Surgical Atrial Septal Patch Endocarditis in a Patient with a Complete Corrected Atrioventricular Canal Defect: A Case Report and Review of the Literature
Abstract
1. Introduction
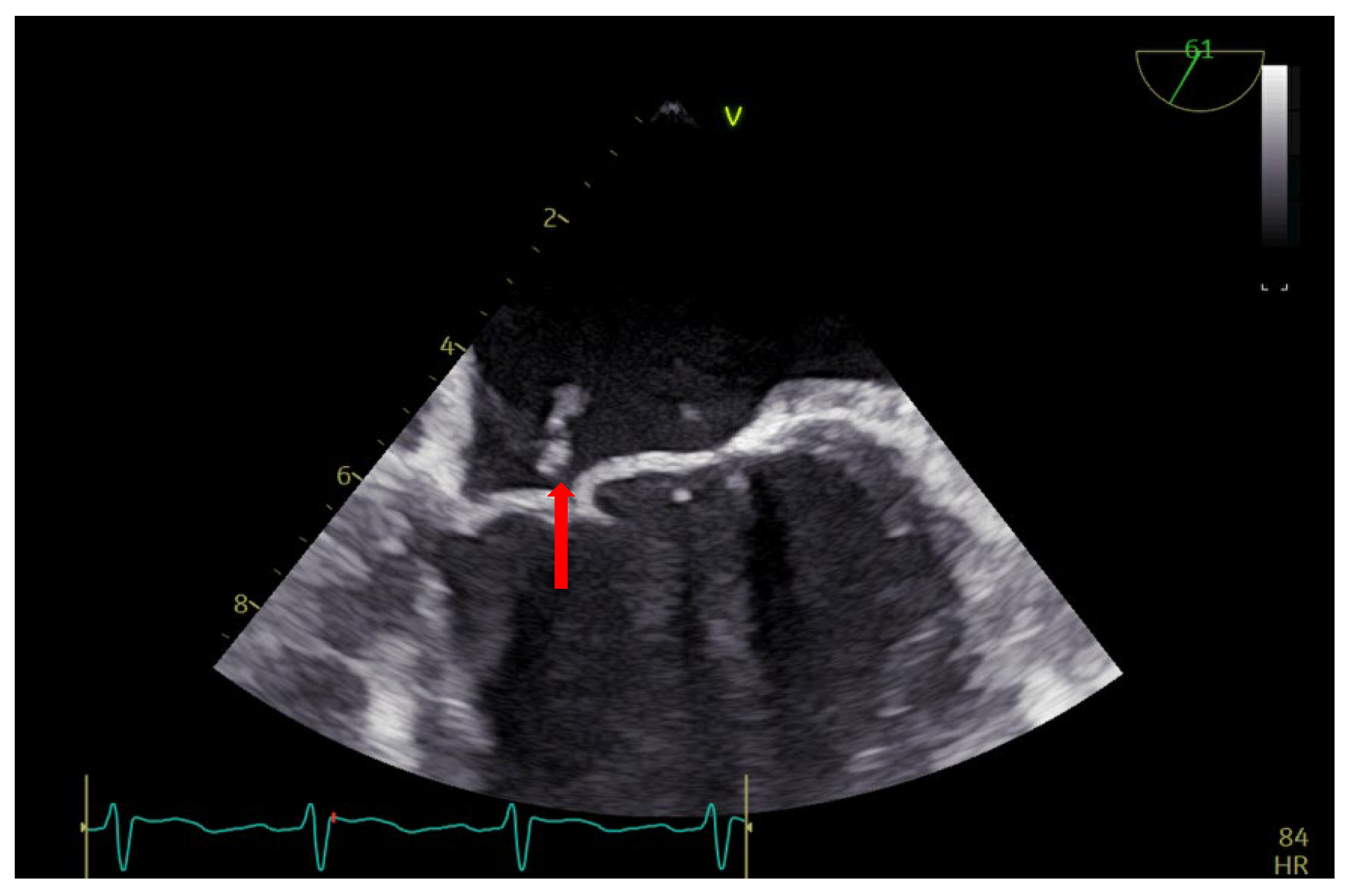
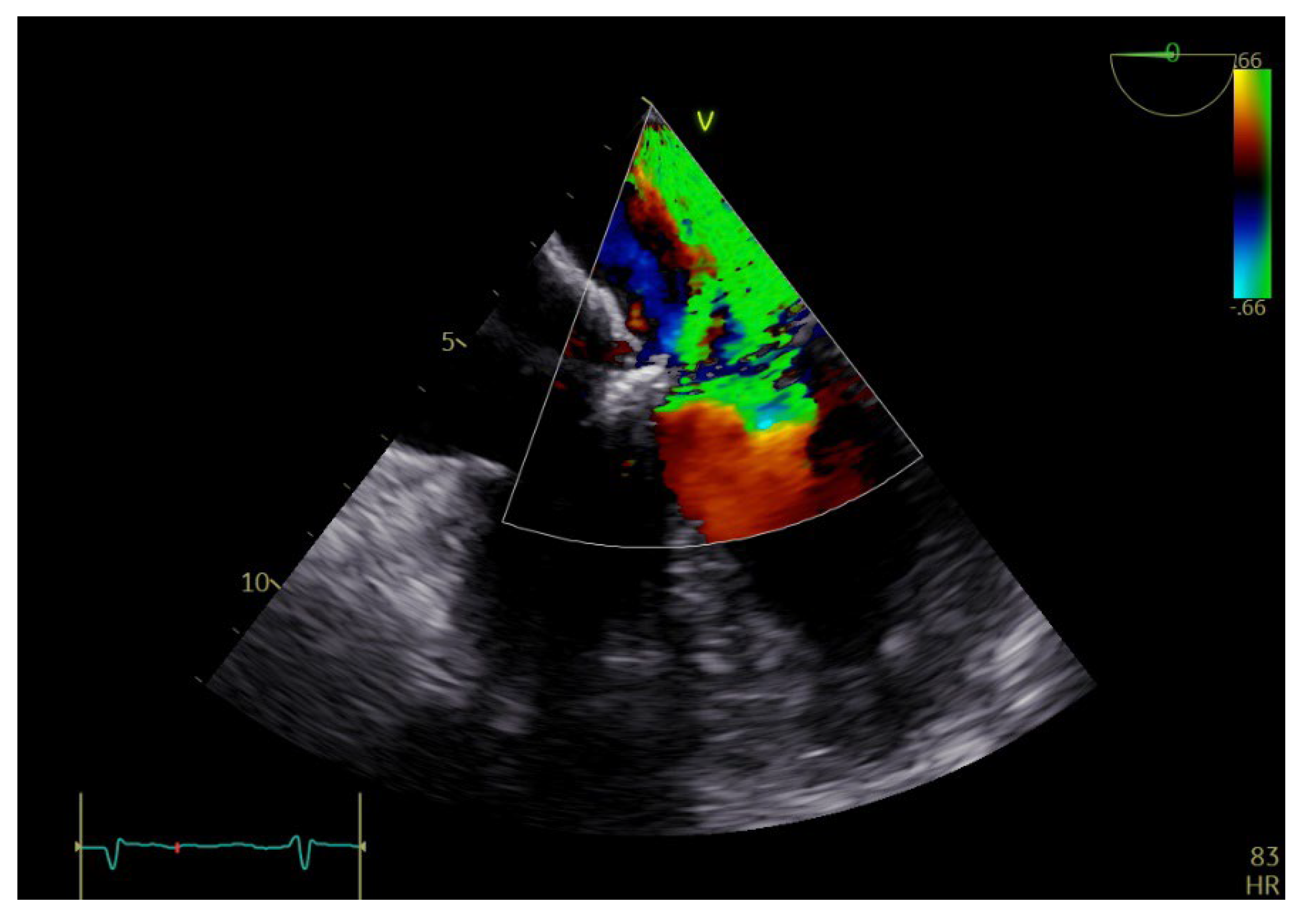
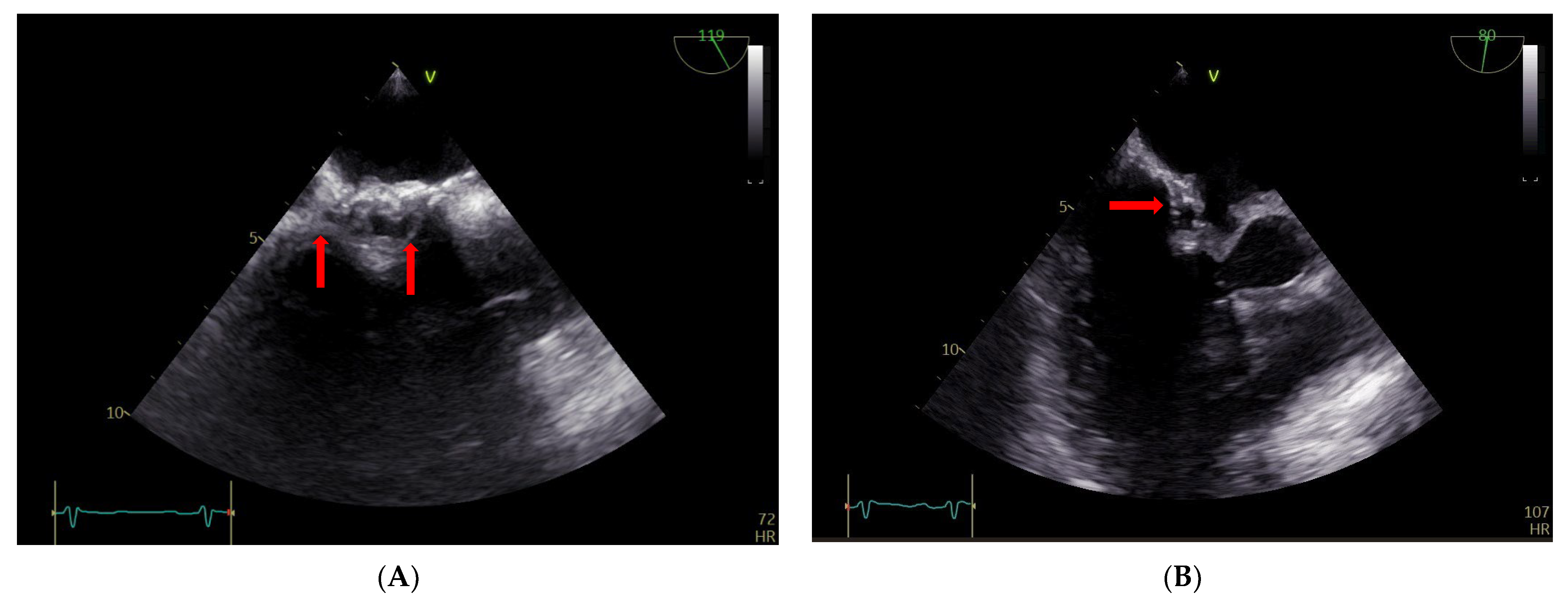
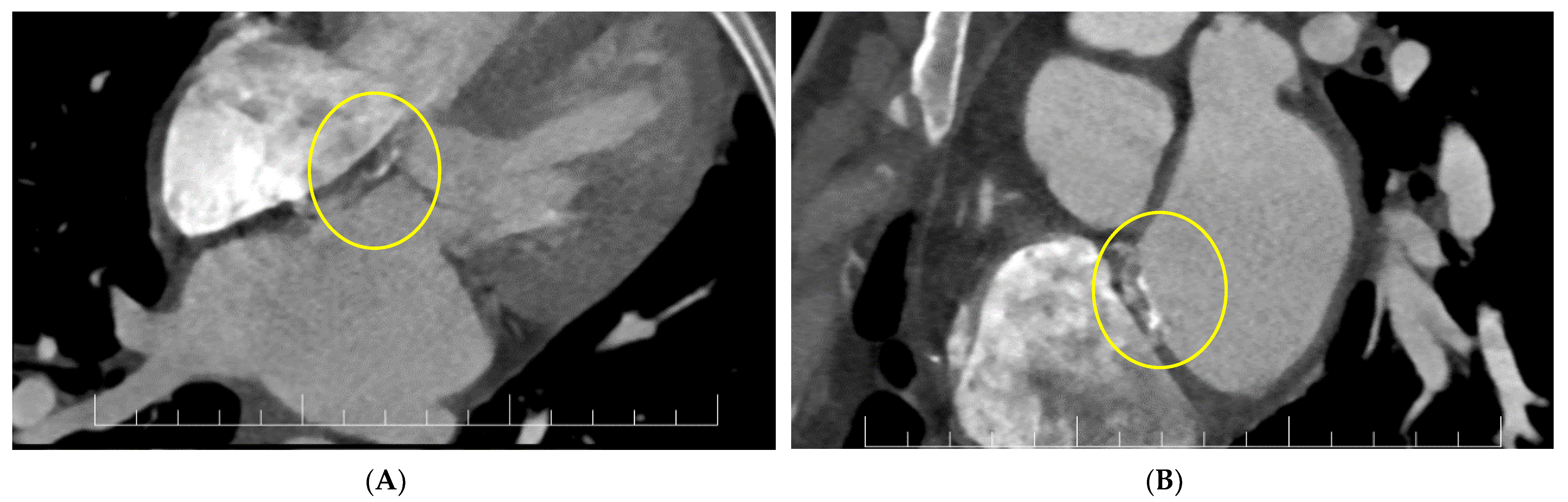
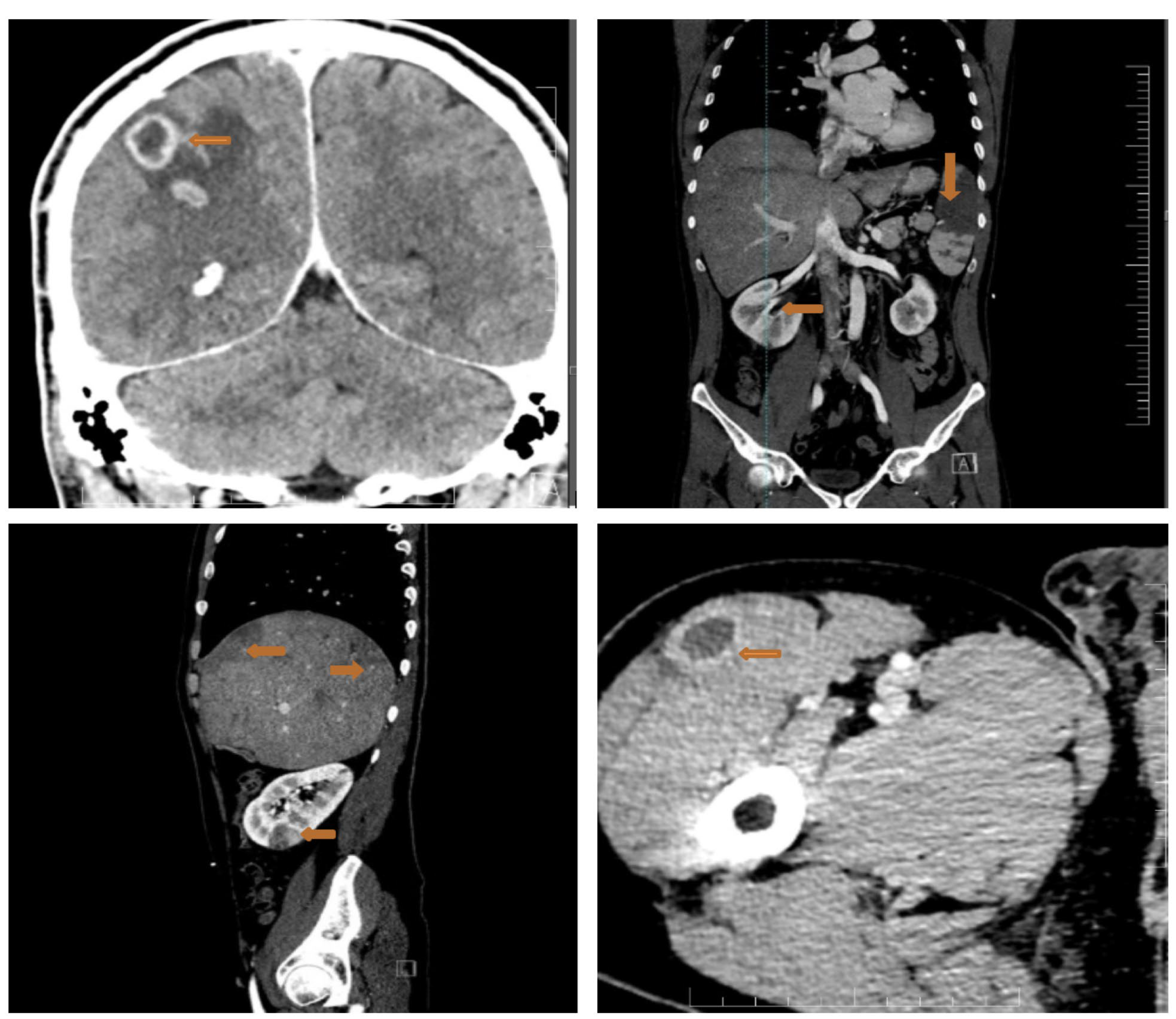
2. Case Report
3. Discussion
3.1. Current Reports and Actual Evidence
3.2. Future Outlooks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulder, B.J. Endocarditis in congenital heart disease: Who is at highest risk? Circulation 2013, 128, 1396–1397. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Snygg-Martin, U.; Giang, K.W.; Dellborg, M.; Robertson, J.; Mandalenakis, Z. Cumulative Incidence of Infective Endocarditis in Patients with Congenital Heart Disease: A Nationwide, Case-Control Study Over Nine Decades. Clin. Infect. Dis. 2021, 73, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.; Taubert, K.A.; Gewitz, M.; Lockhart, P.B.; Baddour, L.M.; Levison, M.; Bolger, A.; Cabell, C.H.; Takahashi, M.; Baltimore, R.S.; et al. Prevention of infective endocarditis: Guidelines from the American Heart Association: A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007, 116, 1736–1754. [Google Scholar] [CrossRef] [PubMed]
- Slesnick, T.C.; Nugent, A.W.; Fraser, C.D., Jr.; Cannon, B.C. Images in cardiovascular medicine. Incomplete endothelialization and late development of acute bacterial endocarditis after implantation of an Amplatzer septal occluder device. Circulation 2008, 117, e326–e327. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, T.; Sanusi, M.; Momin, E.S.; Khan, A.A.; Mannan, V.; Pervaiz, M.A.; Akram, A.; Elshaikh, A.O. Transcatheter Occluder Devices for the Closure of Atrial Septal Defect in Children: How Safe and Effective Are They? A Systematic Review. Cureus 2022, 14, e25402. [Google Scholar] [CrossRef]
- Honnorat, E.; Seng, P.; Riberi, A.; Habib, G.; Stein, A. Late infectious endocarditis of surgical patch closure of atrial septal defects diagnosed by 18F-fluorodeoxyglucose gated cardiac computed tomography (18F-FDG-PET/CT): A case report. BMC Res. Notes 2016, 9, 1–4. [Google Scholar] [CrossRef]
- Al-Terki, H.; Mügge, A.; Gotzmann, M. Infective endocarditis of a left atrial appendage closure device: A case report and literature review. Eur. Heart J. Case Rep. 2022, 6, ytac434. [Google Scholar] [CrossRef]
- Białkowski, J.; Pawlak, S.; Banaszak, P. Incomplete endothelialisation of an Amplatzer Septal Occluder device followed by meningitis and late acute bacterial endocarditis. Cardiol. Young 2016, 26, 808–810. [Google Scholar] [CrossRef]
- Verheugt, C.L.; Uiterwaal, C.S.P.M.; van der Velde, E.T.; Meijboom, F.J.; Pieper, P.G.; Veen, G.; Stappers, J.L.M.; Grobbee, D.E.; Mulder, B.J.M. Turning 18 with congenital heart disease: Prediction of infective endocarditis based on a large population. Eur. Heart J. 2011, 32, 1926–1934. [Google Scholar] [CrossRef]
- Brida, M.; Balint, H.O.; Bence, A.; Panfile, E.; Prokšelj, K.; Kačar, P.; Lebid, I.H.; Šimkova, I.; Bobocka, K.; Meidrops, K.; et al. Infective endocarditis in adults with congenital heart disease: Contemporary management and related outcomes in Central and South-Eastern European region. Int. J. Cardiol. 2023. [Google Scholar] [CrossRef]
- Knirsch, W.; Nadal, D. Infective endocarditis in congenital heart disease. Eur. J. Pediatr. 2011, 170, 1111–1127. [Google Scholar] [CrossRef]
- Jahangir, M.; Nawaz, M.; Jabbar, F.; Khan, F.; Hasnain, N. Infective Endocarditis Associated with Atrial Septal Defect in an Intravenous Drug Abuser: A Case Report. Cureus 2018, 10, e2482. [Google Scholar] [CrossRef]
- Deng, M.; Yang, Q. Efficacy and safety of dacron patch in surgical treatment of congenital disease by echocardiography. J. Infect. Public Health 2020, 13, 2067–2071. [Google Scholar] [CrossRef]
- Kuijpers, J.M.; Koolbergen, D.R.; Groenink, M.; Peels, K.C.H.; Reichert, C.L.A.; Post, M.C.; Bosker, H.A.; Wajon, E.M.C.J.; Zwinderman, A.H.; Mulder, B.J.M. Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: Focus on the use of prosthetic material. Eur. Heart J. 2017, 38, 2048–2056. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, Y.J.; Kim, S.H.; Sun, B.J.; Kim, D.H.; Yun, S.C.; Song, J.M.; Choo, S.J.; Chung, C.H.; Song, J.K.; et al. Early surgery versus conventional treatment for infective endocarditis. N. Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef]
- Anantha Narayanan, M.; Mahfood Haddad, T.; Kalil, A.C.; Kanmanthareddy, A.; Suri, R.M.; Mansour, G.; Destache, C.J.; Baskaran, J.; Mooss, A.N.; Wichman, T.; et al. Early versus late surgical intervention or medical management for infective endocarditis: A systematic review and meta-analysis. Heart 2016, 102, 950–957. [Google Scholar] [CrossRef]
- Morris, C.D.; Reller, M.D.; Menashe, V.D. Thirty-year incidence of infective endocarditis after surgery for congenital heart defect. JAMA 1998, 279, 599–603. [Google Scholar] [CrossRef]
- Vaideeswar, P.; Mishra, P.; Nimbalkar, M. Infective endocarditis of the Dacron patch—A report of 13 cases at autopsy. Cardiovasc. Pathol. 2011, 20, e169–e175. [Google Scholar] [CrossRef]
- Amedro, P.; Soulatges, C.; Fraisse, A. Infective endocarditis after device closure of atrial septal defects: Case report and review of the literature. Catheter. Cardiovasc. Interv. 2017, 89, 324–334. [Google Scholar] [CrossRef]
- Taj, S.; Arshad, M.U.; Khan, H.; Sidhu, G.S.; Singh, R. Infective Endocarditis Leading to Intracranial Abscess: A Case Report and Literature Review. Cureus 2021, 13, e12660. [Google Scholar] [CrossRef]
- Bonaros, N.; Czerny, M.; Pfausler, B.; Müller, S.; Bartel, T.; Thielmann, M.; Shehada, S.-E.; Folliguet, T.; Obadia, J.-F.; Holfeld, J. Infective endocarditis and neurologic events: Indications and timing for surgical interventions. Eur. Hear. J. Suppl. 2020, 22, 19–25. [Google Scholar] [CrossRef]
- Vitali, P.; Savoldi, F.; Segati, F.; Melazzini, L.; Zanardo, M.; Fedeli, M.P.; Benedek, A.; Di Leo, G.; Menicanti, L.; Sardanelli, F. MRI versus CT in the detection of brain lesions in patients with infective endocarditis before or after cardiac surgery. Neuroradiology 2022, 64, 905–913. [Google Scholar] [CrossRef]
- Morris, N.A.; Matiello, M.; Lyons, J.L.; Samuels, M.A. Neurologic complications in infective endocarditis: Identification, management, and impact on cardiac surgery. Neurohospitalist 2014, 4, 213–222. [Google Scholar] [CrossRef]
- Hu, W.; Wang, X.; Su, G. Infective endocarditis complicated by embolic events: Pathogenesis and predictors. Clin. Cardiol. 2021, 44, 307–315. [Google Scholar] [CrossRef]
- Del Rio, A.; Cervera, C.; Moreno, A.; Moreillon, P.; Miró, J.M. Patients at risk of complications of Staphylococcus aureus bloodstream infection. Clin. Infect. Dis. 2009, 48, 246–253. [Google Scholar] [CrossRef]
- Achim, A.; Stanek, A.; Homorodean, C.; Spinu, M.; Onea, H.L.; Lazăr, L.; Marc, M.; Ruzsa, Z.; Olinic, D.M. Approaches to Peripheral Artery Disease in Diabetes: Are There Any Differences? Int. J. Environ. Res. Public Health 2022, 19, 9801. [Google Scholar] [CrossRef]
- Delahaye, F.; M’Hammedi, A.; Guerpillon, B.; de Gevigney, G.; Boibieux, A.; Dauwalder, O.; Bouchiat, C.; Vandenesch, F. Systematic Search for Present and Potential Portals of Entry for Infective Endocarditis. J. Am. Coll. Cardiol. 2016, 67, 151–158. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, K.; Hua, Y.; Wang, C. Very late-onset endocarditis complicated by non-significant aortic regurgitation after device closure of perimembranous ventricular septal defect. Medicine 2020, 99, e20120. [Google Scholar] [CrossRef]
- Mejía Sanjuanelo, A.M.; Manzur Barbur, M.C.; Martínez-Ávila, M.C.; García Domínguez, J.C. Late Infective Endocarditis Associated with an Atrial Septal Defect Occluder Device. Eur. J. Case Rep. Intern. Med. 2022, 9, 003238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Weena, U.; Medamana, J.; Mann, N.; Strachan, P.; Chikwe, J.; Kort, S. Atrial Septal Defect Closure Device-Related Infective Endocarditis in a 20-Week Pregnant Woman. JACC Case Rep. 2021, 3, 300–303. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serban, A.; Achim, A.; Gavan, D.E.; Tomoaia, R.; Molnar, A.; Suceveanu, M.; Axente, D.D.; Mot, S.; Dadarlat-Pop, A. Surgical Atrial Septal Patch Endocarditis in a Patient with a Complete Corrected Atrioventricular Canal Defect: A Case Report and Review of the Literature. Diagnostics 2023, 13, 856. https://doi.org/10.3390/diagnostics13050856
Serban A, Achim A, Gavan DE, Tomoaia R, Molnar A, Suceveanu M, Axente DD, Mot S, Dadarlat-Pop A. Surgical Atrial Septal Patch Endocarditis in a Patient with a Complete Corrected Atrioventricular Canal Defect: A Case Report and Review of the Literature. Diagnostics. 2023; 13(5):856. https://doi.org/10.3390/diagnostics13050856
Chicago/Turabian StyleSerban, Adela, Alexandru Achim, Dana Elena Gavan, Raluca Tomoaia, Adrian Molnar, Mihai Suceveanu, Dan Damian Axente, Stefan Mot, and Alexandra Dadarlat-Pop. 2023. "Surgical Atrial Septal Patch Endocarditis in a Patient with a Complete Corrected Atrioventricular Canal Defect: A Case Report and Review of the Literature" Diagnostics 13, no. 5: 856. https://doi.org/10.3390/diagnostics13050856
APA StyleSerban, A., Achim, A., Gavan, D. E., Tomoaia, R., Molnar, A., Suceveanu, M., Axente, D. D., Mot, S., & Dadarlat-Pop, A. (2023). Surgical Atrial Septal Patch Endocarditis in a Patient with a Complete Corrected Atrioventricular Canal Defect: A Case Report and Review of the Literature. Diagnostics, 13(5), 856. https://doi.org/10.3390/diagnostics13050856





