The Clinical Significance of Salusins in Systemic Sclerosis—A Cross-Sectional Study
Abstract
1. Introduction
1.1. Bioactive Peptide Biomarkers-Salusin-α and Salusin-β
1.2. Salusins and the Vascular Tone Regulation
1.3. Salusins and the Endothelial Disfunction
2. Materials and Methods
2.1. Ethics
2.2. Study Group and Data Collection
2.3. Salusin-α and Salusin-β Concentrations Measurement
2.4. Statistical Analysis
3. Results
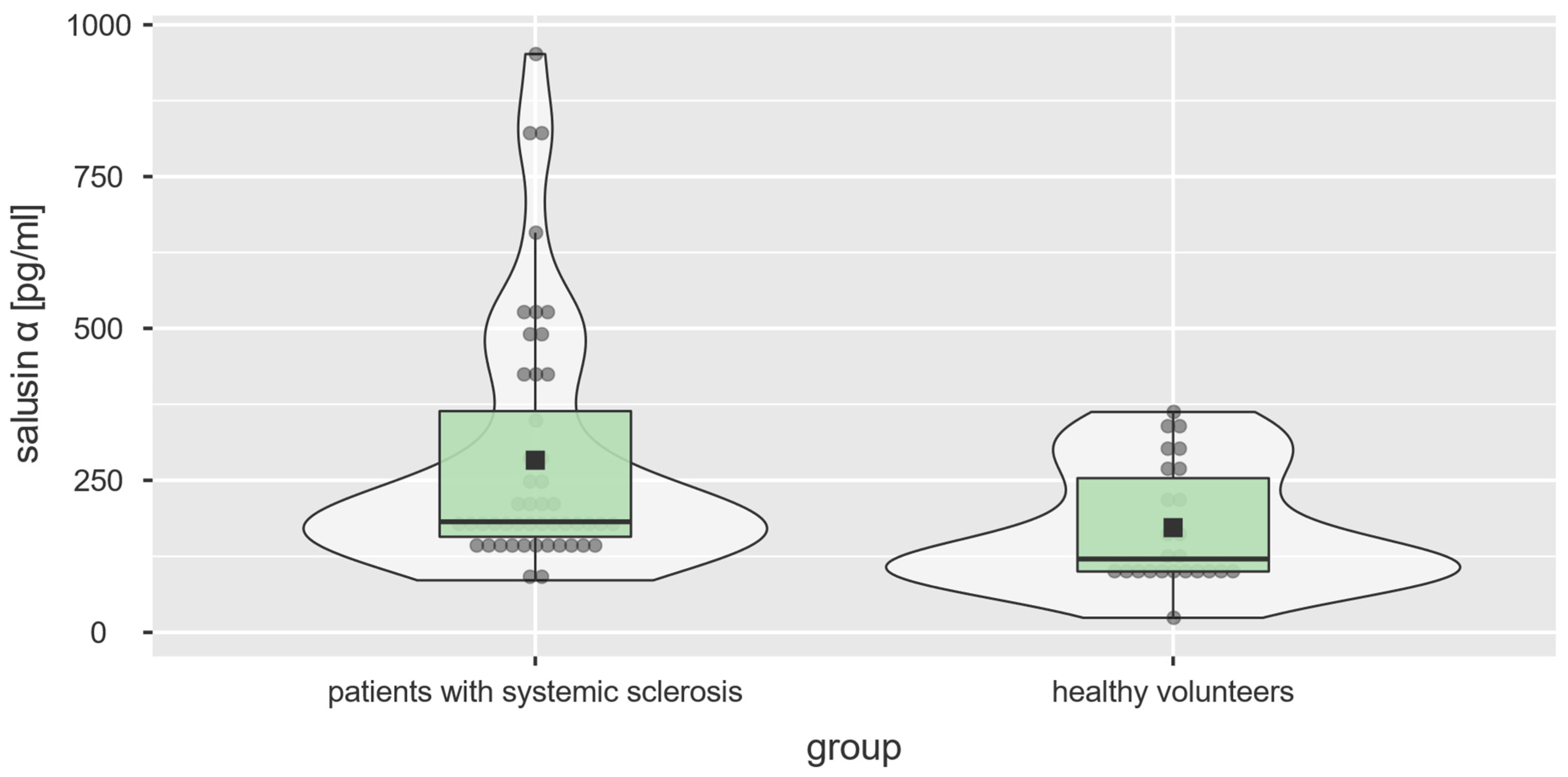
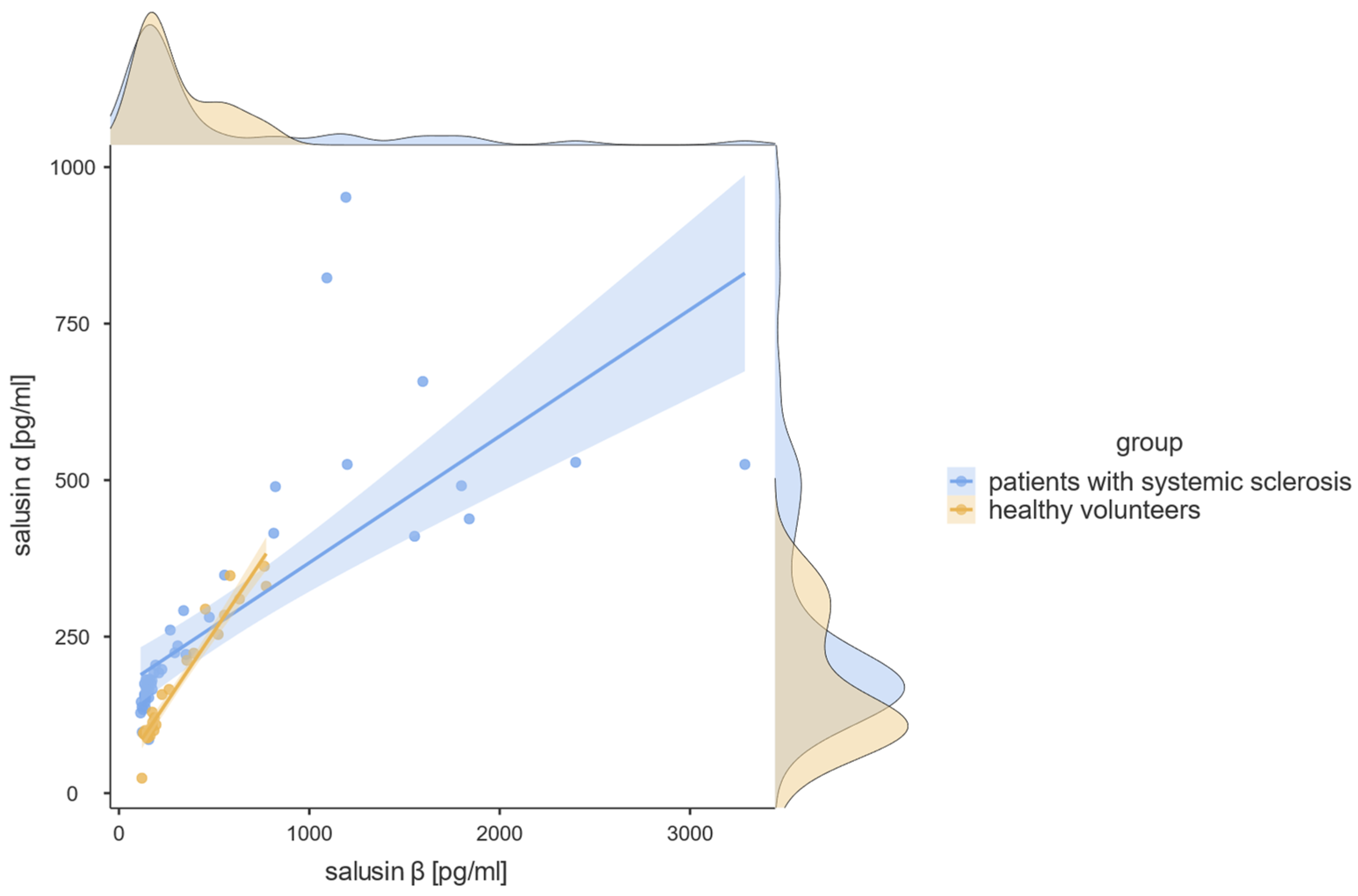
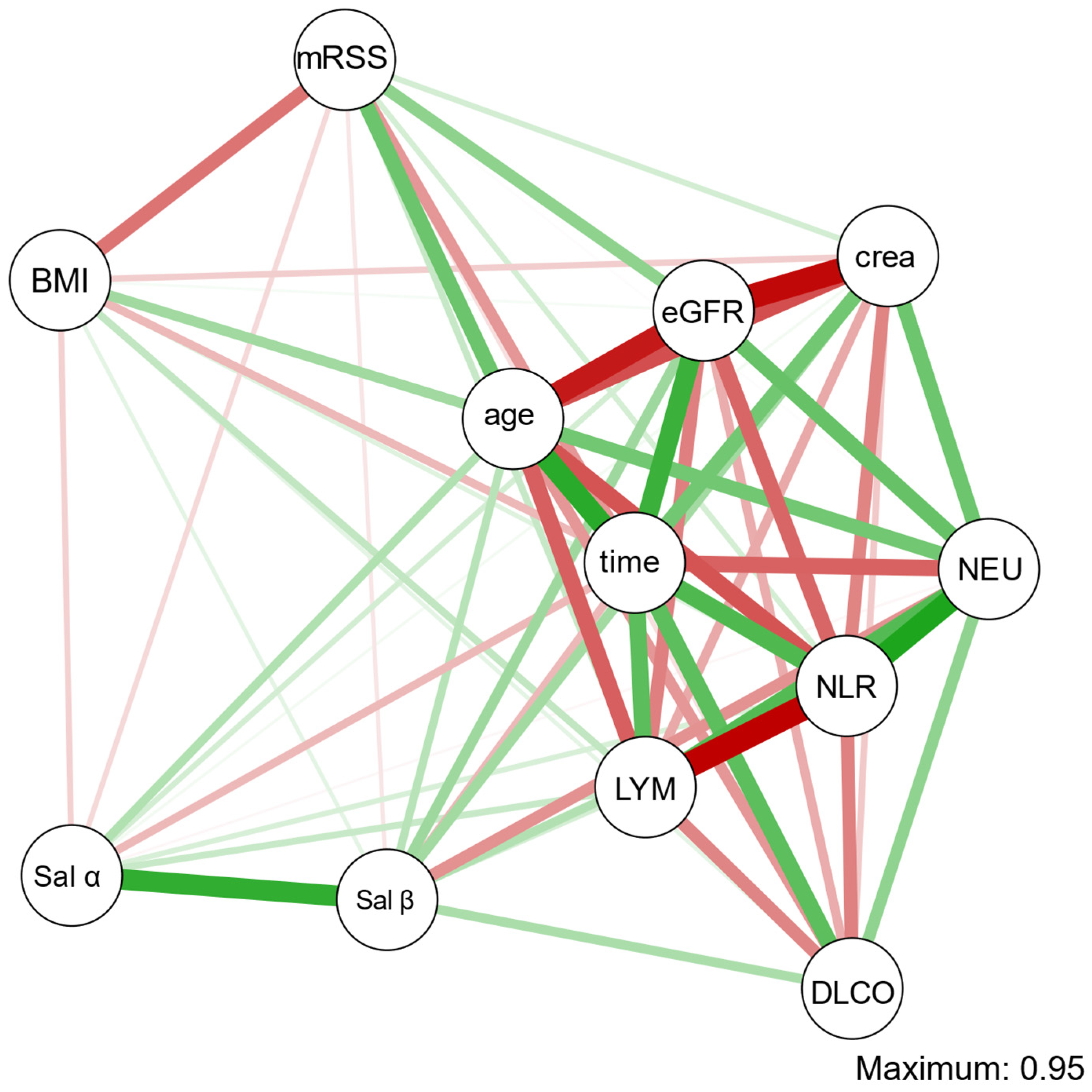
3.1. Salusin-α and Salusin-β Concentrations
3.2. Anti-Topoisomerase I (Scl-70) and Anti-Centromere (ACA) Antibodies
3.3. Skin and Internal Organ Involvement
3.4. Comorbidities
3.5. Treatment
4. Discussion
4.1. Salusin Concentration in the Skin and Connective Tissue Diseases
4.2. The Use of Corticosteroid Therapy
4.3. Non-Corticosteroid Immunosuppressive Medications
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cutolo, M.; Soldano, S.; Smith, V. Pathophysiology of systemic sclerosis: Current understanding and new insights. Expert Rev. Clin. Immunol. 2019, 15, 753–764. [Google Scholar] [CrossRef]
- Zanin-Silva, D.C.; Santana-Gonçalves, M.; Kawashima-Vasconcelos, M.Y.; Oliveira, M.C. Management of Endothelial Dysfunction in Systemic Sclerosis: Current and Developing Strategies. Front. Med. 2021, 8, 788250. [Google Scholar] [CrossRef]
- Affandi, A.J.; Radstake, T.R.D.J.; Marut, W. Update on biomarkers in systemic sclerosis: Tools for diagnosis and treatment. Semin. Immunopathol. 2015, 37, 475–487. [Google Scholar] [CrossRef]
- D’Oria, M.; Gandin, I.; Riccardo, P.; Hughes, M.; Lepidi, S.; Salton, F.; Confalonieri, P.; Confalonieri, M.; Tavano, S.; Ruaro, B. Correlation between Microvascular Damage and Internal Organ Involvement in Scleroderma: Focus on Lung Damage and Endothelial Dysfunction. Diagn. Basel Switz. 2022, 13, 55. [Google Scholar] [CrossRef]
- Mostmans, Y.; Cutolo, M.; Giddelo, C.; Decuman, S.; Melsens, K.; Declercq, H.; Vandecasteele, E.; De Keyser, F.; Distler, O.; Gutermuth, J.; et al. The role of endothelial cells in the vasculopathy of systemic sclerosis: A systematic review. Autoimmun. Rev. 2017, 16, 774–786. [Google Scholar] [CrossRef]
- Smith, V.; Pizzorni, C.; Riccieri, V.; Decuman, S.; Brusselle, G.; De Pauw, M.; Deschepper, E.; Piette, Y.; Ruaro, B.; Sulli, A.; et al. Stabilization of Microcirculation in Patients with Early Systemic Sclerosis with Diffuse Skin Involvement following Rituximab Treatment: An Open-label Study. J. Rheumatol. 2016, 43, 995–996. [Google Scholar] [CrossRef]
- Tieu, A.; Chaigne, B.; Dunogué, B.; Dion, J.; Régent, A.; Casadevall, M.; Cohen, P.; Legendre, P.; Terrier, B.; Costedoat-Chalumeau, N.; et al. Autoantibodies versus Skin Fibrosis Extent in Systemic Sclerosis: A Case-Control Study of Inverted Phenotypes. Diagnostics 2022, 12, 1067. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Zhao, M.; Lu, Q. Meta-analysis of differentially expressed microRNAs in systemic sclerosis. Int. J. Rheum. Dis. 2020, 23, 1297–1304. [Google Scholar] [CrossRef]
- Shichiri, M.; Ishimaru, S.; Ota, T.; Nishikawa, T.; Isogai, T.; Hirata, Y. Salusins: Newly identified bioactive peptides with hemodynamic and mitogenic activities. Nat. Med. 2003, 9, 1166–1172. [Google Scholar] [CrossRef]
- Sato, K.; Watanabe, R.; Itoh, F.; Shichiri, M.; Watanabe, T. Salusins: Potential use as a biomarker for atherosclerotic cardiovascular diseases. Int. J. Hypertens. 2013, 2013, 965140. [Google Scholar] [CrossRef]
- Esfahani, M.; Saidijam, M.; Goodarzi, M.T.; Movahedian, A.; Najafi, R. Salusin-α attenuates inflammatory responses in vascular endothelial cells. Biochem. Mosc. 2017, 82, 1314–1323. [Google Scholar] [CrossRef]
- Sun, H.-J.; Zhao, M.-X.; Liu, T.-Y.; Ren, X.-S.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; Zhu, G.-Q. Salusin-β induces foam cell formation and monocyte adhesion in human vascular smooth muscle cells via miR155/NOX2/NFκB pathway. Sci. Rep. 2016, 6, 23596. [Google Scholar] [CrossRef]
- Sun, H.-J.; Liu, T.-Y.; Zhang, F.; Xiong, X.-Q.; Wang, J.-J.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; Zhou, Y.-B.; Han, Y.; et al. Salusin-β contributes to vascular remodeling associated with hypertension via promoting vascular smooth muscle cell proliferation and vascular fibrosis. Biochim. Biophys. Acta BBA -Mol. Basis Dis. 2015, 1852, 1709–1718. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Liu, L.; Liu, L.; Zhang, M.-X.; Guo, H.; Pan, J.; Yin, X.-X.; Ma, T.-F.; Wu, Y.-Q. Salusin-β not salusin-α promotes vascular inflammation in ApoE-deficient mice via the I-κBα/NF-κB pathway. PLoS ONE 2014, 9, e91468. [Google Scholar] [CrossRef]
- Koya, T.; Miyazaki, T.; Watanabe, T.; Shichiri, M.; Atsumi, T.; Kim-Kaneyama, J.; Miyazaki, A. Salusin-β accelerates inflammatory responses in vascular endothelial cells via NF-κB signaling in LDL receptor-deficient mice in vivo and HUVECs in vitro. Am. J. Physiol.-Heart Circ. Physiol. 2012, 303, H96–H105. [Google Scholar] [CrossRef]
- Ren, X.-S.; Ling, L.; Zhou, B.; Han, Y.; Zhou, Y.-B.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; Zhu, G.-Q. Silencing salusin-β attenuates cardiovascular remodeling and hypertension in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 43259. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Mondal, S.; Saha, A.; Misra, S.; Chatterjee, S.; Rao, A.; Sarkar, A.; Chatterjee, S.; Sinhamahapatra, P.; Ghosh, A. Effect of vasodilator and immunosuppressive therapy on the endothelial dysfunction in patients with systemic sclerosis. Clin. Exp. Med. 2022. [Google Scholar] [CrossRef]
- Cardillo, C.; Kilcoyne, C.M.; Cannon, R.O.; Panza, J.A. Interactions Between Nitric Oxide and Endothelin in the Regulation of Vascular Tone of Human Resistance Vessels In Vivo. Hypertension 2000, 35, 1237–1241. [Google Scholar] [CrossRef]
- Lerman, A.; Zeiher, A.M. Endothelial function: Cardiac events. Circulation 2005, 111, 363–368. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, F.; Pan, Y.; Xu, Y.; Chen, A.; Wang, J.; Tang, H.; Han, Y. A TOR2A Gene Product: Salusin-β Contributes to Attenuated Vasodilatation of Spontaneously Hypertensive Rats. Cardiovasc. Drugs Ther. 2021, 35, 125–139. [Google Scholar] [CrossRef]
- Li, H.-B.; Qin, D.-N.; Cheng, K.; Su, Q.; Miao, Y.-W.; Guo, J.; Zhang, M.; Zhu, G.-Q.; Kang, Y.-M. Central blockade of salusin β attenuates hypertension and hypothalamic inflammation in spontaneously hypertensive rats. Sci. Rep. 2015, 5, 11162. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Z.; Liu, T.; Zhang, W.; Liu, J.; Wang, W.; Wang, J. Salusin-β contributes to vascular inflammation associated with pulmonary arterial hypertension in rats. J. Thorac. Cardiovasc. Surg. 2016, 152, 1177–1187. [Google Scholar] [CrossRef]
- Watanabe, T.; Suguro, T.; Sato, K.; Koyama, T.; Nagashima, M.; Kodate, S.; Hirano, T.; Adachi, M.; Shichiri, M.; Miyazaki, A. Serum salusin-α levels are decreased and correlated negatively with carotid atherosclerosis in essential hypertensive patients. Hypertens. Res. 2008, 31, 463–468. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Pan, J.; Huang, H.; Zhu, Y.; Zhang, M.; Liu, L.; Wu, Y. Salusin-β, but Not Salusin-α, Promotes Human Umbilical Vein Endothelial Cell Inflammation via the p38 MAPK/JNK-NF-κB Pathway. PLoS ONE 2014, 9, e107555. [Google Scholar] [CrossRef]
- Gao, S.; Xu, L.; Zhang, Y.; Yu, Q.; Li, J.; Guan, H.; Wang, X.; Cheng, D.; Liu, Y.; Bai, L.; et al. Salusin-α Inhibits Proliferation and Migration of Vascular Smooth Muscle Cell via Akt/mTOR Signaling. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 50, 1740–1753. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet Lond. Engl. 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Krasowska, D.; Rudnicka, L.; Dańczak-Pazdrowska, A.; Chodorowska, G.; Woźniacka, A.; Lis-Święty, A.; Czuwara, J.; Maj, J.; Majewski, S.; Sysa-Jędrzejowska, A.; et al. Systemic sclerosis—Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Part 2: Treatment. Dermatol. Rev. Dermatol. 2017, 104, 583–596. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2022, 79, 268–288. [Google Scholar] [CrossRef]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef]
- Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research (Version 2.2.9). 2022. Available online: https://CRAN.R-project.org/package=psych (accessed on 15 December 2022).
- Epskamp, S.; Costantini, G.; Haslbeck, J.; Isvoranu, A.; Cramer, A.O.J.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. qgraph: Graph Plotting Methods, Psychometric Data Visualization and Graphical Model Estimation (Version 1.9.3). 2022. Available online: https://CRAN.R-project.org/package=qgraph (accessed on 15 December 2022).
- Seol, H. Seolmatrix. 2022. Available online: https://github.com/hyunsooseol/seolmatrix (accessed on 15 December 2022).
- Kim, S. ppcor: Partial and Semi-Partial (Part) Correlation (Version 1.1). 2015. Available online: https://CRAN.R-project.org/package=ppcor (accessed on 15 December 2022).
- The Jamovi Project Jamovi (Version 2.3). 2022. Available online: https://www.jamovi.org (accessed on 15 December 2022).
- R Core Team. R: A Language and Environment for Statistical Computing (Version 4.1). 2021. Available online: https://www.R-project.org/ (accessed on 15 December 2022).
- Kimoto, S.; Sato, K.; Watanabe, T.; Suguro, T.; Koyama, T.; Shichiri, M. Serum levels and urinary excretion of salusin-α in renal insufficiency. Regul. Pept. 2010, 162, 129–132. [Google Scholar] [CrossRef]
- Watanabe, T.; Nishio, K.; Kanome, T.; Matsuyama, T.; Koba, S.; Sakai, T.; Sato, K.; Hongo, S.; Nose, K.; Ota, H.; et al. Impact of salusin-alpha and -beta on human macrophage foam cell formation and coronary atherosclerosis. Circulation 2008, 117, 638–648. [Google Scholar] [CrossRef]
- SCORE2 working group and ESC Cardiovascular risk collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- Yılmaz, E.; Kurt, D.; Aydın, E.; Çamcı, S.; Vural, A. A New Marker for Determining Cardiovascular Risk: Salusin Alpha. Cureus 2022, 14, e30340. [Google Scholar] [CrossRef]
- Koca, S.S.; Ozgen, M.; Isik, B.; Dagli, M.N.; Ustundag, B.; Isik, A. Serum salusin-α levels in systemic lupus erythematosus and systemic sclerosis. Eur. J. Rheumatol. 2014, 1, 14–17. [Google Scholar] [CrossRef]
- Erden, I.; Uçak, H.; Demir, B.; Cicek, D.; Bakar Dertlioğlu, S.; Oztürk, S.; Aydin, S. Serum salusin-α and salusin-β levels in patients with psoriasis. Eur. J. Dermatol. 2015, 25, 352–353. [Google Scholar] [CrossRef]
- Erden, I.; Demir, B.; Uçak, H.; Cicek, D.; Dertlioğlu, S.B.; Aydin, S. Serum salusin-α and salusin-β levels in patients with Behcet’s disease. Eur. J. Dermatol. 2014, 24, 577–582. [Google Scholar] [CrossRef]
- Ozgen, M.; Koca, S.S.; Dagli, N.; Balin, M.; Ustundag, B.; Isik, A. Serum salusin-alpha level in rheumatoid arthritis. Regul. Pept. 2011, 167, 125–128. [Google Scholar] [CrossRef]
- Kobak, S.; Atabay, T.; Akyildiz, M.; Gokduman, A.; Vural, H. Serum salusin-α and salusin-β levels in patients with psoriatic arthritis. Reumatologia 2022, 60, 306–310. [Google Scholar] [CrossRef]
- Cinoku, I.I.; Mavragani, C.P.; Moutsopoulos, H.M. Atherosclerosis: Beyond the lipid storage hypothesis. The role of autoimmunity. Eur. J. Clin. Investig. 2020, 50, e13195. [Google Scholar] [CrossRef]
- Marks, J.L.; Edwards, C.J. Protective effect of methotrexate in patients with rheumatoid arthritis and cardiovascular comorbidity. Ther. Adv. Musculoskelet. Dis. 2012, 4, 149–157. [Google Scholar] [CrossRef]
- Bălănescu, A.-R.; Bojincă, V.C.; Bojincă, M.; Donisan, T.; Bălănescu, S.M. Cardiovascular effects of methotrexate in immune-mediated inflammatory diseases. Exp. Ther. Med. 2019, 17, 1024–1029. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Zinellu, A.; Sotgia, S.; Carru, C.; Piga, M.; Erre, G.L. Protective Effects of Methotrexate against Proatherosclerotic Cytokines: A Review of the Evidence. Mediators Inflamm. 2017, 2017, 9632846. [Google Scholar] [CrossRef]
- Liu, D.; Lv, H.; Liu, Q.; Sun, Y.; Hou, S.; Zhang, L.; Yang, M.; Han, B.; wang, G.; Wang, X.; et al. Atheroprotective effects of methotrexate via the inhibition of YAP/TAZ under disturbed flow. J. Transl. Med. 2019, 17, 378. [Google Scholar] [CrossRef]
- Yang, D.; Haemmig, S.; Zhou, H.; Pérez-Cremades, D.; Sun, X.; Chen, L.; Li, J.; Haneo-Mejia, J.; Yang, T.; Hollan, I.; et al. Methotrexate attenuates vascular inflammation through an adenosine-microRNA-dependent pathway. eLife 2021, 10, e58064. [Google Scholar] [CrossRef]
- Riksen, N.P.; Barrera, P.; van den Broek, P.H.H.; van Riel, P.L.C.M.; Smits, P.; Rongen, G.A. Methotrexate modulates the kinetics of adenosine in humans in vivo. Ann. Rheum. Dis. 2006, 65, 465–470. [Google Scholar] [CrossRef]
- Verhoeven, F.; Prati, C.; Chouk, M.; Demougeot, C.; Wendling, D. Methotrexate and cardiovascular risk in rheumatic diseases: A comprehensive review. Expert Rev. Clin. Pharmacol. 2021, 14, 1105–1112. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, M.-J.; Lee, C.-K.; Hong, Y.-H. Effects of Methotrexate on Carotid Intima-media Thickness in Patients with Rheumatoid Arthritis. J. Korean Med. Sci. 2015, 30, 1589–1596. [Google Scholar] [CrossRef]
- Plein, S.; Erhayiem, B.; Fent, G.; Horton, S.; Dumitru, R.B.; Andrews, J.; Greenwood, J.P.; Emery, P.; Hensor, E.M.; Baxter, P.; et al. Cardiovascular effects of biological versus conventional synthetic disease-modifying antirheumatic drug therapy in treatment-naïve, early rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 1414–1422. [Google Scholar] [CrossRef]
- Turiel, M.; Tomasoni, L.; Sitia, S.; Cicala, S.; Gianturco, L.; Ricci, C.; Atzeni, F.; De Gennaro Colonna, V.; Longhi, M.; Sarzi-Puttini, P. Effects of long-term disease-modifying antirheumatic drugs on endothelial function in patients with early rheumatoid arthritis. Cardiovasc. Ther. 2010, 28, e53–e64. [Google Scholar] [CrossRef]
- van Leuven, S.I.; Kastelein, J.J.P.; Allison, A.C.; Hayden, M.R.; Stroes, E.S.G. Mycophenolate mofetil (MMF): Firing at the atherosclerotic plaque from different angles? Cardiovasc. Res. 2006, 69, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Bryk, D.; Zapolska-Downar, D.; Małecki, M.; Stachurska, A.; Sitkiewicz, D. Mycophenolic acid attenuates the tumour necrosis factor-α-mediated proinflammatory response in endothelial cells by blocking the MAPK/NF-κB and ROS pathways. Eur. J. Clin. Investig. 2014, 44, 54–64. [Google Scholar] [CrossRef]
- Miljkovic, D.; Cvetkovic, I.; Stosic-Grujicic, S.; Trajkovic, V. Mycophenolic acid inhibits activation of inducible nitric oxide synthase in rodent fibroblasts. Clin. Exp. Immunol. 2003, 132, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Senda, M.; DeLustro, B.; Eugui, E.; Natsumeda, Y. Mycophenolic acid, an inhibitor of IMP dehydrogenase that is also an immunosuppressive agent, suppresses the cytokine-induced nitric oxide production in mouse and rat vascular endothelial cells. Transplantation 1995, 60, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.Y.; Kim, Y.J.; Yang, W.S.; Park, J.S.; Han, N.J.; Lee, J.M.; Park, S.K. Mycophenolic acid regulates spleen tyrosine kinase to repress tumour necrosis factor-alpha-induced monocyte chemotatic protein-1 production in cultured human aortic endothelial cells. Cell Biol. Int. 2013, 37, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.N.; Magder, L.S.; Petri, M. Mycophenolate mofetil (MMF) does not slow the progression of subclinical atherosclerosis in SLE over 2 years. Rheumatol. Int. 2012, 32, 2701–2705. [Google Scholar] [CrossRef] [PubMed]
- Sato-Okabayashi, Y.; Isoda, K.; Heissig, B.; Kadoguchi, T.; Akita, K.; Kitamura, K.; Shimada, K.; Hattori, K.; Daida, H. Low-dose oral cyclophosphamide therapy reduces atherosclerosis progression by decreasing inflammatory cells in a murine model of atherosclerosis. Int. J. Cardiol. Heart Vasc. 2020, 28, 100529. [Google Scholar] [CrossRef]
- Watanabe, T.; Sato, K.; Itoh, F.; Iso, Y.; Nagashima, M.; Hirano, T.; Shichiri, M. The roles of salusins in atherosclerosis and related cardiovascular diseases. J. Am. Soc. Hypertens. JASH 2011, 5, 359–365. [Google Scholar] [CrossRef]

| Variable | Patients with Systemic Sclerosis (N = 48) |
|---|---|
| women, n, % | 44 (92%) |
| age, years | 56.4 (11.4) |
| BMI, kg/m2 | 24.9 (4.1) |
| duration of disease since the first diagnosis, years | 6.5 [1–38] |
| serum creatinine, mg/dl | 0.73 [0.48–1.06] |
| CKD-EPI eGFR, mL/min/1.73 m2 | 92.78 (16.88) |
| mRSS | 6 [2–22] |
| DLCO, n, % | 71.1 [36.0–135.6] |
| neutrophiles, n, % | 63.6 (8.7) |
| lymphocytes, n, % | 25.2 (7.6) |
| NLR | 2.63 [0.94–6.69] |
| coronary artery disease, n, % | 7 (15%) * |
| hyper- or hypothyroidism, n, % | 11 (23%) |
| arterial hypertension, n, % | 17 (35%) |
| gastroesophageal reflux disease, n, % | 11 (23%) * |
| another autoimmune disease, n, % | 11 (23%) * |
| dyslipidemia, n, % | 13 (30%) ᶧ |
| Scl-70 antibodies, n, % | 23 (49%) * |
| ACA antibodies, n, % | 19 (40%) * |
| immunosuppressive treatment, n, % | 27 (57%) * |
| Salusin | Patients with Systemic Sclerosis (N = 48) | Healthy Volunteers (N = 25) | Test Statistic and p Value |
|---|---|---|---|
| salusin-α, pg/mL, median [range] | 181.85 [85.66–951.80] | 120.70 [24.03–362.40] | U = 350.5, p = 0.004 |
| salusin-β, pg/mL, median [range] | 173.70 [113.70–3288.00] | 186.30 [120.30–773.50] | U = 550.0, p = 0.662 |
| Organ/System | Variable | For Salusin-α | For Salusin-β | ||
|---|---|---|---|---|---|
| Test Statistic/Correlation Coefficient | p Value | Test Statistic/Correlation Coefficient | p Value | ||
| skin | mRSS | ρ = −0.05 | 0.772 | ρ = −0.12 | 0.474 |
| fingertip ulcerations | U = 71.0 | 0.101 | U = 65.0 | 0.150 | |
| cardiovascular | Raynaud’s phenomenon | U = 228.5 | 0.227 | U = 184.5 | 0.053 |
| arterial hypertension | U = 244.5 | 0.690 | U = 242.0 | 0.782 | |
| coronary artery disease | U = 112.5 | 0.420 | U = 115.0 | 0.521 | |
| pulmonary | lung fibrosis in HRCT | U = 92.0 | 0.724 | U = 99.0 | 0.986 |
| DLCO | ρ = 0.12 | 0.561 | ρ = 0.22 | 0.305 | |
| gastrointestinal | esophageal dysmotility in X-ray with contrast | U = 65.5 | 0.461 | U = 54.5 | 0.267 |
| gastroesophageal reflux | U = 194.0 | 0.930 | U = 183.5 | 0.725 | |
| renal | serum creatinine | ρ = −0.32 | 0.035 | ρ = −0.37 | 0.015 |
| No Immunosuppression (N = 17), pg/mL, Median [Range] | With Immunosuppression (N = 27), pg/mL, Median [Range] | Test Statistic and p Value |
|---|---|---|
| 174.50 [85.66–525.10] | 235.30 [133.50–951.80] | U = 176.0, p = 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowaczyk, J.; Blicharz, L.; Zawistowski, M.; Sikora, M.; Zaremba, M.; Czuwara, J.; Rudnicka, L. The Clinical Significance of Salusins in Systemic Sclerosis—A Cross-Sectional Study. Diagnostics 2023, 13, 848. https://doi.org/10.3390/diagnostics13050848
Nowaczyk J, Blicharz L, Zawistowski M, Sikora M, Zaremba M, Czuwara J, Rudnicka L. The Clinical Significance of Salusins in Systemic Sclerosis—A Cross-Sectional Study. Diagnostics. 2023; 13(5):848. https://doi.org/10.3390/diagnostics13050848
Chicago/Turabian StyleNowaczyk, Joanna, Leszek Blicharz, Michał Zawistowski, Mariusz Sikora, Michał Zaremba, Joanna Czuwara, and Lidia Rudnicka. 2023. "The Clinical Significance of Salusins in Systemic Sclerosis—A Cross-Sectional Study" Diagnostics 13, no. 5: 848. https://doi.org/10.3390/diagnostics13050848
APA StyleNowaczyk, J., Blicharz, L., Zawistowski, M., Sikora, M., Zaremba, M., Czuwara, J., & Rudnicka, L. (2023). The Clinical Significance of Salusins in Systemic Sclerosis—A Cross-Sectional Study. Diagnostics, 13(5), 848. https://doi.org/10.3390/diagnostics13050848







