yasuo, T., & Kitaya, K. (2023). Precision Medicine for Chronic Endometritis: Computer-Aided Diagnosis Using Deep Learning Model. Diagnostics, 13(5), 936. https://doi.org/10.3390/diagnostics13050936






