Confocal Microscopy for Diagnosis and Management of Cutaneous Malignancies: Clinical Impacts and Innovation
Abstract
1. Introduction
2. Current Application of Confocal Microscopy (CM)
2.1. Current Applications of Reflectance CM (RCM)
2.1.1. Skin Cancer Diagnosis and Management with RCM
Melanoma Diagnosis
Margin Assessment and Surveillance of Melanoma
Basal Cell Carcinoma (BCC) Diagnosis and Management
Squamous Cell Carcinoma (SCC) Diagnosis and Management
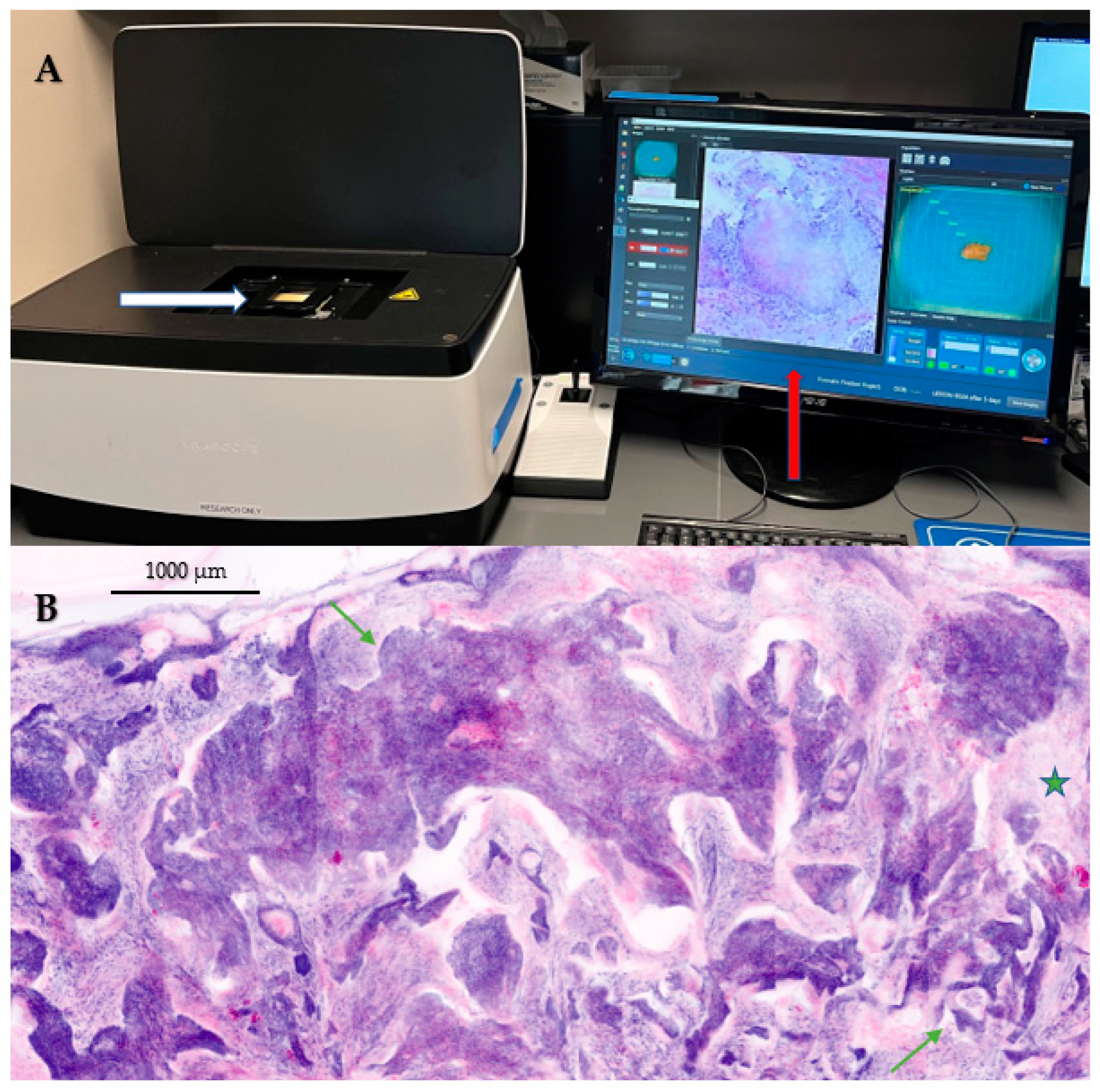
2.2. Current Applications of Ex Vivo Confocal Microscopy (EVCM) in Dermatology
2.2.1. EVCM for Diagnosis and Management of Melanoma
2.2.2. EVCM for the Intra-Operative Margin Assessment of Keratinocyte Carcinomas
3. Advances in the Field of Confocal Microscopy (CM)
3.1. Enlarging the Field of View (FOV) of RCM Images
3.2. Multimodal Imaging
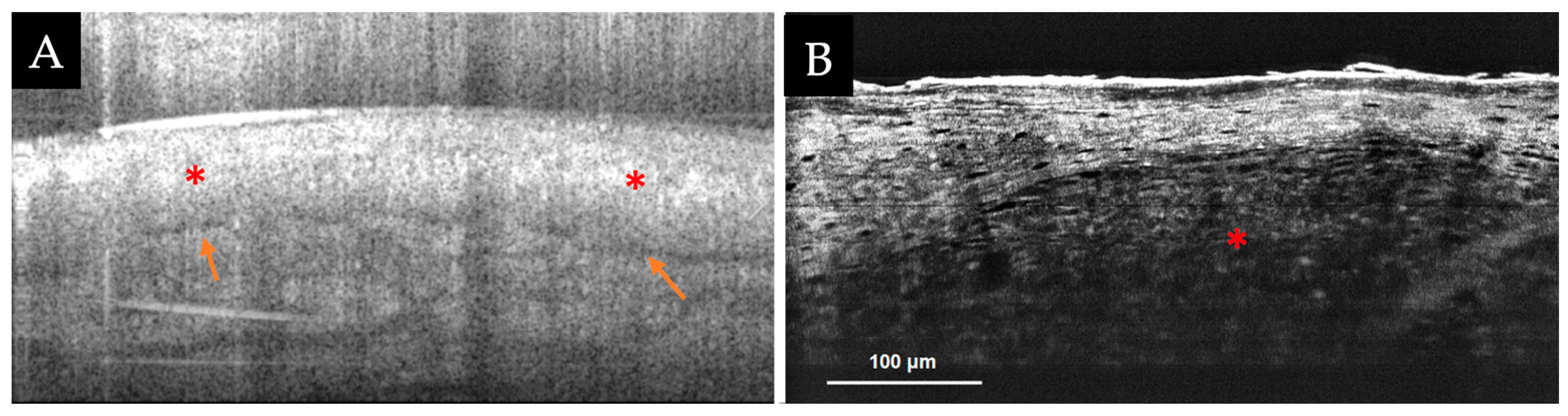
3.2.1. RCM-Optical Coherence Tomography (OCT) Device
3.2.2. High-Resolution Full-Field (FF)-OCT Devices
3.2.3. Line Field (LC)-OCT
3.2.4. Combined Multiphoton Microscopy (MPM)-RCM Device
3.3. Addition of Fluorescent Targeted Molecular Probes to Improve Diagnostic Accuracy of RCM
3.4. Enhancement of Tumor Detection via EVCM with Fluorescent-Labeled Antibodies
3.5. Dynamic Observation of Tumor Microenvironment (TME) to Predict the Response to Immunotherapy
3.6. Building More Affordable and Portable Microscopes for Widespread Use
3.7. Integration of Artificial Intelligence (AI) Algorithms to Aid Novices with Confocal Microscopy Image Interpretation and Diagnosis
3.7.1. AI for Diagnosis and Interpretation of In Vivo RCM Images
3.7.2. AI for EVCM Image Diagnosis and Interpretation
3.8. Remote Reading of Confocal Images
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urban, K.; Mehrmal, S.; Uppal, P.; Giesey, R.L.; Delost, G.R. The global burden of skin cancer: A longitudinal analysis from the Global Burden of Disease Study, 1990–2017. JAAD Int. 2021, 2, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, V.; Stratigos, A. Emerging trends in the epidemiology of melanoma. Br. J. Dermatol. 2014, 170, 11–19. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Ahmed, S.; Ardern-Jones, M.; Bhatti, L.A.; Bleiker, T.O.; Gavin, A.; Hussain, S.; Huws, D.W.; Irvine, L.; Langan, S.M.; et al. An updated report on the incidence and epidemiological trends of keratinocyte cancers in the United Kingdom 2013–2018. Ski. Health Dis. 2021, 1, e61. [Google Scholar] [CrossRef]
- Tripp, M.K.; Watson, M.; Balk, S.J.; Swetter, S.M.; Gershenwald, J.E. State of the science on prevention and screening to reduce melanoma incidence and mortality: The time is now. CA Cancer J. Clin. 2016, 66, 460–480. [Google Scholar] [CrossRef] [PubMed]
- Hoorens, I.; Vossaert, K.; Lanssens, S.; Dierckxsens, L.; Argenziano, G.; Brochez, L. Value of Dermoscopy in a Population-Based Screening Sample by Dermatologists. Dermatol. Pract. Concept. 2019, 9, 200–206. [Google Scholar] [CrossRef]
- Xiong, Y.-Q.; Ma, S.-J.; Mo, Y.; Huo, S.-T.; Wen, Y.-Q.; Chen, Q. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: A meta-analysis. J. Cancer Res. Clin. Oncol. 2017, 143, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Farnetani, F.; Ciardo, S.; Chester, J.; Kaleci, S.; Mazzoni, L.; Bassoli, S.; Casari, A.; Pampena, R.; Mirra, M.; et al. Effect of Reflectance Confocal Microscopy for Suspect Lesions on Diagnostic Accuracy in Melanoma: A Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 754–761. [Google Scholar] [CrossRef]
- Terushkin, V.; Warycha, M.; Levy, M.; Kopf, A.W.; Cohen, D.E.; Polsky, D. Analysis of the Benign to Malignant Ratio of Lesions Biopsied by a General Dermatologist Before and After the Adoption of Dermoscopy. Arch. Dermatol. 2010, 146, 343–344. [Google Scholar] [CrossRef]
- Tromme, I.; Legrand, C.; Devleesschauwer, B.; Leiter, U.; Suciu, S.; Eggermont, A.; Sacré, L.; Baurain, J.-F.; Thomas, L.; Beutels, P.; et al. Cost-effectiveness analysis in melanoma detection: A transition model applied to dermoscopy. Eur. J. Cancer 2016, 67, 38–45. [Google Scholar] [CrossRef]
- Pellacani, G.; Witkowski, A.; Cesinaro, A.; Losi, A.; Colombo, G.; Campagna, A.; Longo, C.; Piana, S.; De Carvalho, N.; Giusti, F.; et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J. Eur. Acad. Dermatol. Venereol. 2015, 30, 413–419. [Google Scholar] [CrossRef]
- Longo, C.; Ragazzi, M.; Rajadhyaksha, M.; Nehal, K.; Bennassar, A.; Pellacani, G.; Guilera, J.M. In Vivo and Ex Vivo Confocal Microscopy for Dermatologic and Mohs Surgeons. Dermatol. Clin. 2016, 34, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Pulijal, S.V.; Rajadhyaksha, M.; Halpern, A.C.; Gonzalez, S. Evaluation of Bedside Diagnostic Accuracy, Learning Curve, and Challenges for a Novice Reflectance Confocal Microscopy Reader for Skin Cancer Detection In Vivo. JAMA Dermatol. 2018, 154, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Yélamos, O.; Manubens, E.; Jain, M.; Chavez-Bourgeois, M.; Pulijal, S.V.; Dusza, S.W.; Marchetti, M.A.; Barreiro, A.; Marino, M.L.; Malvehy, J.; et al. Improvement of diagnostic confidence and management of equivocal skin lesions by integration of reflectance confocal microscopy in daily practice: Prospective study in 2 referral skin cancer centers. J. Am. Acad. Dermatol. 2020, 83, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Saleh, D.; Chuchu, N.; Bayliss, S.E.; Patel, L.; Davenport, C.; Takwoingi, Y.; Godfrey, K.; Matin, R.N.; et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst. Rev. 2018, 12, CD013190. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Chuchu, N.; Saleh, D.; E Bayliss, S.; Takwoingi, Y.; Davenport, C.; Patel, L.; Matin, R.N.; O’Sullivan, C.; et al. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst. Rev. 2018, 2018, CD013191. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Dechent, C.; Cordova, M.; Liopyris, K.; Rishpon, A.; Aleissa, S.; Rossi, A.; Lee, E.; Chen, C.; Busam, K.; Marghoob, A.; et al. Reflectance confocal microscopy and dermoscopy aid in evaluating repigmentation within or adjacent to lentigo maligna melanoma surgical scars. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Guida, S.; Alma, A.; Shaniko, K.; Chester, J.; Ciardo, S.; Proietti, I.; Giuffrida, R.; Zalaudek, I.; Manfredini, M.; Longo, C.; et al. Non-Melanoma Skin Cancer Clearance after Medical Treatment Detected with Noninvasive Skin Imaging: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2836. [Google Scholar] [CrossRef]
- Guitera, P.; Moloney, F.J.; Menzies, S.W.; Stretch, J.R.; Quinn, M.J.; Hong, A.; Fogarty, G.; Scolyer, R.A. Improving Management and Patient Care in Lentigo Maligna by Mapping with In Vivo Confocal Microscopy. JAMA Dermatol. 2013, 149, 692–698. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Cordova, M.; Liopyris, K.; Yélamos, O.; Aleissa, S.; Hibler, B.; Sierra, H.; Sahu, A.; Blank, N.; Rajadhyaksha, M.; et al. Reflectance confocal microscopy-guided carbon dioxide laser ablation of low-risk basal cell carcinomas: A prospective study. J. Am. Acad. Dermatol. 2019, 81, 984–988. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance confocal microscopy: Principles, basic terminology, clinical indications, limitations, and practical considerations. J. Am. Acad. Dermatol. 2020, 84, 1–14. [Google Scholar] [CrossRef]
- Malvehy, J.; Pérez-Anker, J.; Toll, A.; Pigem, R.; Garcia, A.; Alos, L.; Puig, S. Ex vivo confocal microscopy: Revolution in fast pathology in dermatology. Br. J. Dermatol. 2020, 183, 1011–1025. [Google Scholar] [CrossRef]
- Longo, C.; Ragazzi, M.; Gardini, S.; Piana, S.; Moscarella, E.; Lallas, A.; Raucci, M.; Argenziano, G.; Pellacani, G. Ex vivo fluorescence confocal microscopy in conjunction with Mohs micrographic surgery for cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2015, 73, 321–322. [Google Scholar] [CrossRef]
- Pellacani, G.; Scope, A.; Gonzalez, S.; Guitera, P.; Farnetani, F.; Malvehy, J.; Witkowski, A.; De Carvalho, N.; Lupi, O.; Longo, C. Reflectance confocal microscopy made easy: The 4 must-know key features for the diagnosis of melanoma and nonmelanoma skin cancers. J. Am. Acad. Dermatol. 2019, 81, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Dechent, C.; Liopyris, K.; Monnier, J.; Aleissa, S.; Boyce, L.M.; Longo, C.; Oliviero, M.; Rabinovitz, H.; Marghoob, A.A.; Halpern, A.C.; et al. Reflectance confocal microscopy terminology glossary for melanocytic skin lesions: A systematic review. J. Am. Acad. Dermatol. 2020, 84, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Dechent, C.; DeRosa, A.P.; Longo, C.; Liopyris, K.; Oliviero, M.; Rabinovitz, H.; Marghoob, A.A.; Halpern, A.C.; Pellacani, G.; Scope, A.; et al. Reflectance confocal microscopy terminology glossary for nonmelanocytic skin lesions: A systematic review. J. Am. Acad. Dermatol. 2018, 80, 1414–1427.e3. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, N.; Rabinovitz, H.; Oliviero, M.; Grant-Kels, J.M. Reflectance confocal microscopy: Melanocytic and nonmelanocytic. Clin. Dermatol. 2021, 39, 643–656. [Google Scholar] [CrossRef]
- Pezzini, C.; Kaleci, S.; Chester, J.; Farnetani, F.; Longo, C.; Pellacani, G. Reflectance confocal microscopy diagnostic accuracy for malignant melanoma in different clinical settings: Systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2268–2279. [Google Scholar] [CrossRef]
- Lan, J.; Wen, J.; Cao, S.; Yin, T.; Jiang, B.; Lou, Y.; Zhu, J.; An, X.; Suo, H.; Li, D.; et al. The diagnostic accuracy of dermoscopy and reflectance confocal microscopy for amelanotic/hypomelanotic melanoma: A systematic review and meta-analysis. Br. J. Dermatol. 2020, 183, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, A.G.; Farnetani, F.; Ciardo, S.; Chester, J.; Kaleci, S.; Stanganelli, I.; Mazzoni, L.; Magi, S.; Mandel, V.D.; Mirra, M.; et al. Dynamic dermoscopic and reflectance confocal microscopic changes of melanocytic lesions excised during follow up. J. Am. Acad. Dermatol. 2021, 86, 1049–1057. [Google Scholar] [CrossRef]
- Borsari, S.; Pampena, R.; Lallas, A.; Kyrgidis, A.; Moscarella, E.; Benati, E.; Raucci, M.; Pellacani, G.; Zalaudek, I.; Argenziano, G.; et al. Clinical Indications for Use of Reflectance Confocal Microscopy for Skin Cancer Diagnosis. JAMA Dermatol. 2016, 152, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Matas-Nadal, C.; Malvehy, J.; Ferreres, J.R.; Boada, A.; Bodet, D.; Segura, S.; Salleras, M.; Azon, A.; Bel-Pla, S.; Bigata, X.; et al. Increasing incidence of lentigo maligna and lentigo maligna melanoma in Catalonia. Int. J. Dermatol. 2018, 58, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Guitera, P.; Pellacani, G.; Crotty, K.A.; Scolyer, R.A.; Li, L.-X.L.; Bassoli, S.; Vinceti, M.; Rabinovitz, H.; Longo, C.; Menzies, S.W. The Impact of In Vivo Reflectance Confocal Microscopy on the Diagnostic Accuracy of Lentigo Maligna and Equivocal Pigmented and Nonpigmented Macules of the Face. J. Investig. Dermatol. 2010, 130, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Gouveia, B.M.; Rawson, R.; Guitera, P. Complex management of lentigo maligna in the setting of chrysiasis, argyriasis, and tattoo using in vivo reflectance confocal microscopy. J. Dermatol. 2022, 49, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Coco, V.; Farnetani, F.; Cesinaro, A.M.; Ciardo, S.; Argenziano, G.; Peris, K.; Pellacani, G.; Longo, C. False-Negative Cases on Confocal Microscopy Examination: A Retrospective Evaluation and Critical Reappraisal. Dermatology 2016, 232, 189–197. [Google Scholar] [CrossRef]
- Zoutendijk, J.; Koljenovic, S.; Wakkee, M.; Mooyaart, A.; Nijsten, T.; Bos, R.V.D. Clinical findings are not helpful in detecting lentigo maligna melanoma in patients with biopsy-proven lentigo maligna. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2325–2330. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Aleissa, S.; Cordova, M.; Liopyris, K.; Lee, E.H.; Rossi, A.M.; Hollman, T.; Pulitzer, M.; Lezcano, C.; Busam, K.J.; et al. Incompletely excised lentigo maligna melanoma is associated with unpredictable residual disease: Clinical features and the emerging role of reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Elshot, Y.S.; Zupan-Kajcovski, B.; Klop, W.M.C.; Bekkenk, M.W.; Crijns, M.B.; Rie, M.A.; Balm, A.J.M. Handheld reflectance confocal microscopy: Personalized and accurate presurgical delineation of lentigo maligna (melanoma). Head Neck 2020, 43, 895–902. [Google Scholar] [CrossRef]
- Hibler, B.P.; Yélamos, O.; Cordova, M.; Sierra, H.; Rajadhyaksha, M.; Nehal, K.S.; Rossi, A.M. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis 2017, 99, 346–352. [Google Scholar]
- Gao, J.M.; Garioch, J.J.; Fadhil, M.; Tan, E.; Shah, N.; Moncrieff, M. Planning slow Mohs excision margins for lentigo maligna: A retrospective nonrandomized cohort study comparing reflectance confocal microscopy margin mapping vs. visual inspection with dermoscopy. Br. J. Dermatol. 2020, 184, 1182–1183. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Cordova, M.; Aleissa, S.; Shoushtari, A.; Hollmann, T.J.; Leitao, M.M., Jr.; Rossi, A.M. Monitoring vulvar melanoma response to combined immunotherapy and radiotherapy with in vivo reflectance confocal microscopy. Dtsch. Dermatol. Ges. 2021, 19, 768–770. [Google Scholar] [CrossRef]
- Ho, G.; Schwartz, R.; Pereira, A.R.; Dimitrou, F.; Paver, E.; McKenzie, C.; Saw, R.; Scolyer, R.; Long, G.; Guitera, P. Reflectance confocal microscopy—A non-invasive tool for monitoring systemic treatment response in stage III unresectable primary scalp melanoma. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e583–e585. [Google Scholar] [CrossRef]
- Lupu, M.; Popa, I.M.; Voiculescu, V.M.; Caruntu, A.; Caruntu, C. A Systematic Review and Meta-Analysis of the Accuracy of In Vivo Reflectance Confocal Microscopy for the Diagnosis of Primary Basal Cell Carcinoma. J. Clin. Med. 2019, 8, 1462. [Google Scholar] [CrossRef]
- Woliner–van der Weg, W.; Peppelman, M.; Elshot, Y.S.; Visch, M.B.; Crijns, M.B.; Alkemade, H.A.C.; Bronkhorst, E.M.; Adang, E.; Amir, A.; Gerritsen, M.J.P.; et al. Biopsy outperforms reflectance confocal microscopy in diagnosing and subtyping basal cell carcinoma: Results and experiences from a randomized controlled multicentre trial*. Br. J. Dermatol. 2021, 184, 663–671. [Google Scholar] [CrossRef]
- Kadouch, D.; Elshot, Y.; Zupan-Kajcovski, B.; van Haersma de With, A.S.E.; van der Wal, A.; Leeflang, M.; Jóźwiak, K.; Wolkerstorfer, A.; Bekkenk, M.; Spuls, P.; et al. One-stop-shop with confocal microscopy imaging vs. standard care for surgical treatment of basal cell carcinoma: An open-label, noninferiority, randomized controlled multicentre trial. Br. J. Dermatol. 2017, 177, 735–741. [Google Scholar] [CrossRef]
- Witkowski, A.M.; Łudzik, J.; De Carvalho, N.T.; Ciardo, S.; Longo, C.; Dinardo, A.; Pellacani, G. Non-invasive diagnosis of pink basal cell carcinoma: How much can we rely on dermoscopy and reflectance confocal microscopy? Ski. Res. Technol. 2015, 22, 230–237. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Marmol, V.D.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus–based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Cordova, M.; Aleissa, S.; Liopyris, K.; Dusza, S.W.; Phillips, W.; Rossi, A.M.; Lee, E.H.; Marghoob, A.A.; Nehal, K.S. Reflectance confocal microscopy confirms residual basal cell carcinoma on clinically negative biopsy sites before Mohs micrographic surgery: A prospective study. J. Am. Acad. Dermatol. 2019, 81, 417–426. [Google Scholar] [CrossRef]
- Flores, E.S.; Córdova, M.; Kose, K.; Phillips, W.; Rossi, A.; Nehal, K.; Rajadhyaksha, M. Intraoperative imaging during Mohs surgery with reflectance confocal microscopy: Initial clinical experience. J. Biomed. Opt. 2015, 20, 61103. [Google Scholar] [CrossRef]
- Shavlokhova, V.; Vollmer, M.; Vollmer, A.; Gholam, P.; Saravi, B.; Hoffmann, J.; Engel, M.; Elsner, J.; Neumeier, F.; Freudlsperger, C. In vivo reflectance confocal microscopy of wounds: Feasibility of intraoperative basal cell carcinoma margin assessment. Ann. Transl. Med. 2021, 9, 1716. [Google Scholar] [CrossRef]
- Rao, B.K.; Mateus, R.; Wassef, C.; Pellacani, G. In vivo confocal microscopy in clinical practice: Comparison of bedside diagnostic accuracy of a trained physician and distant diagnosis of an expert reader. J. Am. Acad. Dermatol. 2013, 69, e295–e300. [Google Scholar] [CrossRef]
- Vladimirova, G.; Ruini, C.; Kapp, F.; Kendziora, B.; Ergün, E.Z.; Bağcı, I.S.; Krammer, S.; Jastaneyah, J.; Sattler, E.C.; Flaig, M.J.; et al. Ex vivo confocal laser scanning microscopy: A diagnostic technique for easy real-time evaluation of benign and malignant skin tumours. J. Biophotonics 2022, 15, e202100372. [Google Scholar] [CrossRef]
- Bennàssar, A.; Vilata, A.; Puig, S.; Malvehy, J. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br. J. Dermatol. 2014, 170, 360–365. [Google Scholar] [CrossRef]
- Cinotti, E.; Belgrano, V.; Labeille, B.; Grivet, D.; Douchet, C.; Chauleur, C.; Cambazard, F.; Thomas, A.; Prade, V.; Tognetti, L.; et al. In vivo and ex vivo confocal microscopy for the evaluation of surgical margins of melanoma. J. Biophotonics 2020, 13, e202000179. [Google Scholar] [CrossRef]
- Hartmann, D.; Krammer, S.; Ruini, C.; Ruzicka, T.; von Braunmühl, T. Correlation of histological and ex-vivo confocal tumor thickness in malignant melanoma. Lasers Med. Sci. 2016, 31, 921–927. [Google Scholar] [CrossRef]
- Longo, C.; Pampena, R.; Bombonato, C.; Gardini, S.; Piana, S.; Mirra, M.; Raucci, M.; Kyrgidis, A.; Pellacani, G.; Ragazzi, M. Diagnostic accuracy of ex vivo fluorescence confocal microscopy in Mohs surgery of basal cell carcinomas: A prospective study on 753 margins. Br. J. Dermatol. 2019, 180, 1473–1480. [Google Scholar] [CrossRef]
- Kose, K.; Fox, C.A.; Rossi, A.; Jain, M.; Cordova, M.; Dusza, S.W.; Ragazzi, M.; Gardini, S.; Moscarella, E.; Diaz, A.; et al. An international 3-center training and reading study to assess basal cell carcinoma surgical margins with ex vivo fluorescence confocal microscopy. J. Cutan. Pathol. 2021, 48, 1010–1019. [Google Scholar] [CrossRef]
- Grizzetti, L.; Kuonen, F. Ex vivo confocal microscopy for surgical margin assessment: A histology-compared study on 109 specimens. Ski. Health Dis. 2022, 2, e91. [Google Scholar] [CrossRef]
- Horn, M.; Gerger, A.; Koller, S.; Weger, W.; Langsenlehner, U.; Krippl, P.; Kerl, H.; Samonigg, H.; Smolle, J. The use of confocal laser-scanning microscopy in microsurgery for invasive squamous cell carcinoma. Br. J. Dermatol. 2007, 156, 81–84. [Google Scholar] [CrossRef]
- Kose, K.; Gou, M.; Yélamos, O.; Cordova, M.; Rossi, A.M.; Nehal, K.S.; Flores, E.S.; Camps, O.; Dy, J.G.; Brooks, D.H.; et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: An approach to address challenges in imaging living tissue and extend field of view. Sci. Rep. 2017, 7, 10759. [Google Scholar] [CrossRef]
- Gambichler, T.; Jaedicke, V.; Terras, S. Optical coherence tomography in dermatology: Technical and clinical aspects. Arch. Dermatol. Res. 2011, 303, 457–473. [Google Scholar] [CrossRef]
- Harris, U.; Rajadhyaksha, M.; Jain, M. Combining Reflectance Confocal Microscopy with Optical Coherence Tomography for Noninvasive Diagnosis of Skin Cancers via Image Acquisition. J. Vis. Exp. 2022, 186. [Google Scholar] [CrossRef]
- Jung, J.M.; Cho, J.Y.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Won, C.H. Emerging Minimally Invasive Technologies for the Detection of Skin Cancer. J. Pers. Med. 2021, 11, 951. [Google Scholar] [CrossRef]
- Monnier, J.; De Carvalho, N.; Harris, U.; Garfinkel, J.; Saud, A.; Navarrete-Dechent, C.; Liopyris, K.; Reiter, O.; Rubinstien, G.; Iftimia, N.; et al. Combined reflectance confocal microscopy and optical coherence tomography to improve the diagnosis of equivocal lesions for basal cell carcinoma. J. Am. Acad. Dermatol. 2021, 86, 934–936. [Google Scholar] [CrossRef]
- Sahu, A.; Yélamos, O.; Iftimia, N.; Cordova, M.; Alessi-Fox, C.; Gill, M.; Maguluri, G.; Dusza, S.W.; Navarrete-Dechent, C.; Gonzalez, S.; et al. Evaluation of a Combined Reflectance Confocal Microscopy–Optical Coherence Tomography Device for Detection and Depth Assessment of Basal Cell Carcinoma. JAMA Dermatol. 2018, 154, 1175–1183. [Google Scholar] [CrossRef]
- Iftimia, N.; Yélamos, O.; Chen, C.-S.J.; Maguluri, G.; Cordova, M.A.; Sahu, A.; Park, J.; Fox, W.; Alessi-Fox, C.; Rajadhyaksha, M. Handheld optical coherence tomography–reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J. Biomed. Opt. 2017, 22, 76006. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Aleissa, S.; Cordova, M.; Liopyris, K.; Sahu, A.; Rossi, A.M.; Lee, E.H.; Nehal, K.S. Management of complex head-and-neck basal cell carcinomas using a combined reflectance confocal microscopy/optical coherence tomography: A descriptive study. Arch. Dermatol. Res. 2020, 313, 193–200. [Google Scholar] [CrossRef]
- Aleissa, S.; Navarrete-Dechent, C.; Cordova, M.; Sahu, A.; Dusza, S.W.; Phillips, W.; Rossi, A.; Lee, E.; Nehal, K.S. Presurgical evaluation of basal cell carcinoma using combined reflectance confocal microscopy–optical coherence tomography: A prospective study. J. Am. Acad. Dermatol. 2020, 82, 962–968. [Google Scholar] [CrossRef]
- Bang, A.; Monnier, J.; Harris, U.; Garfinkel, J.; Rubinstein, G.; Iftimia, N.; Pulitzer, M.; Murray, M.; Lacouture, M.; Jain, M. Non-invasive, in vivo, characterization of cutaneous metastases using a novel multimodal RCM-OCT imaging device: A case-series. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2051–2054. [Google Scholar] [CrossRef]
- Ho, T.-S.; Tsai, M.-R.; Lu, C.-W. In vivo Mirau-type optical coherence microscopy with symmetrical illumination. In Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XXIV; SPIE: Bellingham, WA, USA, 2020; Volume 11228. [Google Scholar]
- Wang, Y.-J.; Wang, J.-Y.; Wu, Y.-H. Application of Cellular Resolution Full-Field Optical Coherence Tomography in vivo for the Diagnosis of Skin Tumours and Inflammatory Skin Diseases: A Pilot Study. Dermatology 2022, 238, 121–131. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wu, Y. In vivo characterization of extramammary Paget’s disease by ultra-high cellular resolution optical coherence tomography. Ski. Res. Technol. 2020, 27, 114–117. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, W.; Wang, J.; Wu, Y. Ex vivo full-field cellular-resolution optical coherence tomography of basal cell carcinomas: A pilot study of quality and feasibility of images and diagnostic accuracy in subtypes. Ski. Res. Technol. 2020, 26, 308–316. [Google Scholar] [CrossRef]
- Dubois, A.; Levecq, O.; Azimani, H.; Siret, D.; Barut, A.; Suppa, M.; Del Marmol, V.; Malvehy, J.; Cinotti, E.; Rubegni, P.; et al. Line-field confocal optical coherence tomography for high-resolution noninvasive imaging of skin tumors. J. Biomed. Opt. 2018, 23, 106007. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Sattler, E.; Welzel, J. Line-field confocal optical coherence tomography—Practical applications in dermatology and comparison with established imaging methods. Ski. Res. Technol. 2021, 27, 340–352. [Google Scholar] [CrossRef]
- Schuh, S.; Ruini, C.; Perwein, M.K.E.; Daxenberger, F.; Gust, C.; Sattler, E.C.; Welzel, J. Line-Field Confocal Optical Coherence Tomography: A New Tool for the Differentiation between Nevi and Melanomas? Cancers 2022, 14, 1140. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E. Line-field optical coherence tomography: In vivo diagnosis of basal cell carcinoma subtypes compared with histopathology. Clin. Exp. Dermatol. 2021, 46, 1471–1481. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E.C. Line-field confocal optical coherence tomography for the in vivo real-time diagnosis of different stages of keratinocyte skin cancer: A preliminary study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2388–2397. [Google Scholar] [CrossRef]
- Cinotti, E.; Tognetti, L.; Cartocci, A.; Lamberti, A.; Gherbassi, S.; Cano, C.O.; Lenoir, C.; Dejonckheere, G.; Diet, G.; Fontaine, M.; et al. Line-field confocal optical coherence tomography for actinic keratosis and squamous cell carcinoma: A descriptive study. Clin. Exp. Dermatol. 2021, 46, 1530–1541. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Hartmann, D.; French, L.; Sattler, E.; Welzel, J. In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study. Cancers 2021, 13, 2856. [Google Scholar] [CrossRef]
- Majdzadeh, A.; Lee, A.M.D.; Wang, H.; Lui, H.; McLean, D.I.; Crawford, R.I.; Zloty, D.; Zeng, H. Real-time visualization of melanin granules in normal human skin using combined multiphoton and reflectance confocal microscopy. Photodermatol. Photoimmunol. Photomed. 2015, 31, 141–148. [Google Scholar] [CrossRef]
- Sendín-Martín, M.; Posner, J.; Harris, U.; Moronta, M.; Sánchez, J.C.; Mukherjee, S.; Rajadhyaksha, M.; Kose, K.; Jain, M. Quantitative collagen analysis using second harmonic generation images for the detection of basal cell carcinoma with ex vivo multiphoton microscopy. Exp. Dermatol. 2022. [Google Scholar] [CrossRef]
- Rajadhyaksha, M.; Gonzalez, S.; Zavislan, J.M. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J. Biomed. Opt. 2004, 9, 323–331. [Google Scholar] [CrossRef]
- Sahu, A.; Cordero, J.; Wu, X.; Kossatz, S.; Harris, U.; Franca, P.D.D.; Kurtansky, N.R.; Everett, N.; Dusza, S.; Monnier, J.; et al. Combined PARP1-Targeted Nuclear Contrast and Reflectance Contrast Enhance Confocal Microscopic Detection of Basal Cell Carcinoma. J. Nucl. Med. 2022, 63, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.; Krammer, S.; Vural, S.; Bachmann, M.R.; Ruini, C.; Sárdy, M.; Ruzicka, T.; Berking, C.; Von Braunmühl, T. Immunofluorescence and confocal microscopy for ex-vivo diagnosis of melanocytic and non-melanocytic skin tumors: A pilot study. J. Biophotonics 2018, 11, e201700211. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Huang, X.; Zhang, G.; Hong, Z.; Bai, X.; Liang, T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 72. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Kose, K.; Kraehenbuehl, L.; Byers, C.; Holland, A.; Tembo, T.; Santella, A.; Alfonso, A.; Li, M.; Cordova, M.; et al. In vivo tumor immune microenvironment phenotypes correlate with inflammation and vasculature to predict immunotherapy response. Nat. Commun. 2022, 13, 5312. [Google Scholar] [CrossRef]
- Curiel-Lewandrowski, C.; Stratton, D.B.; Gong, C.; Kang, D. Preliminary imaging of skin lesions with near-infrared, portable, confocal microscopy. J. Am. Acad. Dermatol. 2021, 85, 1624–1625. [Google Scholar] [CrossRef]
- Freeman, E.E.; Semeere, A.; Laker-Oketta, M.; Namaganda, P.; Osman, H.; Lukande, R.; McMahon, D.; Seth, D.; Oyesiku, L.; Tearney, G.J.; et al. Feasibility and implementation of portable confocal microscopy for point-of-care diagnosis of cutaneous lesions in a low-resource setting. J. Am. Acad. Dermatol. 2021, 84, 499–502. [Google Scholar] [CrossRef]
- Larson, B.; Abeytunge, S.; Rajadhyaksha, M. Performance of full-pupil line-scanning reflectance confocal microscopy in human skin and oral mucosa in vivo. Biomed. Opt. Express 2011, 2, 2055–2067. [Google Scholar] [CrossRef]
- Freeman, E.; Semeere, A.; Osman, H.; Peterson, G.; Rajadhyaksha, M.; González, S.; Martin, J.N.; Anderson, R.R.; Tearney, G.J.; Kang, D. Smartphone confocal microscopy for imaging cellular structures in human skin in vivo. Biomed. Opt. Express 2018, 9, 1906–1915. [Google Scholar] [CrossRef]
- Gong, C.; Stratton, D.B.; Curiel-Lewandrowski, C.N.; Kang, D. Speckle-free, near-infrared portable confocal microscope. Appl. Opt. 2020, 59, G41–G46. [Google Scholar] [CrossRef] [PubMed]
- Yelamos, O.; Cordova, M.; Peterson, G.; Pulitzer, M.; Singh, B.; Rajadhyaksha, M.; DeFazio, J. In vivo intraoral reflectance confocal microscopy of an amalgam tattoo. Dermatol. Pract. Concept. 2017, 7, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.; Zanoni, D.K.; Ardigo, M.; Migliacci, J.C.; Patel, S.G.; Rajadhyaksha, M. Feasibility of a Video-Mosaicking Approach to Extend the Field-of-View For Reflectance Confocal Microscopy in the Oral Cavity In Vivo. Lasers Surg. Med. 2019, 51, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, G.; Taralli, S.; Vaccaro, M.; Indovina, L.; Mattoli, M.; Capotosti, A.; Scolozzi, V.; Calcagni, M.; Giordano, A.; De Spirito, M.; et al. Automated detection and classification of tumor histotypes on dynamic PET imaging data through machine-learning driven voxel classification. Comput. Biol. Med. 2022, 145, 105423. [Google Scholar] [CrossRef]
- Combalia, M.; Garcia, S.; Malvehy, J.; Puig, S.; Mülberger, A.G.; Browning, J.; Garcet, S.; Krueger, J.G.; Lish, S.R.; Lax, R.; et al. Deep learning automated pathology in ex vivo microscopy. Biomed. Opt. Express 2021, 12, 3103–3116. [Google Scholar] [CrossRef]
- Sendín-Martín, M.; Lara-Caro, M.; Harris, U.; Moronta, M.; Rossi, A.; Lee, E.; Chen, C.-S.J.; Nehal, K.; Sánchez, J.C.-M.; Pereyra-Rodríguez, J.-J.; et al. Classification of Basal Cell Carcinoma in Ex Vivo Confocal Microscopy Images from Freshly Excised Tissues Using a Deep Learning Algorithm. J. Investig. Dermatol. 2022, 142, 1291–1299.e2. [Google Scholar] [CrossRef]
- Wodzinski, M.; Skalski, A.; Witkowski, A.; Pellacani, G.; Ludzik, J. Convolutional Neural Network Approach to Classify Skin Lesions Using Reflectance Confocal Microscopy. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 4754–4757. [Google Scholar] [CrossRef]
- Campanella, G.; Navarrete-Dechent, C.; Liopyris, K.; Monnier, J.; Aleissa, S.; Minhas, B.; Scope, A.; Longo, C.; Guitera, P.; Pellacani, G.; et al. Deep Learning for Basal Cell Carcinoma Detection for Reflectance Confocal Microscopy. J. Investig. Dermatol. 2021, 142, 97–103. [Google Scholar] [CrossRef]
- Soenen, A.; Vourc’H, M.; Dréno, B.; Chiavérini, C.; Alkhalifah, A.; Dessomme, B.K.; Roussel, K.; Chambon, S.; Debarbieux, S.; Monnier, J.; et al. Diagnosis of congenital pigmented macules in infants with reflectance confocal microscopy and machine learning. J. Am. Acad. Dermatol. 2021, 85, 1308–1309. [Google Scholar] [CrossRef]
- Kose, K.; Bozkurt, A.; Alessi-Fox, C.; Gill, M.; Longo, C.; Pellacani, G.; Dy, J.G.; Brooks, D.H.; Rajadhyaksha, M. Segmentation of cellular patterns in confocal images of melanocytic lesions in vivo via a multiscale encoder-decoder network (MED-Net). Med. Image Anal. 2021, 67, 101841. [Google Scholar] [CrossRef]
- Li, J.; Garfinkel, J.; Zhang, X.; Di Wu, D.; Zhang, Y.; de Haan, K.; Wang, H.; Liu, T.; Bai, B.; Rivenson, Y.; et al. Biopsy-free in vivo virtual histology of skin using deep learning. Light. Sci. Appl. 2021, 10, 233. [Google Scholar] [CrossRef]
- Malciu, A.M.; Lupu, M.; Voiculescu, V.M. Artificial Intelligence-Based Approaches to Reflectance Confocal Microscopy Image Analysis in Dermatology. J. Clin. Med. 2022, 11, 429. [Google Scholar] [CrossRef]
- Kose, K.; Bozkurt, A.; Alessi-Fox, C.; Brooks, D.H.; Dy, J.G.; Rajadhyaksha, M.; Gill, M. Utilizing Machine Learning for Image Quality Assessment for Reflectance Confocal Microscopy. J. Investig. Dermatol. 2020, 140, 1214–1222. [Google Scholar] [CrossRef]
- Zhao, J.; Jain, M.; Harris, U.G.; Kose, K.; Curiel-Lewandrowski, C.; Kang, D. Deep Learning-Based Denoising in High-Speed Portable Reflectance Confocal Microscopy. Lasers Surg. Med. 2021, 53, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Schlingmann, S.; Jonke, Ž.; Avci, P.; Padrón-Laso, V.; Neumeier, F.; Koveshazi, I.; Ikeliani, I.U.; Patzer, K.; Kunrad, E.; et al. Machine Learning Based Prediction of Squamous Cell Carcinoma in Ex Vivo Confocal Laser Scanning Microscopy. Cancers 2021, 13, 5522. [Google Scholar] [CrossRef] [PubMed]
- Sendín-Martín, M.; Kose, K.; Harris, U.; Rossi, A.; Lee, E.; Nehal, K.; Rajadhyaksha, M.; Jain, M. Complete visualization of epidermal margin during ex vivo confocal microscopy of excised tissue with 3-dimensional mosaicking and intensity projection. J. Am. Acad. Dermatol. 2022, 86, e13–e14. [Google Scholar] [CrossRef]
- Cowen, E.A.; Sun, M.D.; Gu, L.; Acevedo, C.; Rotemberg, V.; Halpern, A.C. Store-and-forward mobile application as an accessible method of study participant assessment. J. Eur. Acad. Dermatol. Venereol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Scope, A.; Dusza, S.; Pellacani, G.; Gill, M.; Gonzalez, S.; Marchetti, M.; Rabinovitz, H.; Marghoob, A.; Alessi-Fox, C.; Halpern, A. Accuracy of tele-consultation on management decisions of lesions suspect for melanoma using reflectance confocal microscopy as a stand-alone diagnostic tool. J. Eur. Acad. Dermatol. Venereol. 2018, 33, 439–446. [Google Scholar] [CrossRef]
- Rubinstein, G.; Garfinkel, J.; Jain, M. Live, remote control of an in vivo reflectance confocal microscope for diagnosis of basal cell carcinoma at the bedside of a patient 2500 miles away: A novel tele-reflectance confocal microscope approach. J. Am. Acad. Dermatol. 2019, 81, e41–e42. [Google Scholar] [CrossRef]
- Witkowski, A.; Łudzik, J.; Soyer, H.P. Telediagnosis with Confocal Microscopy: A Reality or a Dream? Dermatol. Clin. 2016, 34, 505–512. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atak, M.F.; Farabi, B.; Navarrete-Dechent, C.; Rubinstein, G.; Rajadhyaksha, M.; Jain, M. Confocal Microscopy for Diagnosis and Management of Cutaneous Malignancies: Clinical Impacts and Innovation. Diagnostics 2023, 13, 854. https://doi.org/10.3390/diagnostics13050854
Atak MF, Farabi B, Navarrete-Dechent C, Rubinstein G, Rajadhyaksha M, Jain M. Confocal Microscopy for Diagnosis and Management of Cutaneous Malignancies: Clinical Impacts and Innovation. Diagnostics. 2023; 13(5):854. https://doi.org/10.3390/diagnostics13050854
Chicago/Turabian StyleAtak, Mehmet Fatih, Banu Farabi, Cristian Navarrete-Dechent, Gennady Rubinstein, Milind Rajadhyaksha, and Manu Jain. 2023. "Confocal Microscopy for Diagnosis and Management of Cutaneous Malignancies: Clinical Impacts and Innovation" Diagnostics 13, no. 5: 854. https://doi.org/10.3390/diagnostics13050854
APA StyleAtak, M. F., Farabi, B., Navarrete-Dechent, C., Rubinstein, G., Rajadhyaksha, M., & Jain, M. (2023). Confocal Microscopy for Diagnosis and Management of Cutaneous Malignancies: Clinical Impacts and Innovation. Diagnostics, 13(5), 854. https://doi.org/10.3390/diagnostics13050854






