Development, Implementation and Application of Confocal Laser Endomicroscopy in Brain, Head and Neck Surgery—A Review
Abstract
1. Introduction
2. Technology and Methodology
2.1. Cellvizio 400 and 800
2.2. Five-1 and Five-2
2.3. Other Clinically Available Confocal Systems
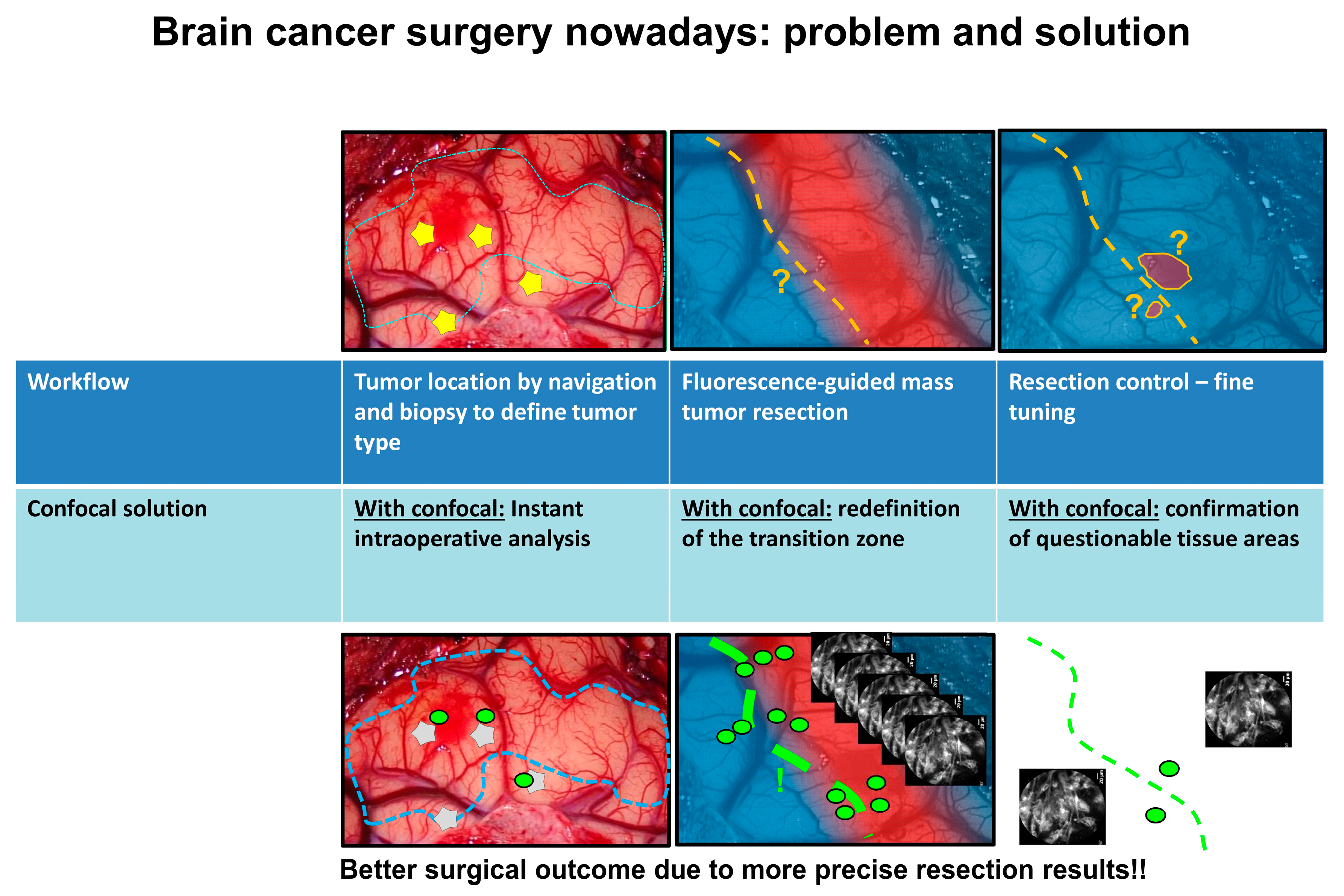
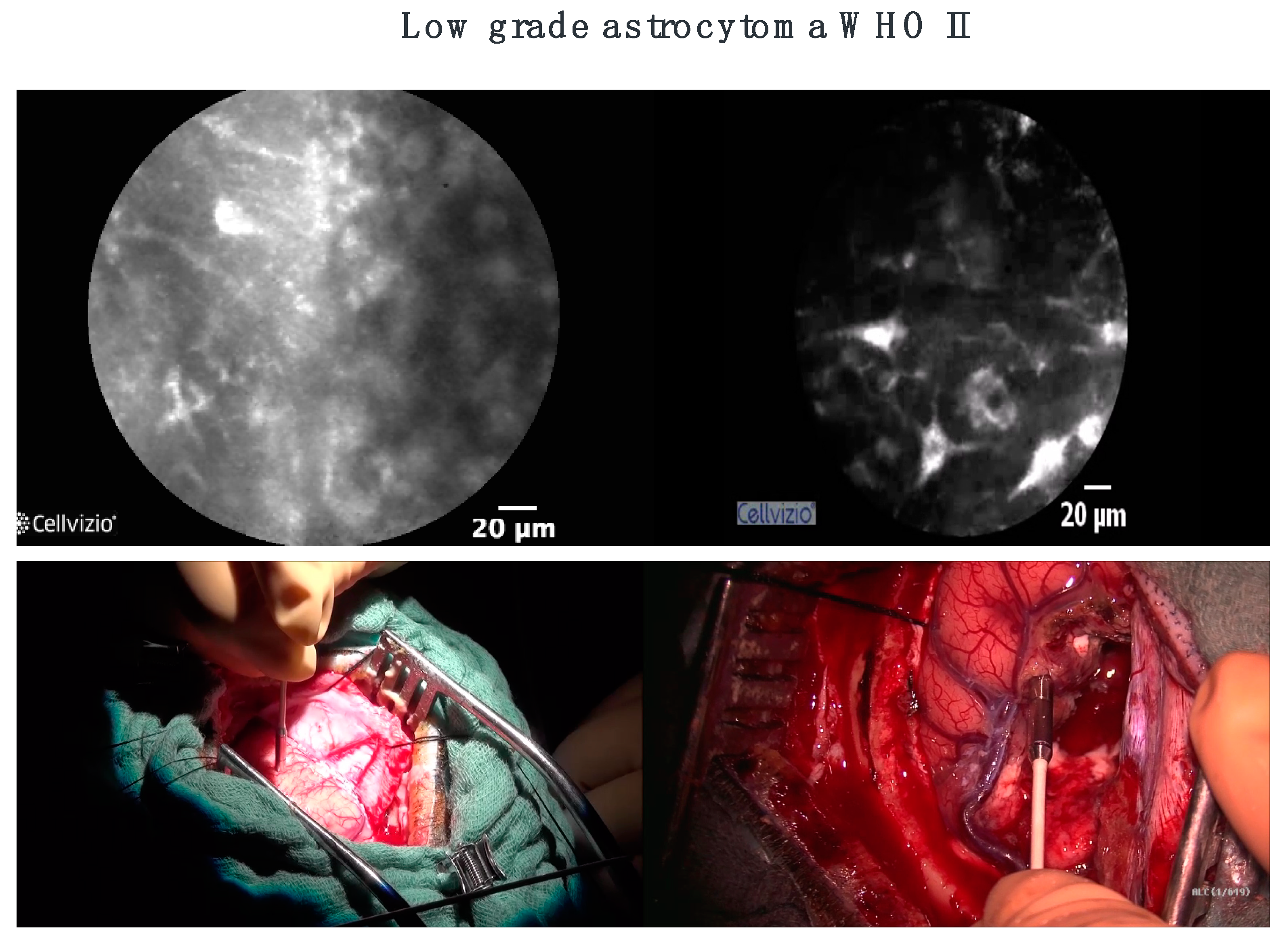
3. CLE in Neurosurgery
4. CLE on Ent Applications
5. Limitations of CLE
- (1)
- There are some fluorescent agents for instance cresyl violet and acriflavine that have no approval for their use in a clinical setting in neurosurgery. For this reason, we prefer fluorescein and indocyanine green for intravenous application, since the use of both fluorescent agents is well known for a lot of years now in clinical practice. Systems that are working label free will be the best outlook for the future.
- (2)
- Even if we found out during our observations that the strongest signal of the applied fluorescent agent was on the surface of the tumor, the infiltration depth of the endomicroscope is limited, which could represent a mentionable disadvantage. Nevertheless, the next generation of confocal systems (e.g., with near-infrared probes) could possibly provide a solution to this problem. Furthermore, confocal systems which have numerous excitation wavelengths, will make clinical use easier in the future.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. ALA-Glioma Study Group. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- MacAulay, C.; Lane, P.; Richards-Kortum, R. In vivo pathology: Microendoscopy as a new endoscopic imaging modality. Gastrointest. Endosc. Clin. N. Am. 2004, 14, 595–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.D.; Van Dam, J. Optical biopsy: A new frontier in endoscopic detection and diagnosis. Clin. Gastroenterol. Hepatol. 2004, 2, 744–753. [Google Scholar] [CrossRef]
- Charalampaki, P.; Kakaletri, I. Confocal Laser Endomicroscopy in Oncological Surgery. Diagnostics 2021, 11, 1813. [Google Scholar] [CrossRef]
- Becker, V.; Wallace, M.B.; Fockens, P.; von Delius, S.; Woodward, T.A.; Raimondo, M.; Voermans, R.P.; Meining, A. Needle-based confocal endomicroscopy for in vivo histology of intra-abdominal organs: First results in a porcine model (with videos). Gastrointest. Endosc. 2010, 71, 1260–1266. [Google Scholar] [CrossRef]
- Wijmans, L.; Bonta, P.I.; Rocha-Pinto, R.; de Bruin, D.M.; Brinkman, P.; Jonkers, R.E.; Roelofs, J.J.; Poletti, V.; Hetzel, J.; Annema, J.T. Confocal Laser Endomicroscopy as a Guidance Tool for Transbronchial Lung Cryobiopsies in Interstitial Lung Disorder. Respiration 2018, 97, 259–263. [Google Scholar] [CrossRef]
- Chen, S.P.; Liao, J.C. Confocal laser endomicroscopy of bladder and upper tract urothelial carcinoma: A new era of optical diagnosis? Curr. Urol. Rep. 2014, 15, 437. [Google Scholar] [CrossRef]
- Chang, T.P.; Leff, D.R.; Shousha, S.; Hadjiminas, D.J.; Ramakrishnan, R.; Hughes, M.R.; Yang, G.Z.; Darzi, A. Imaging breast cancer morphology using probe-based confocal laserendomicroscopy: Towards a real-time intraoperative imaging tool for cavity scanning. Breast Cancer Res. Treat. 2015, 153, 299–310. [Google Scholar] [CrossRef]
- Minsky, M. Memoir on inventing the confocal scanning microscope. Scanning 1988, 10, 128–138. [Google Scholar] [CrossRef]
- Sankar, T.; Delaney, P.M.; Ryan, R.W.; Eschbacher, J.; Abdelwahab, M.; Nakaji, P.; Coons, S.W.; Scheck, A.C.; Smith, K.A.; Spetzler, R.F.; et al. Miniaturized Handheld Confocal Microscopy for Neurosurgery. Neurosurgery 2010, 66, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Georges, J.; Zehri, A.; Carlson, E.; Nichols, J.; Mooney, M.A.; Martirosyan, N.L.; Ghaffari, L.; Kalani, M.Y.; Eschbacher, J.; Feuerstein, B.; et al. Label-free microscopic assessment of glioblastoma biopsy specimens prior to biobanking. Neurosurg. Focus 2014, 36, E8. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, N.L.; Georges, J.; Eschbacher, J.M.; Cavalcanti, D.D.; Elhadi, A.M.; Abdelwahab, M.G.; Scheck, A.C.; Nakaji, P.; Spetzler, R.F.; Preul, M.C. Potential application of a handheld confocal endomicroscope imaging system using a variety of fluorophores in experimental gliomas and normal brain. Neurosurg. Focus 2014, 36, E16. [Google Scholar] [CrossRef]
- Foersch, S.; Heimann, A.; Ayyad, A.; Spoden, G.A.; Florin, L.; Mpoukouvalas, K.; Kiesslich, R.; Kempski, O.; Goetz, M.; Charalampaki, P. Confocal Laser Endomicroscopy for Diagnosis and Histomorphologic Imaging of Brain Tumors In Vivo. PLoS ONE 2012, 7, e41760. [Google Scholar] [CrossRef]
- Peyre, M.; Clermont-Taranchon, E.; Stemmer-Rachamimov, A.; Kalamarides, M. Miniaturized Handheld Confocal Microscopy Identifies Focal Brain Invasion in a Mouse Model of Aggressive Meningioma. Brain Pathol. 2013, 23, 371–377. [Google Scholar] [CrossRef]
- Sanai, N.; Eschbacher, J.; Hattendorf, G.; Coons, S.W.; Preul, M.C.; Smith, K.A.; Nakaji, P.; Spetzler, R.F. Intraoperative confocal microscopy for brain tumors: A feasibility analysis in humans. Neurosurgery 2011, 68, 282–290. [Google Scholar] [CrossRef]
- Charalampaki, P.; Javed, M.; Daali, S.; Heiroth, H.J.; Igressa, A.; Weber, F. Confocal laser endomicroscopy for real-time histomorphological diagnosis: Our clinical experience with 150 brain and spinal tumor cases. Clin. Neurosurg. 2015, 62, 171–176. [Google Scholar] [CrossRef]
- Eschbacher, J.; Martirosyan, N.L.; Nakaji, P.; Sanai, N.; Preul, M.C.; Smith, K.A.; Coons, S.W.; Spetzler, R.F. In vivo intraoperative confocal microscopy for real-time histopathological imaging of brain tumors. J. Neurosurg. 2012, 116, 854–860. [Google Scholar] [CrossRef]
- Pavlov, V.; Meyronet, D.; Meyer-Bisch, V.; Armoiry, X.; Pikul, B.; Dumot, C.; Beuriat, P.A.; Signorelli, F.; Guyotat, J. Intraoperative Probe-Based Confocal Laser Endomicroscopy in Surgery and Stereotactic Biopsy of Low-Grade and High-Grade Gliomas: A Feasibility Study in Humans. J. Neurosurg. 2016, 79, 604–612. [Google Scholar] [CrossRef]
- Acerbi, F.; Pollo, B.; De Laurentis, C.; Restelli, F.; Falco, J.; Vetrano, I.G.; Broggi, M.; Schiariti, M.; Tramacere, I.; Ferroli, P.; et al. Ex Vivo Fluorescein-Assisted Confocal Laser Endomicroscopy (CONVIVO® System) in Patients With Glioblastoma: Results From a Prospective Study. Front. Oncol. 2020, 10, 606574. [Google Scholar] [CrossRef]
- Restelli, F.; Pollo, B.; Vetrano, I.G.; Cabras, S.; Broggi, M.; Schiariti, M.; Falco, J.; de Laurentis, C.; Raccuia, G.; Ferroli, P.; et al. Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications. J. Clin. Med. 2021, 10, 2035. [Google Scholar] [CrossRef] [PubMed]
- Cheyuo, C.; Grand, W.; Balos, L.L. Near-Infrared Confocal Laser Reflectance Cytoarchitectural Imaging of the Substantia Nigra and Cerebellum in the Fresh Human Cadaver. World Neurosurg. 2017, 97, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Charalampaki, P.; Nakamura, M.; Athanasopoulos, D.; Heimann, A. Confocal-Assisted Multispectral Fluorescent Microscopy for Brain Tumor Surgery. Front. Oncol. 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Turcotte, R.; Hampson, K.M.; Wincott, M.; Schmidt, C.C.; Emptage, N.J.; Charalampaki, C.; Booth, M.J. Compact and contactless reflectance confocal microscope for neurosurgery. Biomed. Opt. Express 2020, 11, 4772–4785. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Charalampaki, P.; Liu, Y.; Yang, G.-Z.; Giannarou, S. Context aware decision support in neurosurgical oncology based on an efficient classification of endomicroscopic data. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Meza, D.; Wang, D.; Wang, Y.; Borwege, S.; Sanai, N.; Liu, J.T. Comparing high-resolution microscopy techniques for potential intraoperative use in guiding low-grade glioma resections. Lasers Surg. Med. 2015, 47, 289–295. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.; Turner, J.D.; Belykh, E.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: Experience with 74 cases. Neurosurg. Focus 2016, 40, E11. [Google Scholar] [CrossRef]
- Belykh, E.; Zhao, X.; Ngo, B.; Farhadi, D.S.; Kindelin, A.; Ahmad, S.; Martirosyan, N.L.; Lawton, M.T.; Preul, M.C. Visualization of brain microvasculature and blood flow in vivo: Feasibility study using confocal laser endomicroscopy. Microcirculation 2021, 28, e12678. [Google Scholar] [CrossRef]
- Belykh, E.; Patel, A.A.; Miller, E.J.; Bozkurt, B.; Yağmurlu, K.; Woolf, E.C.; Scheck, A.C.; Eschbacher, J.M.; Nakaji, P.; Preul, M.C. Probe-based three-dimensional confocal laser endomicroscopy of braintumors: Technical note. Cancer Manag. Res. 2018, 10, 3109–3123. [Google Scholar] [CrossRef]
- Belykh, E.; Zhao, X.; Ngo, B.; Farhadi, D.S.; Byvaltsev, V.A.; Eschbacher, J.M.; Nakaji, P.; Preul, M.C. Intraoperative Confocal Laser Endomicroscopy Ex Vivo Examination of Tissue Microstructure During Fluorescence-Guided Brain Tumor Surgery. Front. Oncol. 2020, 10, 599250. [Google Scholar] [CrossRef]
- Höhne, J.; Schebesch, K.M.; Zoubaa, S.; Proescholdt, M.; Riemenschneider, M.J.; Schmidt, N.O. Intraoperative imaging of brain tumors with fluorescein: Confocal laser endomicroscopy in neurosurgery. Clinical and user experience. Neurosurg. Focus 2021, 50, E19. [Google Scholar] [CrossRef] [PubMed]
- Abramov, I.; Park, M.T.; Belykh, E.; Dru, A.B.; Xu, Y.; Gooldy, T.C.; Scherschinski, L.; Farber, S.H.; Little, A.S.; Porter, R.W.; et al. Intraoperative confocal laser endomicroscopy: Prospective in vivo feasibility study of a clinical-grade system for brain tumors. J. Neurosurg. 2022, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abramov, I.; Park, M.T.; Gooldy, T.C.; Xu, Y.; Lawton, M.T.; Little, A.S.; Porter, R.W.; Smith, K.A.; Eschbacher, J.M.; Preul, M.C. Real-time intraoperative surgical telepathology using confocal laser endomicroscopy. Neurosurg. Focus 2022, 52, E9. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, P.D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Cancer: Changing Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef]
- Chiesa, F.; Mauri, S.; Tradati, N.; Calabrese, L.; Guigliano, G.; Ansarin, M.; Andrle, J.; Zurrida, S.; Orecchia, R.; Scully, C. Surfing prognostic factors in head and neck cancer at the millenium. Oral. Oncol. 1999, 35, 590–596. [Google Scholar] [CrossRef]
- Wallace, M.B.; Sharma, P.; Lightdale, C.; Wolfsen, H.; Coron, E.; Buchner, B.M.; Bansal, A.; Rastogi, A.; Abrams, J.; Crook, J.; et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe-based confocal laser endomicroscopy. Gastrointest. Endosc. 2010, 72, 19–24. [Google Scholar] [CrossRef][Green Version]
- Wallace, M.B.; Meining, A.; Canto, M.I.; Fockens, P.; Miehlke, S.; Roesch, T.; Lightdale, C.J.; Pohl, H.; Carr-Locke, D.; Löhr, M.; et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2010, 31, 548–552. [Google Scholar] [CrossRef]
- Polglase, A.L.; McLaren, W.J.; Skinner, S.A.; Kiesslich, R.; Neurath, M.F.; Delaney, P.M. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest. Endosc. 2005, 62, 686–695. [Google Scholar] [CrossRef]
- Kiesslich, R.; Burg, J.; Vieth, M.; Gnaendiger, J.; Enders, M.; Delany, P.; Polglase, A.; McLaren, W.; Janell, D.; Thomas, S.; et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 2004, 127, 706–713. [Google Scholar] [CrossRef]
- Goetz, M.; Ziebart, A.; Foersch, S.; Vieth, M.; Waldner, M.J.; Delaney, P.; Galle, P.R.; Neurath, M.F.; Kiesslich, R. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology 2010, 138, 435–446. [Google Scholar] [CrossRef]
- Foersch, S.; Kiesslich, R.; Waldner, M.J.; Delaney, P.; Galle, P.R.; Neurath, M.F.; Goetz, M. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut 2010, 59, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Meining, A.; Saur, D.; Bajbouj, M.; Becker, V.; Peltier, E.; Höfler, H.; Hann von Weyhern, C.; Schmid, R.M.; Prinz, C. In vivo histopathology for detection of gastrointestinal neoplasia with a portable, confocal miniprobe: An examiner blinded analysis. Clin. Gastroenterol. Hepatol. 2007, 5, 1261–1267. [Google Scholar] [CrossRef]
- Carlson, K.; Pavlova, I.; Collier, T.; Descour, M.; Follen, M.; Richards-Kortum, R. Confocal microscopy: Imaging cervical precancerous lesions. Gynecol. Oncol. 2005, 99, S84–S88. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Quinn, M.; Pyman, J.M.; Delaney, P.M.; McLaren, W.J. Detection of cervical intraepithelial neoplasia in vivo using confocal endomicroscopy. BJOG 2009, 116, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, C.; Jäger, W.; Salzer, A.; Biesterfeld, S.; Kiesslihc, R.; Hampel, C.; Thüroff, J.W.; Goetz, M. Confocal laser endomicroscopy for the diagnosis of urothelial bladder neoplasia: A technology of the future? BJU Int. 2011, 107, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Sonn, G.A.; Jones, S.E.; Tarin, T.V.; Du, C.B.; Mach, K.E.; Jensen, K.C.; Liao, J.C. Optical biopsy of human bladder neoplasia with in vivo confocal laser endomicroscopy. J. Urol. 2009, 128, 1299–1305. [Google Scholar] [CrossRef]
- Fuchs, F.S.; Zirlik, S.; Schubert, J.; Vieth, M.; Neurath, M.F. Confocal laser endomicroscopy for diagnosing lung cancer in vivo. Eur. Respir. J. 2013, 41, 1401–1408. [Google Scholar] [CrossRef]
- Lane, P.M.; Lam, S.; McWilliams, A.; Le Riche, J.C.; Anderson, M.W.; MacAulay, C. Confocal fluorescence microendoscopy of bronchial epithelium. J. Biomed. Opt. 2009, 14, 024008. [Google Scholar] [CrossRef]
- Thiberville, L.; Moreno-Swirc, S.; Vercauteren, T.; Peltier, E.; Cavé, C.; Heckly, G.B. In Vivo Imaging of the Bronchial Wall Microstructure Using Fibered Confocal Fluorescence Microscopy. Am. J. Respir. Crit. Care Med. 2007, 175, 22–31. [Google Scholar] [CrossRef]
- Snuderl, M.; Wirth, D.; Sheth, S.A.; Bourne, S.K.; Kwon, C.-S.; Ancukiewicz, M.; Curry, W.T.; Frosch, M.P.; Yaroslavsky, A.N. Dye-Enhanced Multimodal Confocal Imaging as a Novel Approach to Intraoperative Diagnosis of Brain Tumors. Brain Pathol. 2013, 23, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Abbaci, M.; Breuskin, I.; Casiraghi, O.; De Leeuw, F.; Ferchiou, M.; Temam, S.; Laplace- Builhé, C. Confocal laser endomicroscopy for non-invasive head and neck cancer imaging: A comprehensive review. Oral. Oncol. 2014, 50, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Volgger, V.; Conderman, C.; Betz, C.S. Confocal laser endomicroscopy in head and neck cancer: Steps forward? Curr. Opin. Otolaryngol. Head Neck Surg. 2013, 21, 164–170. [Google Scholar] [CrossRef]
- White, W.M.; Rajadhyaksha, M.; González, S.; Fabian, R.L.; Anderson, R.R. Noninvasive Imaging of Human Oral Mucosa in Vivo by Confocal Reflectance Microscopy. Laryngoscope 1999, 109, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.L.; Gillenwater, A.M.; Collier, T.G.; Alizadeh-Naderi, R.; El-Naggar, A.K.; Richards-Kortum, R.R. Confocal microscopy for real-time detection of oral cavity neoplasia. Clin. Cancer Res. 2003, 9, 4714–4721. [Google Scholar]
- Just, T.; Stave, J.; Boltze, C.; Wree, A.; Kramp, B.; Guthoff, R.F.; Pau, H.W. Laser Scanning Microscopy of the Human Larynx Mucosa: A Preliminary, Ex Vivo Study. Laryngoscope 2006, 116, 1136–1141. [Google Scholar] [CrossRef]
- Abbaci, M.; Casiraghi, O.; Temam, S.; Ferchiou, M.; Bosq, J.; Dartigues, P.; De Leeuw, F.; Breuskin, I.; Laplace-Builhé, C. Red and far-red fluorescent dyes for the characterization of head and neck cancer at the cellular level. J. Oral Pathol. Med. 2015, 44, 831–841. [Google Scholar] [CrossRef]
- Muldoon, T.J.; Roblyer, D.; Williams, M.D.; Stepanek, V.M.; Richards-Kortum, R.; Gillenwater, A.M. Noninvasive imaging of oral neoplasia with a high-resolution fiber-optic microendoscope. Head Neck 2012, 34, 305–312. [Google Scholar] [CrossRef]
- Vila, P.M.; Park, C.W.; Pierce, M.; Goldstein, G.H.; Levy, L.; Gurudutt, V.V.; Polydorides, A.D.; Godbold, J.H.; Teng, M.S.; Genden, E.M.; et al. Discrimination of Benign and Neoplastic Mucosa with a High-Resolution Microendoscope (HRME) in Head and Neck Cancer. Ann. Surg. Oncol. 2012, 19, 3534–3539. [Google Scholar] [CrossRef]
- Linxweiler, M.; Al Kadah, B.; Bozzato, A.; Bozzato, V.; Hasenfus, A.; Kim, Y.-J.; Wagner, M.; Igressa, A.; Schick, B.; Charalampaki, P. Noninvasive histological imaging of head and neck squamous cell carcinomas using confocal laser endomicroscopy. Eur. Arch. Otorhinolaryngol. 2016, 273, 4473–4478. [Google Scholar] [CrossRef]
- Farahati, B.; Stachs, O.; Prall, F.; Stave, J.; Guthoff, R.; Pau, H.W.; Just, T. Rigid confocal endoscopy for in vivo imaging of experimental oral squamous intraepithelial lesions. J. Oral Pathol. Med. 2010, 39, 318–327. [Google Scholar] [PubMed]
- Zheng, W.; Harris, M.; Kho, K.W.; Thong, P.S.; Hibbs, A.; Olivo, M.; Soo, K.C. Confocal endomicroscopic imaging of normal and neoplastic human tongue tissue using ALA-induced-PPIX fluorescence: A preliminary study. Oncol. Rep. 2004, 12, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Thong, P.S.P.; Kho, K.W.; Zheng, W.; Harris, M.; Soo, K.C.; Olivo, M. Development of a laser confocal endomicroscope for in vivo fluorescence imaging. J. Mech. Med. Biol. 2007, 7, 11–18. [Google Scholar] [CrossRef]
- Thong, P.S.-P.; Olivo, M.C.; Kho, K.-W.; Zheng, W.; Mancer, K.; Harris, M.R.; Soo, K.-C. Laser confocal endomicroscopy as a novel technique for fluorescence diagnostic imaging of the oral cavity. J. Biomed. Opt. 2007, 12, 014007. [Google Scholar] [CrossRef] [PubMed]
- Maitland, K.C.; Gillenwater, A.M.; Williams, M.D.; El-Naggar, A.K.; Descour, M.R.; Richards-Kortum, R.R. In vivo imaging of oral neoplasia using a miniaturized fiber optic confocal reflectance microscope. Oral Oncol. 2008, 44, 1059–1066. [Google Scholar] [CrossRef]
- Haxel, B.R.; Goetz, M.; Kiesslich, R.; Gosepath, J. Confocal endomicroscopy: A novel application for imaging of oral and oropharyngeal mucosa in human. Eur. Arch. Otorhinolaryngol. 2010, 267, 443–448. [Google Scholar] [CrossRef]
- Pogorzelski, B.; Hanenkamp, U.; Goetz, M.; Kiesslich, R.; Gosepath, J. Systematic intraoperative application of confocal endomicroscopy for early detection and resection of squamous cell carcinoma of the head and neck: A preliminary report. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 404–411. [Google Scholar]
- Thong, P.S.P.; Tandjung, S.S.; Movania, M.M.; Chiew, W.-M.; Olivo, M.; Bhuvaneswari, R.; Seah, H.-S.; Lin, F.; Qian, K.; Soo, K.-C. Toward real-time virtual biopsy of oral lesions using confocal laser endomicroscopy interfaced with embedded computing. J. Biomed. Opt. 2012, 17, 0560091–05600910. [Google Scholar] [CrossRef]
- Pierce, M.C.; Schwarz, R.A.; Bhattar, V.S.; Mondrik, S.; Williams, M.D.; Lee, J.J.; Richards-Kortum, R.; Gillenwater, A.M. Accuracy of In Vivo Multimodal Optical Imaging for Detection of Oral Neoplasia. Cancer Prev. Res. 2012, 5, 801–809. [Google Scholar] [CrossRef]
- Just, T.; Pau, H.W. Intra-operative application of confocal endomicroscopy using a rigid endoscope. J. Laryngol. Otol. 2013, 127, 599–604. [Google Scholar] [CrossRef]
- Contaldo, M.; Poh, C.F.; Guillaud, M.; Lucchese, A.; Rullo, R.; Lam, S.; Serpico, R.; MacAulay, C.E.; Lane, P.M. Oral mucosa optical biopsy by a novel handheld fluorescent confocal microscope specifically developed: Technologic improvements and future prospects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.A.O.; Kaskas, N.M.; Ma, X.; Chaudhery, S.; Lian, T.; Moore-Medlin, T.; Shi, R.; Mehta, V. Confocal laser endomicroscopy in the detection of head and neck precancerous lesions. Otolaryngol. Head Neck Surg. 2014, 151, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Dittberner, A.; Rodner, E.; Ortmann, W.; Stadler, J.; Schmidt, C.; Petersen, I.; Stallmach, A.; Denzler, J.; Guntinas-Lichius, O. Automated analysis of confocal laser endomicroscopy images to detect head and neck cancer. Head Neck 2015, 38, E1419–E1426. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Mehta, V.; Ma, X.; Chaudhery, S.; Shi, R.; Moore-Medlin, T.; Lian, T.; Nathan, C.-A.O. Interobserver agreement of confocal laser endomicroscopy for detection of head and neck neoplasia. Laryngoscope 2015, 126, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Volgger, V.; Girschnick, S.; Ihrler, S.; Englhard, A.S.; Stepp, H.; Betz, C.S. Evaluation of confocal laser endomicroscopy flat lesions of the larynx: A prospective clinical study. Head Neck 2016, 38, E1695–E1704. [Google Scholar] [CrossRef]
- Goncalves, M.; Iro, H.; Dittberner, A.; Agaimy, A.; Bohr, C. Value of confocal laser endomicroscopy in the diagnosis of vocal cord lesions. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3990–3997. [Google Scholar]
- Aubreville, M.; Knipfer, C.; Oetter, N.; Jaremenko, C.; Rodner, E.; Denzler, J.; Bohr, C.; Neumann, H.; Stelzle, F.; Maier, A. Automatic classification of cancerous tissue in laser endomicroscopy images of the oral cavity using deep learning. Sci. Rep. 2017, 7, 11979. [Google Scholar] [CrossRef]
- Englhard, A.S.; Palaras, A.; Volgger, V.; Stepp, H.; Mack, B.; Libl, D.; Gires, O.; Betz, C.S. Confocal laser endomicroscopy in head and neck malignancies using FITC-labelled EpCAM- and EGF-R-antibodies in cell lines and tumor biopsies. J. Biophotonics 2017, 10, 1365–1376. [Google Scholar] [CrossRef]
- Goncalves, M.; Aubreville, M.; Mueller, S.; Sievert, M.; Maier, A.; Iro, H.; Bohr, C. Probe-based confocal laser endomicroscopy in detecting malignant lesions of vocal folds. Acta Otorhinolaryngol. Ital. 2019, 39, 389–395. [Google Scholar] [CrossRef]
- Shahid, M.W.; Crook, J.E.; Meining, A.; Perchant, A.; Buchner, A.; Gomerz, V.; Wallace, M.B. Exploring the optimal fluorescein dose in probe-based confocal laser endomicroscopy for colonic imaging. Interv. Gastroenterol. 2011, 1, 166–171. [Google Scholar] [CrossRef][Green Version]
- Sievert, M.; Mantsopoulos, K.; Mueller, S.K.; Eckstein, M.; Rupp, R.; Aubreville, M.; Stelzle, F.; Oetter, N.; Maier, A.; Iro, H.; et al. Systematic interpretation of confocal laser endomicroscopy: Larynx and pharynx confocal imaging score. Acta Otorhinolaryngol. Ital. 2022, 42, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Sievert, M.; Mantsopoulos, K.; Mueller, S.K.; Rupp, R.; Eckstein, M.; Stelzle, F.; Oetter, N.; Maier, A.; Aubreville, M.; Iro, H.; et al. Validation of a classification and scoring system for the diagnosis of laryngeal and pharyngeal squamous cell carcinomas by confocal laser endomicroscopy. Braz. J. Otorhinolaryngol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sievert, M.; Oetter, N.; Aubreville, M.; Stelzle, F.; Maier, A.; Eckstein, M.; Mantsopoulos, K.; Gostian, A.-O.; Mueller, S.K.; Koch, M.; et al. Feasibility of intraoperative assessment of safe surgical margins during laryngectomy with confocal laser endomicroscopy: A pilot study. Auris Nasus Larynx 2021, 48, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Sievert, M.; Stelzle, F.; Aubreville, M.; Mueller, S.K.; Eckstein, M.; Oetter, N.; Maier, A.; Mantsopoulos, K.; Iro, H.; Goncalves, M. Intraoperative free margins assessment of oropharyngeal squamous cell carcinoma with confocal laser endomicroscopy: A pilot study. Eur. Arch. Otorhinolaryngol. 2021, 278, 4433–4439. [Google Scholar] [CrossRef] [PubMed]
- Wenda, N.; Kiesslich, R.; Gosepath, J. Confocal laser endomicroscopy - first application and validation of malignancy criteria. Laryngorhinootologie 2021, 100, 818–823. [Google Scholar]
- Shinohara, S.; Funabiki, K.; Kikuchi, M.; Takebayashi, S.; Hamaguchi, K.; Hara, S.; Yamashita, D.; Imai, Y.; Mizoguchi, A. Real-time imaging of head and neck squamous cell carcinomas using confocal micro-endoscopy and applicable dye: A preliminary study. Auris Nasus Larynx 2020, 47, 668–675. [Google Scholar] [CrossRef]
- Dittberner, A.; Ziadat, R.; Hoffmann, F.; Pertzborn, D.; Gassler, N.; Guntinas-Lichius, O. Fluorescein-Guided Panendoscopy for Head and Neck Cancer Using Handheld Probe-Based Confocal Laser Endomicroscopy: A Pilot Study. Front. Oncol. 2021, 11, 671880. [Google Scholar] [CrossRef]
- Abbaci, M.; Casiraghi, O.; Vergez, S.; Maillard, A.; Ben Lakhdar, A.; De Leeuw, F.; Crestani, S.; Ngo, C.; Koscielny, S.; Ferchiou, M.; et al. Diagnostic accuracy of in vivo early tumor imaging from probe-based confocal laser endomicroscopy versus histologic examination in head and neck squamous cell carcinoma. Clin. Oral Investig. 2021, 26, 1823–1833. [Google Scholar] [CrossRef]
- Sievert, M.; Aubreville, M.; Gostian, A.-O.; Mantsopoulos, K.; Koch, M.; Mueller, S.K.; Eckstein, M.; Rupp, R.; Stelzle, F.; Oetter, N.; et al. Validity of tissue homogeneity in confocal laser endomicroscopy on the diagnosis of laryngeal and hypopharyngeal squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2022, 279, 4147–4156. [Google Scholar] [CrossRef]
- Sievert, M.; Eckstein, M.; Mantsopoulos, K.; Mueller, S.K.; Stelzle, F.; Aubreville, M.; Oetter, N.; Maier, A.; Iro, H.; Goncalves, M. Impact of intraepithelial capillary loops and atypical vessels in confocal laser endomicroscopy for the diagnosis of laryngeal and hypopharyngeal squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2022, 279, 2029–2037. [Google Scholar] [CrossRef]
- Sievert, M.; Oetter, N.; Mantsopoulos, K.; Gostian, A.-O.; Mueller, S.K.; Koch, M.; Balk, M.; Thimsen, V.; Stelzle, F.; Eckstein, M.; et al. Systematic classification of confocal laser endomicroscopy for the diagnosis of oral cavity carcinoma. Oral Oncol. 2022, 132, 105978. [Google Scholar] [CrossRef] [PubMed]
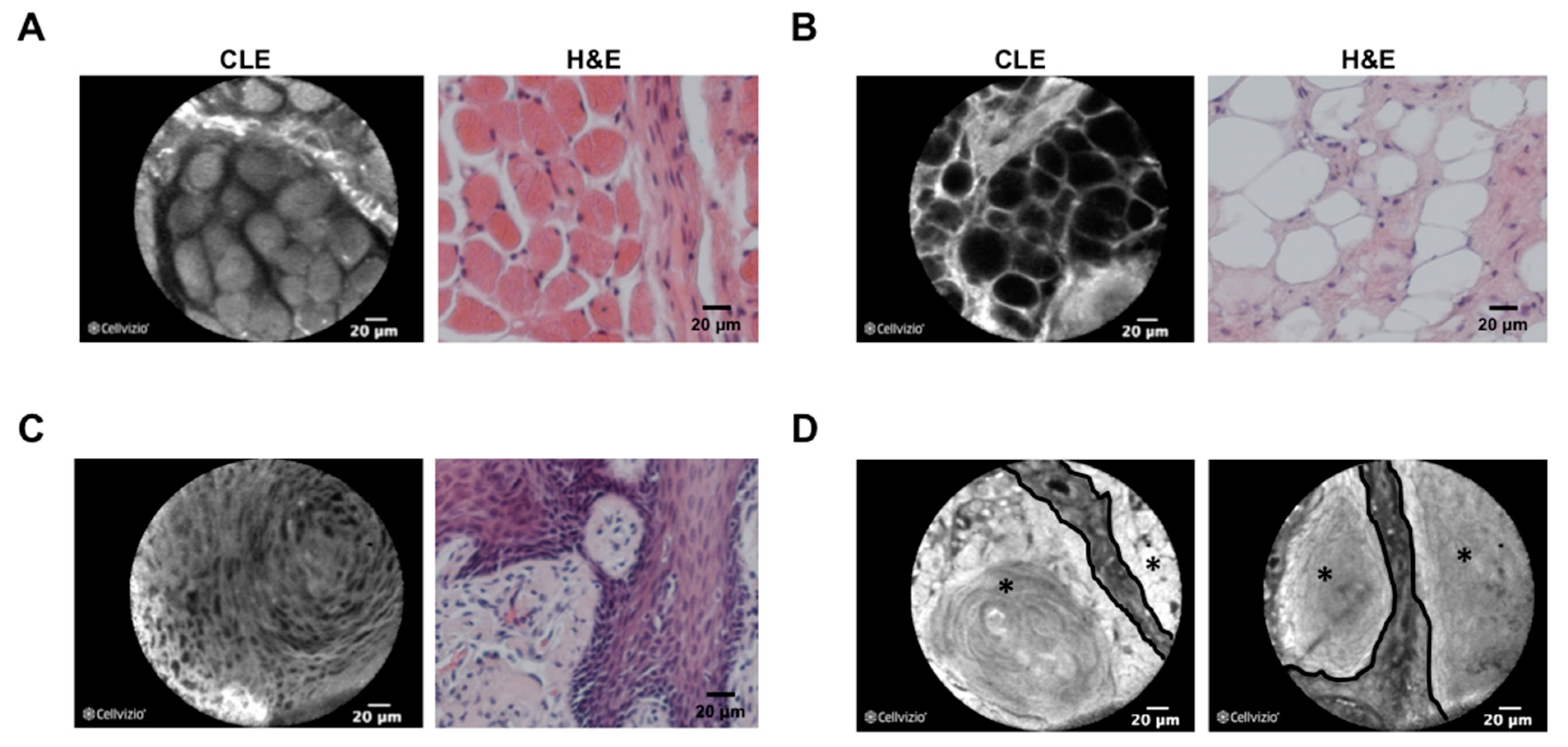
| Name of the Study | Date | Author | Number of Patients | Stain Used | Results |
|---|---|---|---|---|---|
| Laser-Scanning Confocal Endomicroscopy (LSCE) in the Neurosurgical Operating Room: a review and Discussion of future applications | 2011 | Sanai, N. [16] | 10 | Fluorescein 5-ALA |
|
| Comparing High-Resolution Microscopy Techniques for Potential Intraoperative use in Guided Low-Grade Glioma Resections | 2015 | Meza, D. [26] | 7 | 5-ALA |
|
| Confocal Laser Endomicroscopy for Real-time Histomorphological Diagnosis: Our Clinical Experience with 150 Brain and Spinal Tumor Cases | 2015 | Charalampaki, P. [17] | 150 | Acriflavine, Fluorescein |
|
| Intraoperative Probe-Based Confocal Laser Endomicroscopy in Surgery and Stereotactic Biopsy of Low-Grade and High-Grade Gliomas: A Feasibility Study in Humans | 2016 | Pavlov, V. [19] | 9 | 5- ALA (3) FNa (6) |
|
| Prospective Evaluation of the Utility of Intraoperative Confocal Laser Endomicroscopy in Patients with Brain Neoplasms using Fluorescein Sodium: Experience with 74 Cases | 2016 | Martirosyan, NL. [27] | 74 | FNa |
|
| Visualization of Brain Microvasculature and Blood flowin Vivo: Feasibility Study using Confocal Laser Endomicroscopy | 2021 | Belykh, E. [28] | 20 | FNa |
|
| Probe-Based Three-Dimensional Confocal Laser Endomicroscopy of Brain Tumors: Technical Note | 2018 | Belykh, E. [29] | Mice: 19 Patients: 31 | FNa |
|
| Confocal-Assisted Multispectral Fluorescent Microscopy for Brain Tumor Surgery | 2019 | Charalampaki, P. [23] | Rats: 22 Patients: 13 | ICG |
|
| Intraoperative Confocal Laser EndomicroscopyEx VivoExamination of Tissue Microstructure During Fluorescence-Guided Brain Tumor Surgery | 2020 | Belykh, E. [30] | 47 | FNa |
|
| Ex VivoFluorescein-Assisted Confocal Laser Endomicroscopy (CONVIVO System) in Patients with Glioblastoma: Results from a Prospective Study | 2020 | Acerbi, F. [20] | 15 | FNa |
|
| Intraoperative Imaging of Brain Tumors with Fluorescein: Confocal Laser Endomicroscopy in Neurosurgery. Clinical and User experience | 2021 | Höhne, J. [31] | 12 | FNa |
|
| Intraoperative Confocal Laser Endomicroscopy: Prospectivein VivoFeasibility Study of a Clinical-Grade System for Brain Tumors | 2022 | Abramov, I. [32] | 30 | FNa |
|
| Real-Time Intraoperative Surgical Telepathology using Confocal Laser Endomicroscopy | 2022 | Abramov, I. [33] | 11 | FNa |
|
| Study | Year | No. of Cases/Samples | Fluorescent Dye | Main Results | |
|---|---|---|---|---|---|
| ex vivo | Clark et al. [55] | 2003 | 17 | none | HNSCC patients; good visualization of tumor morphology as well as adjacent tumor-free tissue |
| Just et al. [56] | 2006 | 26 | none | larynx biopsies (healthy, dysplasia, benign + malignant tumors); good correlation with histology; primary endpoints Se/Sp | |
| Abbaci et al. [57] | 2009 | 27 | AF/F/5-ALA | laryngectomy specimens; tumor, dysplastic and healthy tissue portions of each specimen were examined; description of CLE morphology compared with HE staining | |
| Muldoon et al. [58] | 2012 | 13 | none | HNSCC samples; primary endpoints Se/Sp; good correlation with histology | |
| Vila et al. [59] | 2012 | 38 | P | HNSCC samples; 7 examiners for the evaluation of CLE images after initial training; primary endpoints Se/Sp/IRR/Ac | |
| Linxweiler et al. [60] | 2016 | 185 | AF/none | HNSCC samples (n = 135) + healthy controls (n = 50); visualization and discrimination between neoplastic and non-neoplastic tissue; identification of the tumor border; evaluation of CLE images by ENT surgeons, pathologists and laymen after initial training; primary endpoint: correct identification of tumor border and tumor localization | |
| in vivo (M) | Farahati et al. [61] | 2010 | 60 | none | 10 healthy mice, 50 mice with chemically induced tongue cancer; description of CLE morphology; primary endpoints Se/Sp/IRR |
| in vivo (H) | White et al. [54] | 1999 | 6 | none | healthy controls; description of CLE morphology; good correlation with histology |
| Zheng et al. [62] | 2004 | 5 | 5-ALA | 2 healthy controls; 3 tongue cancer patients; description of morphology, good correlation with histology | |
| Thong et al. [63] | 2007 | not indicated | 5-ALA/F/H | tissue samples + in vivo measurements in humans and mice; good correlation with histology; differentiation between healthy tissue and tumor tissue | |
| Thong et al. [64] | 2007 | 2 | 5-ALA/F | healthy control patient + tongue cancer patient; description of morphology; good correlation with histology | |
| Maitland et al. [65] | 2008 | 8 | none | HNSCC patients; description of CLE morphology; good correlation with histology | |
| Haxel et al. [66] | 2010 | 5 | AF/F | healthy controls; description of CLE morphology; good correlation with histology | |
| Pogorzelski et al. [67] | 2012 | 15 | none | HNSCC patients; development of a diagnostic score; good differentiation between healthy tissue and tumor tissue | |
| Thong et al. [68] | 2012 | 6 | F/H | healthy controls; description of CLE morphology; good correlation with morphology; application of a 3D fluorescence imaging prototype | |
| Pierce et al. [69] | 2012 | 30 | none | moderate to severe dysplasia + HNSCC patients; good correlation with histology; primary endpoints Se/Sp/PPV/NPV | |
| Just & Pau [70] | 2013 | 10 | none | visualization of laryngeal mucosa from healthy controls and patients with premalignant lesions | |
| Contaldo et al. [71] | 2013 | 6 | AF | healthy controls; visualization of different histological structures | |
| Nathan et al. [72] | 2014 | 21 | F i.v. | visualization of premalignant and malignant lesions of the head and neck mucosa (12 dysplasias, 9) | |
| (9 carcinomas); good correlation with histology; primary endpoints Se/Sp/PPV/NPV | |||||
| Dittberner et al. [73] | 2016 | 12 | F i.v. | automated analysis of CLE images from neoplastic and non-neoplastic oral tissue; primary endpoint AUC | |
| Moore et al. [74] | 2016 | 24 | F i.v. | visualization and discrimination between benign, precancerous and malignant lesions of the head and neck; primary endpoint interobserver agreement; good correlation with histology | |
| Volgger et al. [75] | 2016 | 19 | F i.v. | visualization and discrimination between healthy tissue and various grades of dysplasia up to squamous cell carcinomas of the laryngeal mucosa; primary endpoints Se/Sp; CLE helpful for the discrimination between noninvasive laryngeal lesions | |
| Goncalves et al. [76] | 2017 | 7 | F i.v. | visualization and differentiation between severe dysplasia to invasive carcinoma (n = 3) and benign tumors (n = 4) of the vocal cords; primary endpoints Se/Sp/PPV/NPV/IRR | |
| Aubreville et al. [77] | 2017 | 12 | F i.v. | automated analysis of CLE images of the cancerous and tumor-free oral mucosa from 12 HNSCC patients using a deep learning approach; primary endpoints Se/Sp/AUC | |
| Englhard et al. [78] | 2017 | 11 | FITC-labeled Ab | visualization and differentiation between HNSCC and tumor-free tissue using CLE in combination with FITC-labeled EpCAM and EGF-R-antibodies; in vitro (cell lines) + in vivo (HNSCC samples, n = 11; healthy mucosa samples, n = 5); primary endpoint antigen specificity of the Abs | |
| Goncalves et al. [79] | 2019 | 7 | F i.v. | visualization and differentiation between squamous cell carcinomas (n = 3) and benign tumors (n = 4) of the vocal folds; primary endpoints Se/Sp/PPV/NPV/IRR | |
| Shinohara et al. [86] | 2020 | 10 | AF Food Red No. 106 | visualization of and differentiation between HNSCC and adjacent healthy tissue using autofluorescence, topical AF, or AF + Food Red No. 106; best results with AF only | |
| Sievert et al. [84] | 2021 | 5 | F i.v. | visualization and differentiation between oropharyngeal squamous cell carcinomas and adjacent healthy tissue; assessment of free resection margins; primary endpoints Se/Sp/PPV/NPV/Ac | |
| Wenda et al. [85] | 2021 | 2 | F i.v. | Visualization of tumor tissue in one patient with sinonasal inverted papilloma and one patient with sinonasal squamous cell carcinoma | |
| Dittberner et al. [87] | 2021 | 13 | F i.v. | visualization of and differentiation between HNSCC and adjacent healthy tissue; primary endpoints Se/Sp/Ac/; concordance between CLE imaging and histology | |
| Sievert et al. [82] | 2021 | 13 | F i.v. | generation and evaluation of an eight-point score for correct assessment of malignancy in laryngeal and pharyngeal squamous cell carcinoma; primary endpoints Se/Sp/Ac/NPV/PPV/AUC | |
| Sievert et al. [83] | 2021 | 5 | F i.v. | CLE-based assessment of safe surgical margins in laryngeal cancer patients; primary endpoints Se/Sp/NPV/PPV/Ac | |
| Sievert et al. [81] | 2022 | 13 | F i.v. | generation and evaluation of a larynx and pharynx confocal imaging score for correct assessment of malignancy in laryngeal and pharyngeal squamous cell carcinomas; comparison between CLE experts and CLE nonexperts; primary endpoints Se/Sp/Ac | |
| Abbaci et al. [88] | 2022 | 44 | patent blue V | visualization of and differentiation between HNSCC tumor core and its margins; primary endpoints Se/Sp | |
| Sievert et al. [89] | 2022 | 5 | F i.v. | visualization of and differentiation between tumor and adjacent healthy tissue in 5 laryngectomy patients; primary endpoints Se/Sp, ROI of tumor and healthy tissue | |
| Sievert et al. [90] | 2022 | 10 | F i.v. | visualization and evaluation of diagnostic value of intraepithelial capillary loops and atypical vessels in 10 laryngectomy patients; comparison between tumor vs. healthy tissue; primary endpoints Se/Sp/NPV/PPV/Ac | |
| Sievert et al. [91] | 2022 | 12 | F i.v. | generation and evaluation of a confocal imaging score for correct assessment of malignancy in oral cavity squamous cell carcinomas; primary endpoints Se/Sp/Ac/NPV/PPV/AUC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakaletri, I.; Linxweiler, M.; Ajlouni, S.; Charalampaki, P. Development, Implementation and Application of Confocal Laser Endomicroscopy in Brain, Head and Neck Surgery—A Review. Diagnostics 2022, 12, 2697. https://doi.org/10.3390/diagnostics12112697
Kakaletri I, Linxweiler M, Ajlouni S, Charalampaki P. Development, Implementation and Application of Confocal Laser Endomicroscopy in Brain, Head and Neck Surgery—A Review. Diagnostics. 2022; 12(11):2697. https://doi.org/10.3390/diagnostics12112697
Chicago/Turabian StyleKakaletri, Irini, Maximilian Linxweiler, Serine Ajlouni, and Patra Charalampaki. 2022. "Development, Implementation and Application of Confocal Laser Endomicroscopy in Brain, Head and Neck Surgery—A Review" Diagnostics 12, no. 11: 2697. https://doi.org/10.3390/diagnostics12112697
APA StyleKakaletri, I., Linxweiler, M., Ajlouni, S., & Charalampaki, P. (2022). Development, Implementation and Application of Confocal Laser Endomicroscopy in Brain, Head and Neck Surgery—A Review. Diagnostics, 12(11), 2697. https://doi.org/10.3390/diagnostics12112697





