Clinically Relevant Anatomical Variations in the Brachial Plexus
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Data Collection and Analysis
3. Results
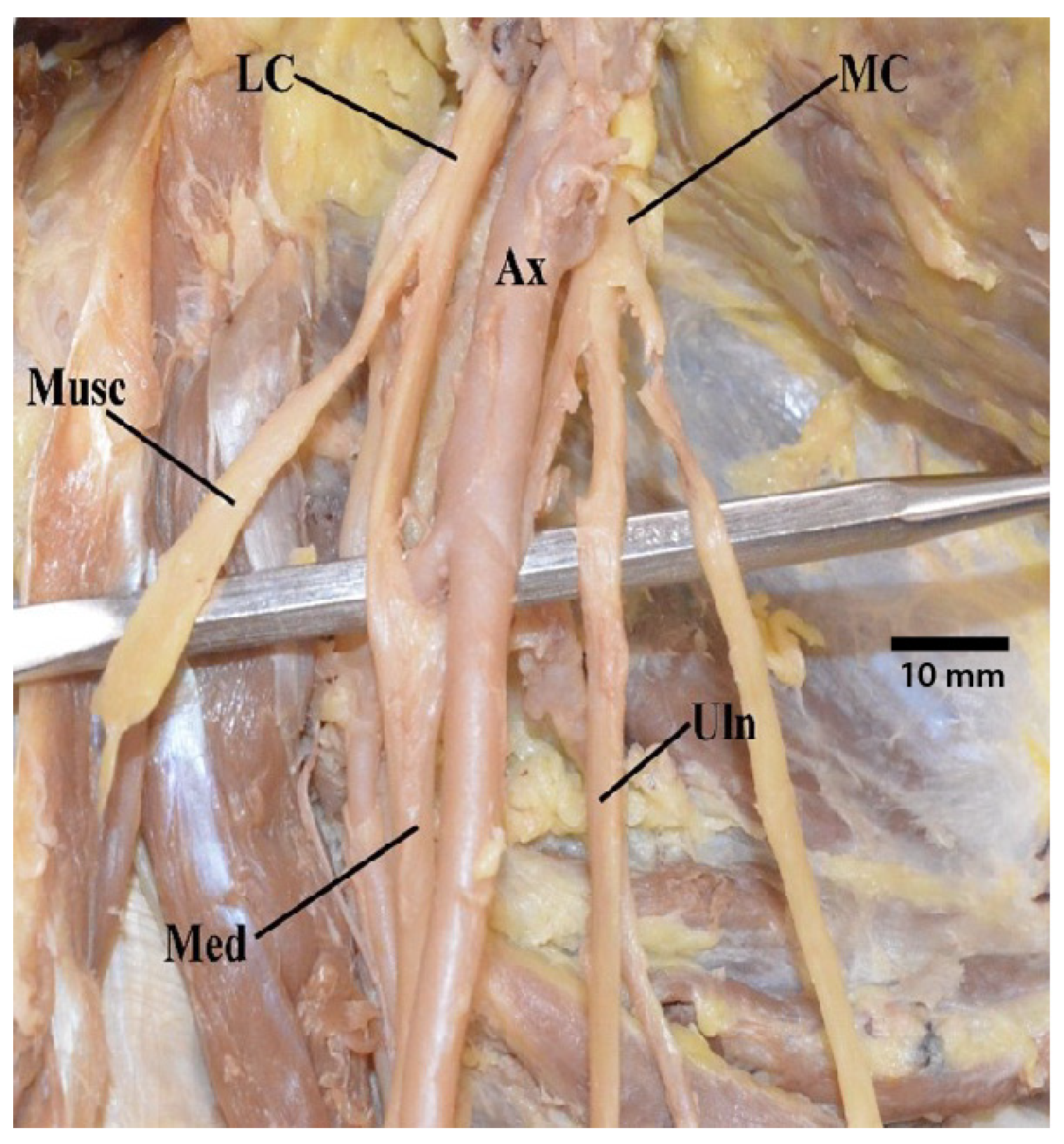
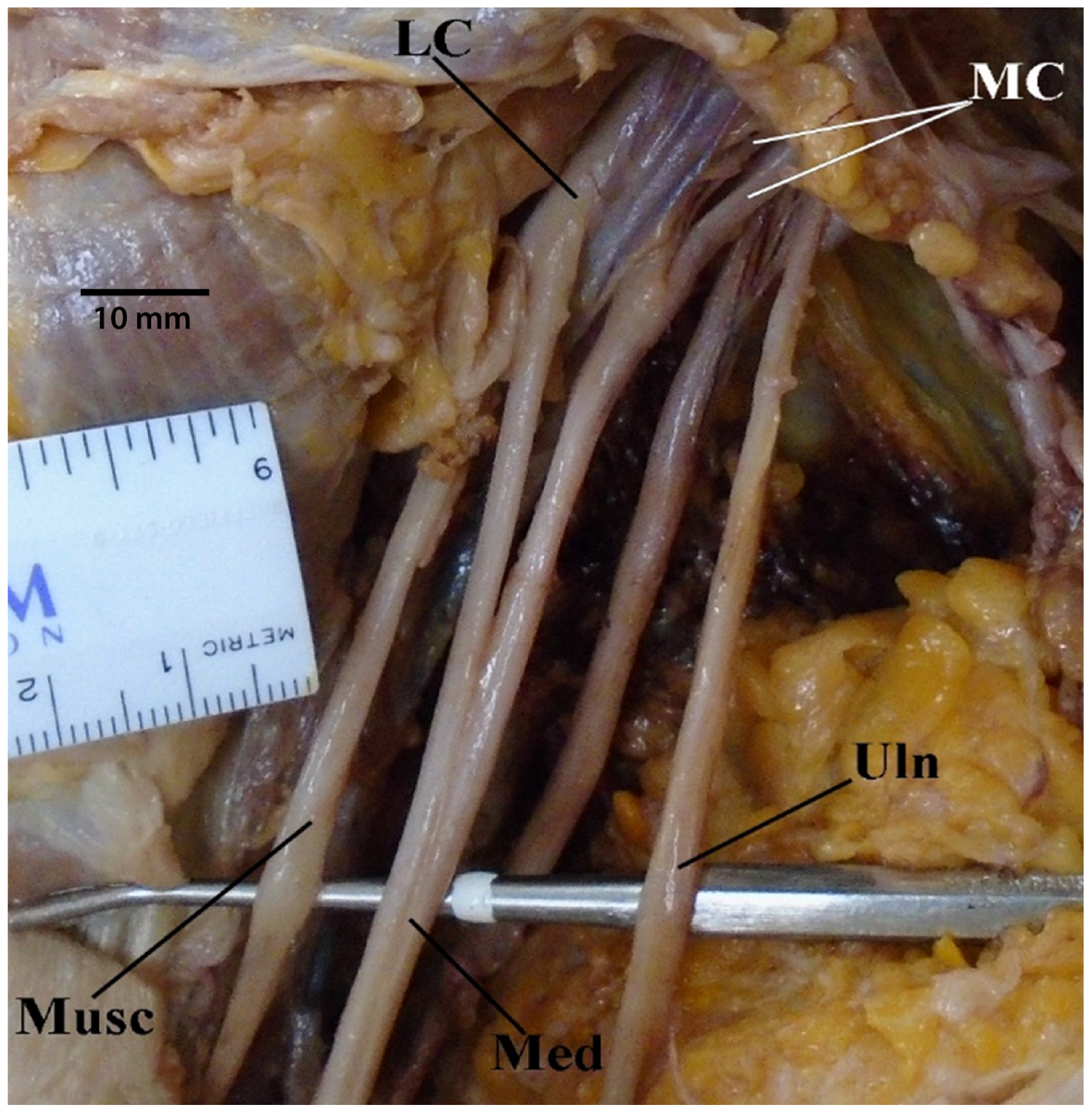
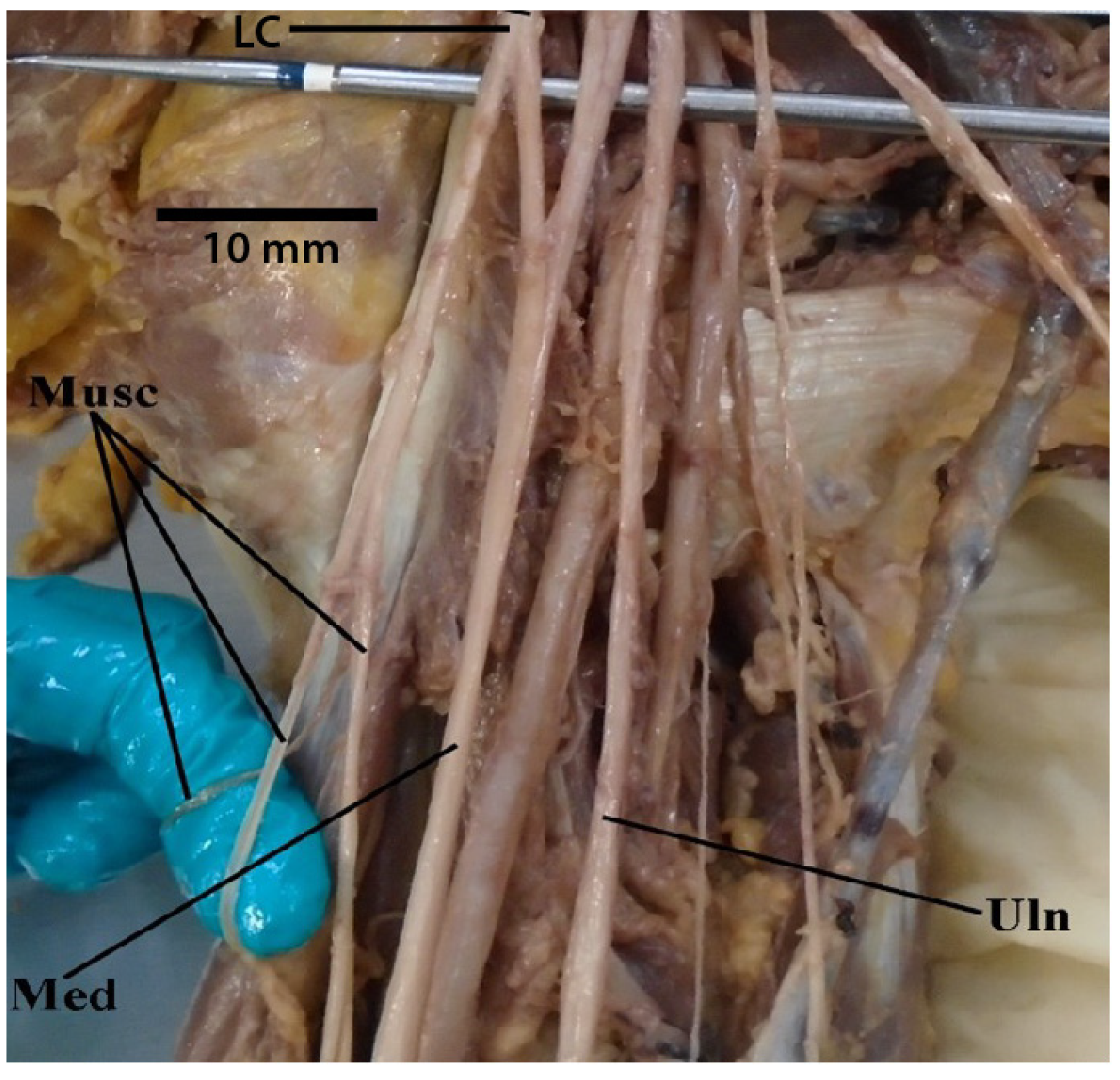
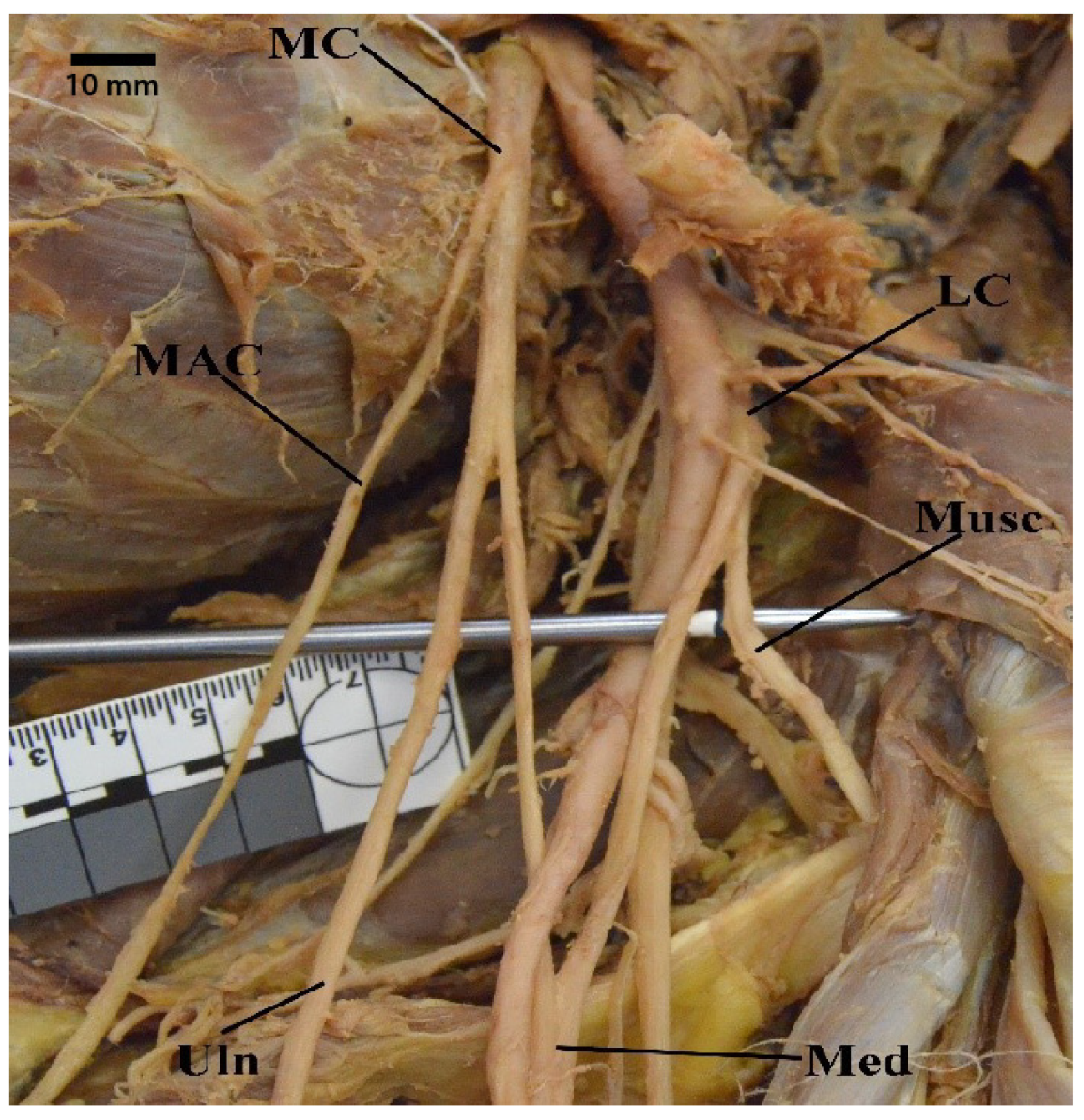
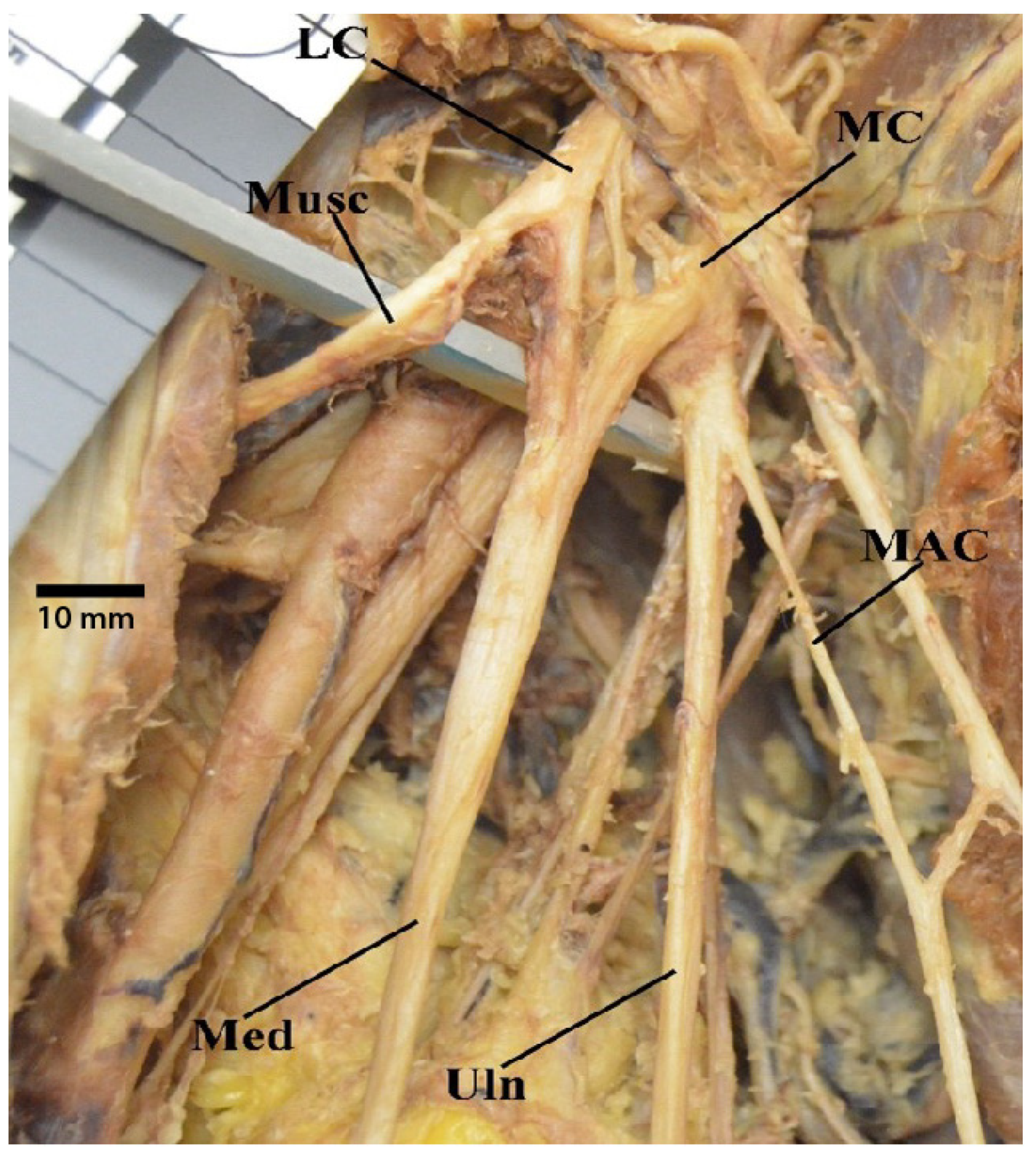
3.1. “M” Branching
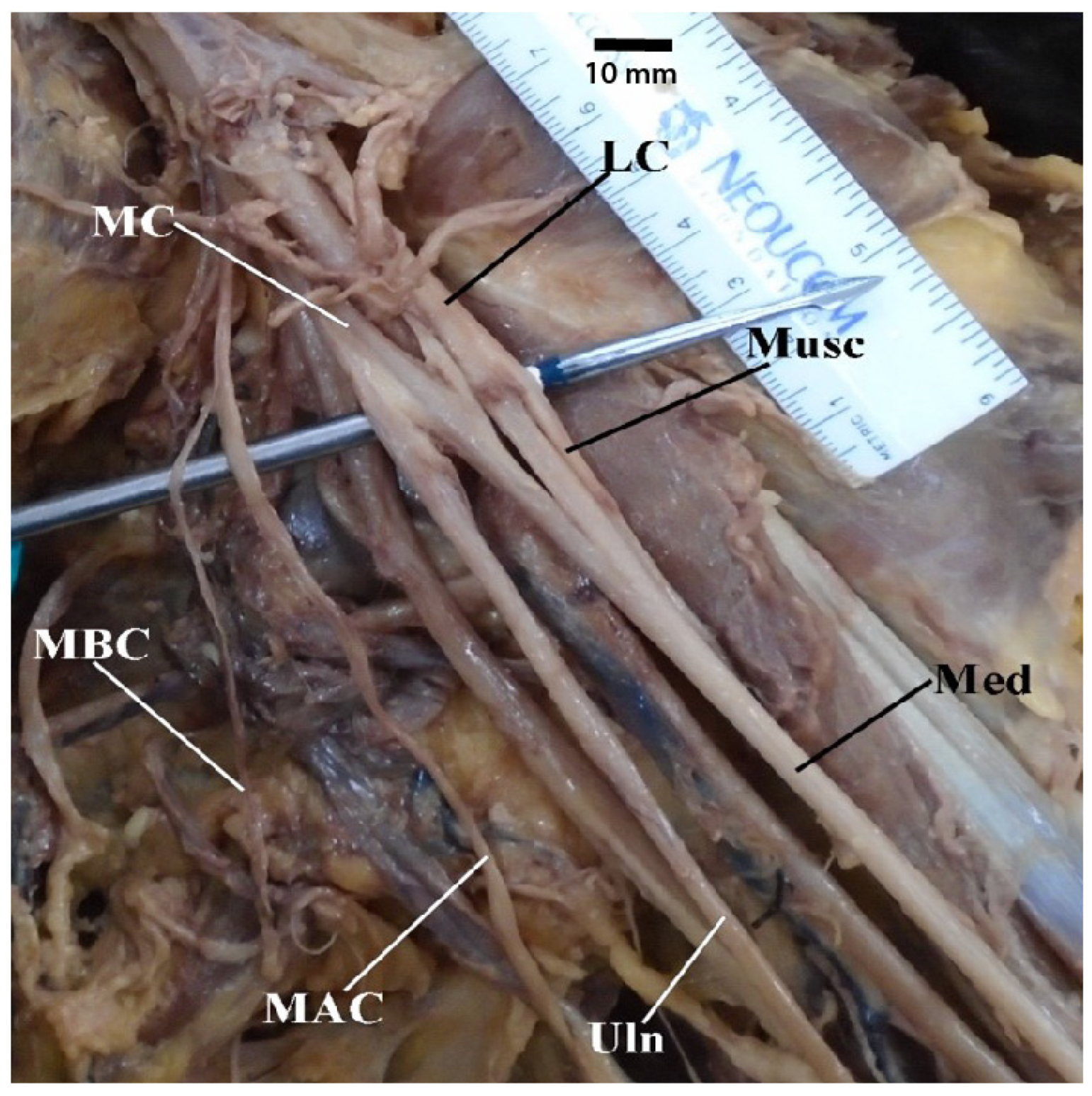
3.2. Common Trunk of Medial Brachial Cutaneous Nerve and Medial Antebrachial Cutaenous Nerve
3.3. Medial Antebrachial Cutaneous Nerve Arising from Inferior Trunk or Ulnar Nerve
3.4. Medial Pectoral Nerve Receives Contributions from Both Medial and Lateral Cords or Just Lateral Cord
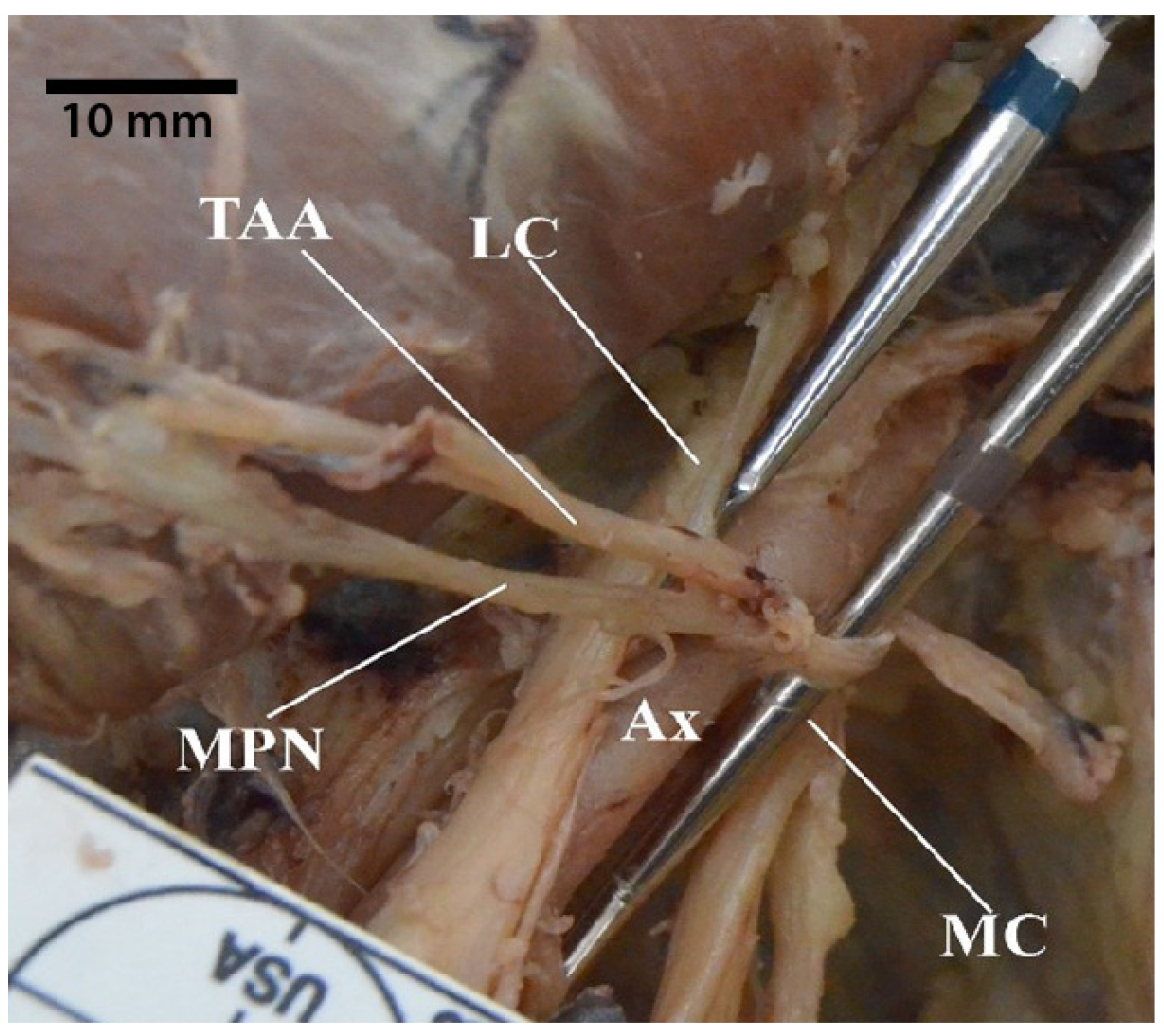
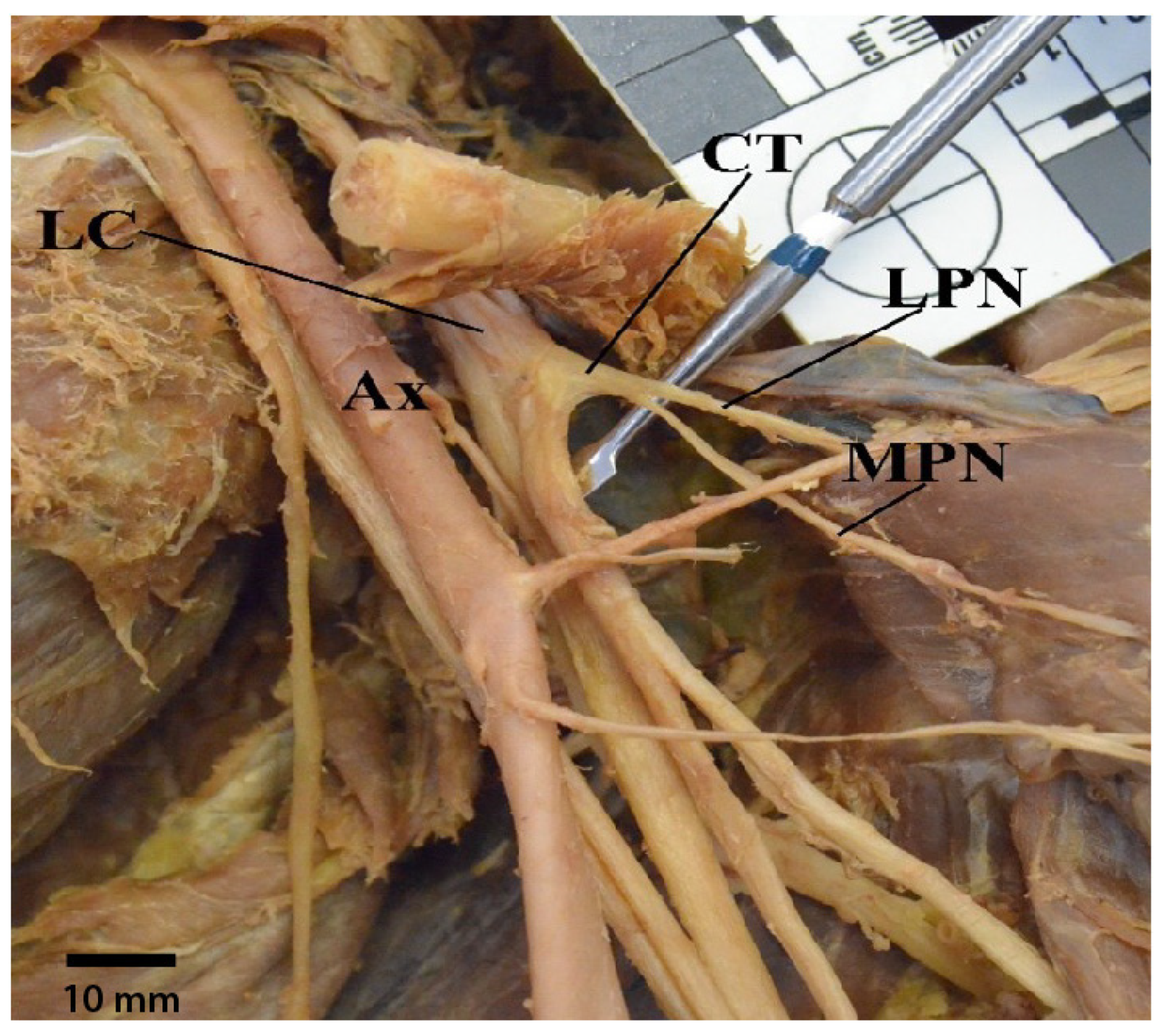
3.5. Thoracodorsal Nerve Originating from the Axillary Nerve
3.6. Ulnar Nerve Receiving Communicating Branches from the Lateral Cord
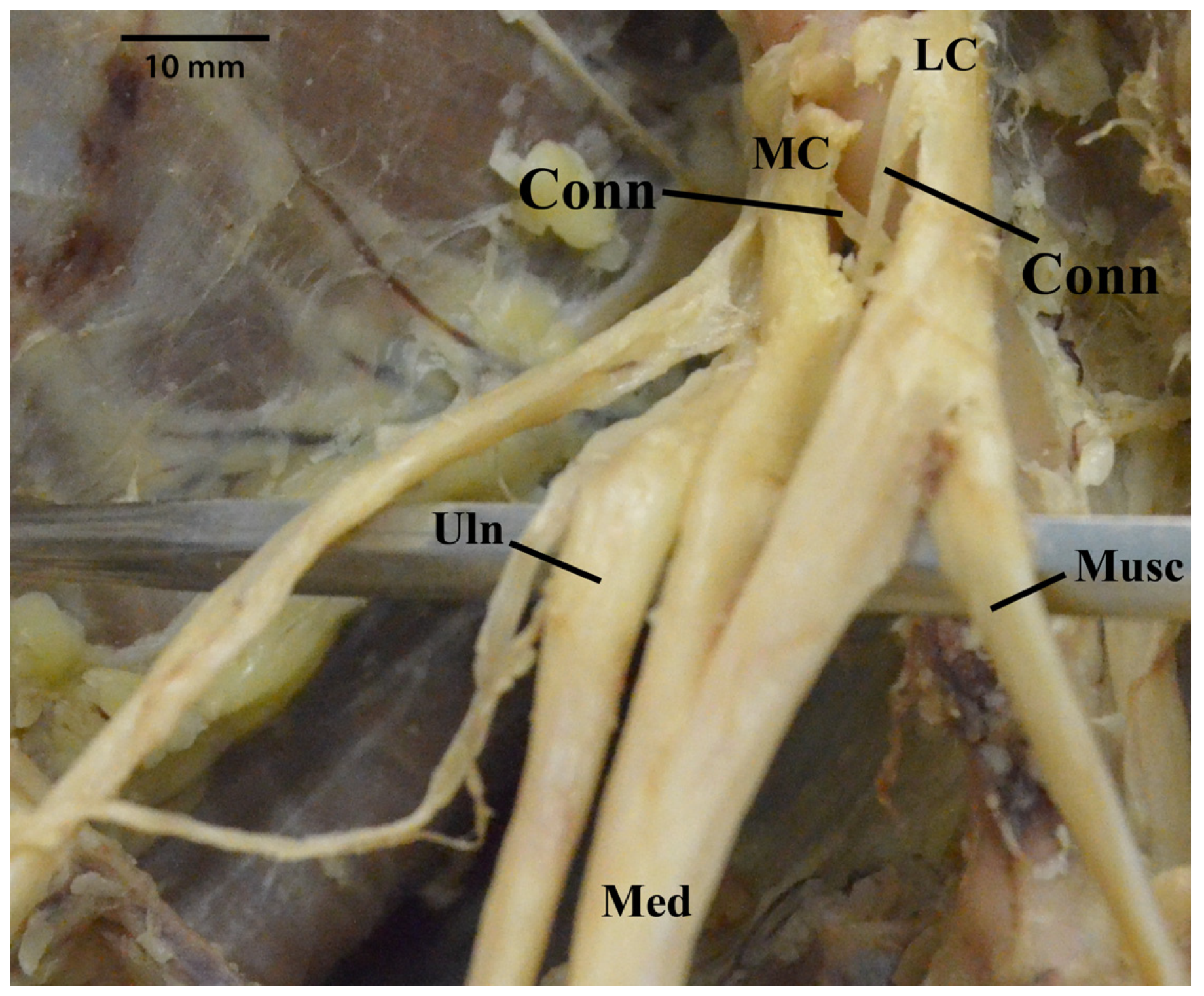
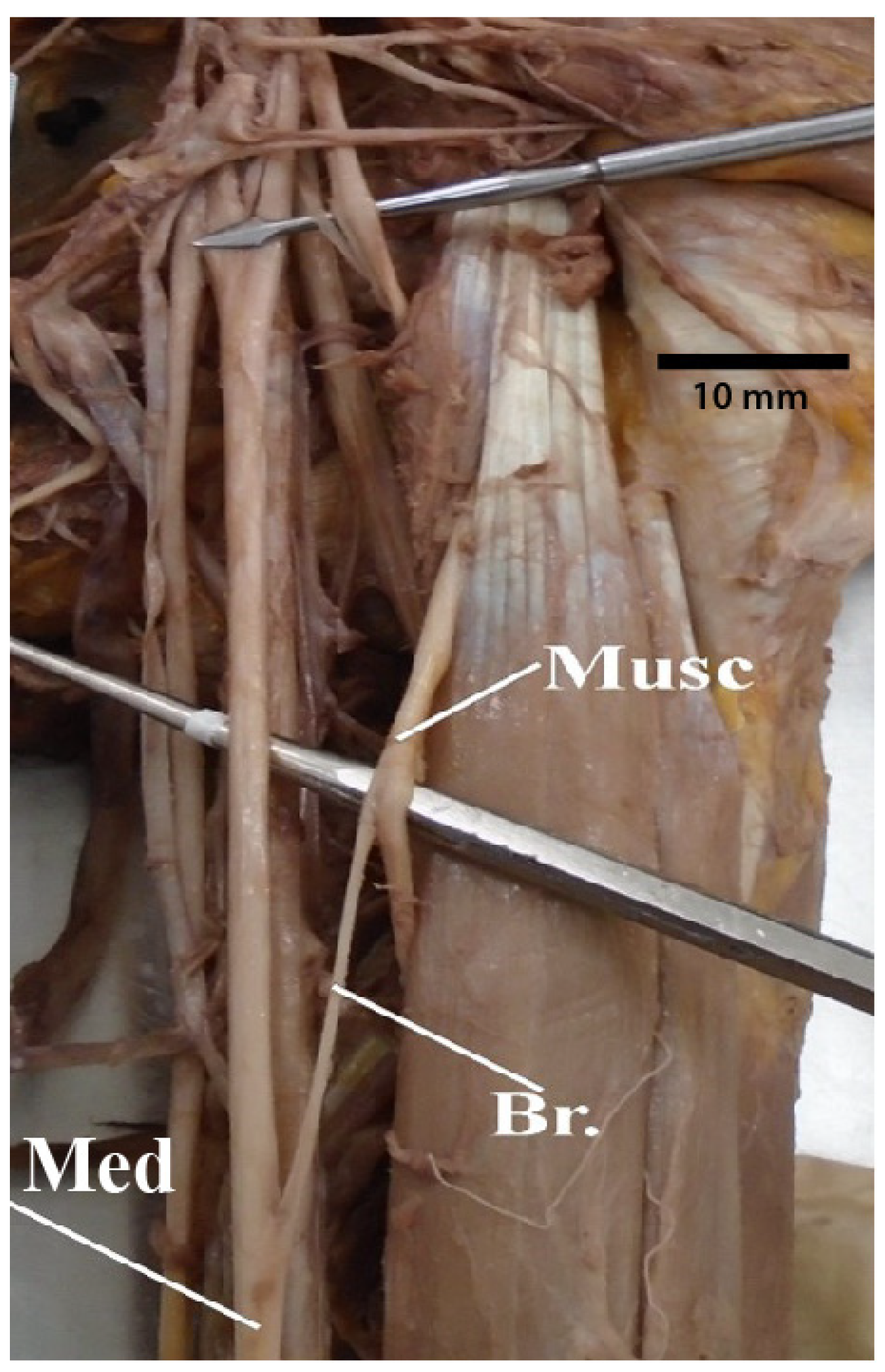
3.7. Median Nerve Receiving Branches from the Musculocutaneous Nerve
3.8. Median Nerve Receiving Branches from the Posterior Cord
3.9. Results of Statistical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Sobhi, M.G.; Zaki, A.I.; El Hamid, F.A.A.; Alshali, R.A.; Mustafa, H.N. The pattern of branching and intercommunications of the musculocutaneous nerve for surgical issues: Anatomical study. Folia Morphol. 2021, in press. [CrossRef]
- Arad, E.; Li, Z.; Sitzman, T.J.; Agur, A.M.; Clarke, H.M. Anatomic sites of origin of the suprascapular and lateral pectoral nerves within the brachial plexus. J. Plast. Reconstr. Surg. 2014, 133, 20e–27e. [Google Scholar] [CrossRef]
- Arquez, H.F.; Hurtado, D.K.A. An anatomical study of formation of the median nerve. J. Chem. Pharm. Res. 2016, 8, 22–26. [Google Scholar]
- Ballesteros, D.R.L.; Forero, P.L.P.; Ballesteros, L.E.A. Anatomic variations in relation to the origin of the musculocutaneous nerve: Absence and non-perforation of the coracobrachialis muscle: Anatomical study and clinical significance. Int. J. Morphol. 2018, 36, 425–429. [Google Scholar] [CrossRef]
- Balsurkar, S.K.; Ramdas, S.G.; Pathan, F.J.; Mahesh, U.; Anand, R. A cadaveric study of variations in branching pattern of brachial plexus. IJRTSAT 2015, 16, 83–87. [Google Scholar]
- Benes, M.; Kachlik, D. Atypical branching of the musculocutaneous and median nerves with associated unusual innervation of muscles in the anterior compartment of the arm: Case report and plea for extension of the current classification system. Surg. Radiol. Anat. 2021, 43, 671–678. [Google Scholar] [CrossRef]
- Boers, N.; Bleys, R.L.A.W.; Schellekens, P.P.A. The nerve supply to the pectoralis major: An anatomical study and clinical application of the denervation in subpectoral breast implant surgery. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 415–423. [Google Scholar] [CrossRef]
- Chaudhary, P.; Singla, R.; Arora, K.; Kalsey, G. Formation and branching pattern of cords of brachial plexus-a cadaveric study in north Indian population. Int. J. Anat. Res. 2014, 2, 225–233. [Google Scholar]
- Emamhadi, M.; Chabok, S.Y.; Samini, F.; Alijani, B.; Behzadnia, H.; Firozabadi, F.A.; Reihanian, Z. Anatomical variations of brachial plexus in adult cadavers; a descriptive study. Arch. Bone Jt. Surg. 2016, 4, 253–258. [Google Scholar]
- Farashahi, M.S.G.; Mohammadi, A.H.; Abolhasani, F. Anatomic variations of brachial plexus: A cadaveric study. Anat. Sci. 2014, 11, 127–130. [Google Scholar]
- Fazan, V.P.S.; Amadeu, A.D.S.; Caleffi, A.L.; Rodrigues Filho, O.A. Brachial plexus variations in its formation and main branches. Acta Cir. Bras. 2002, 18, 14–18. [Google Scholar] [CrossRef]
- Flatow, E.L.; Bigliani, L.U.; April, E.W. An anatomic study of the musculocutaneous nerve and its relationship to the coracoid process. Clin. Orthop. Relat. Res. 1989, 244, 166–171. [Google Scholar] [CrossRef]
- Fuss, F.K.; Matula, C.W.; Tschabitscher, M. Die Arteria brachialis superficialis. Anat. Anz. 1985, 160, 285–294. [Google Scholar]
- Gaur, S.; Katariya, S.K.; Wani, I.N.; Bondre, K.V.; Shah, G.V. A cadaveric study of variation of posterior cord of brachial plexus. Int. J. Biol. Med. Res. 2000, 3, 2214–2217. [Google Scholar]
- Hada, S.; Kadel, M.; Pandit, T.K.; Basnet, K.S. Variations in formation of median nerve: A cadaveric study. JCMC 2020, 10, 66–68. [Google Scholar] [CrossRef]
- Jamuna, M.; Amudha, G. A cadaveric study on the anatomic variations of the musculocutaneous nerve in the infraclavicular part of the brachial plexus. J. Clin. Diagn Res. 2011, 5, 1144–1147. [Google Scholar]
- Kaur, P.; Kumar, R.; Jain, A. Variations in innervation of muscles in anterior compartment of arm—A cadaveric study. J. Clin. Diagn Res. 2014, 8, AC01. [Google Scholar] [CrossRef]
- Keen, J.A. Study of the arterial variations in the limbs, with special reference to symmetry of vascular patterns. Am. J. Anat. 1961, 108, 245–261. [Google Scholar] [CrossRef]
- Khan, G.A.; Yadav, S.K.; Gautam, A.; Shakya, S.; Chetri, R. Anatomical variation in branching pattern of brachial plexus and its clinical significance. Int. J. Anat. Res. 2017, 5, 3324–3328. [Google Scholar] [CrossRef]
- Klepps, S.J.; Goldfarb, C.; Flatow, E.; Galatz, L.M.; Yamaguchi, K. Anatomic evaluation of the subcoracoid pectoralis major transfer in human cadavers. J. Shoulder Elb. Surg. 2001, 10, 453–459. [Google Scholar] [CrossRef]
- Leonhard, V.; Smith, R.; Caldwell, G.; Smith, H.F. Anatomical variations in the brachial plexus roots: Implications for diagnosis of neurogenic thoracic outlet syndrome. Ann. Anat. 2016, 206, 21–26. [Google Scholar] [CrossRef]
- Leonhard, V.; Caldwell, C.; Goh, M.; Reeder, S.; Smith, H.F. Ultrasonographic diagnosis of Thoracic Outlet Syndrome secondary to brachial plexus piercing variation. Diagnostics 2017, 7, 40. [Google Scholar] [CrossRef]
- Malukar, O.; Rathva, A. A study of 100 cases of brachial plexus. Nat. J. Comm. Med. 2011, 2, 166–170. [Google Scholar]
- Ozturk, A.; Kutlu, C.; Taskara, N.; Kale, A.C.; Bayraktar, B.; Cecen, A. Anatomic and morphometric study of the arcade of Frohse in cadavers. Surg. Radiol. Anat. 2005, 27, 171–175. [Google Scholar] [CrossRef]
- Pabst, R.; Lippert, H. Beiderseitiges Vorkommen von A. brachialis superficialis, A. ulnaris superficialis und A. mediana. Anat. Anz. 1968, 123, 223–226. [Google Scholar]
- Porzionato, A.; Macchi, V.; Stecco, C.; Loukas, M.; Tubbs, R.S.; De Caro, R. Surgical anatomy of the pectoral nerves and the pectoral musculature. Clin. Anat. 2012, 25, 559–575. [Google Scholar] [CrossRef]
- Singh, R. Variations of cords of brachial plexus and branching pattern of nerves emanating from them. J. Craniofac. Surg. 2017, 28, 543–547. [Google Scholar] [CrossRef]
- Taib, C.N.M.; Hassan, S.N.A.; Esa, N.; Moklas, M.A.M.; San, A.A. Anatomical variations of median nerve formation, distribution and possible communication with other nerves in preserved human cadavers. Folia Morphol. 2017, 76, 38–43. [Google Scholar] [CrossRef]
- Benes, M.; Kachlik, D.; Belbla, M.; Havlikova, S.; Kuncc, V.; Whitley, A.; Kaiser, R.; Kunca, V. A meta-analysis on the anatomical variability of the brachial plexus: Part III—Branching of the infraclavicular part. Ann. Anat. 2022, 244, 151976. [Google Scholar] [CrossRef]
- Harry, W.G.; Bennett, J.D.; Guha, S.C. Scalene muscles and the brachial plexus: Anatomical variations and their clinical significance. Clin. Anat. 1997, 10, 250–252. [Google Scholar] [CrossRef]
- Sakamoto, Y. Spatial relationships between the morphologies and innervations of the scalene and anterior vertebral muscles. Ann. Anat. 2012, 194, 381–388. [Google Scholar] [CrossRef]
- Williams, A.A.; Smith, H.F. Anatomical entrapment of the dorsal scapular and long thoracic nerves, secondary to brachial plexus piercing variation. Anat. Sci. Int. 2020, 95, 67–75. [Google Scholar] [CrossRef]
- Loukas, M.; Louis, R.G.; Fitzsimmons, J.; Colborn, G. The surgical anatomy of the ansa pectoralis. Clin. Anat. 2006, 19, 685–693. [Google Scholar] [CrossRef]
- Aszmann, O.C.; Rab, M.; Kamolz, L.; Frey, M. The anatomy of the pectoral nerves and their significance in brachial plexus reconstruction. J. Hand. Surg. 2000, 25A, 942–947. [Google Scholar] [CrossRef]
- Benes, M.; Kachlik, D.; Belbla, M.; Havlikova, S.; Kuncc, V.; Whitley, A.; Kunca, V. A meta-analysis on the anatomical variability of the brachial plexus: Part I—Roots, trunks, divisions and cords. Ann. Anat. 2021, 238, 151751. [Google Scholar] [CrossRef]
- Johnson, D.; Collins, P. Pectoral girdle, shoulder region and axilla. In Gray’s Anatomy, 40th ed.; Standring, S., Ed.; Elsevier/Churchil Livingstone: Edinburgh, UK, 2005; pp. 818–821. [Google Scholar]
- Lee, K.S. Anatomic variation of the spinal origins of lateral and medial pectoral nerves. Clin. Anat. 2007, 20, 915–918. [Google Scholar] [CrossRef]
- Al-Hubaity, A.Y.; AL-Ashou, H.A.; AL-Annaz, M.S. Anatomical study of the brachial plexus variations in Iraqi cadavers. Raf. J. Sci. 2005, 16, 23–27. [Google Scholar]
- Rastogi, R.; Budhiraja, V.; Bansal, K. Posterior cord of brachial plexus and its branches: Anatomical variations and clinical implication. ISRN Anat. 2013, 2013, 501813. [Google Scholar] [CrossRef]
- Klaassen, Z.; Sorenson, E.; Tubbs, R.S.; Arya, R.; Meloy, P.; Shah, R.; Shirk, S.; Loukas, M. Thoracic outlet syndrome: A neurological and vascular disorder. Clin. Anat. 2014, 27, 724–732. [Google Scholar] [CrossRef]
- Molina, J.E.; D’Cuhna, J. The vascular component in neurogenic arterial thoracic outlet syndrome. Int. J. Angiol. 2008, 17, 83–87. [Google Scholar] [CrossRef]
- Sanders, R.J.; Hammond, S.L.; Rao, N.M. Diagnosis of thoracic outlet syndrome. J. Vasc. Surg. 2007, 46, 601–604. [Google Scholar] [CrossRef]



| Variable |
|---|
| 1. Is the “M” of the brachial plexus a classic anatomical arrangement? |
| 2. Do the medial brachial cutaneous nerve and medial antebrachial cutaneous nerve arise from a common trunk? |
| 3. Does the medial antebrachial cutaneous nerve arise from the inferior trunk or ulnar nerve? |
| 4. Does the medial pectoral nerve receive fibers from only the lateral cord or from both medial and lateral cords? |
| 5. Does the thoracodorsal nerve originate from the axillary nerve? |
| 6. Does the ulnar nerve receive communicating branches from the lateral cord? |
| 7. Does the median nerve receive branches from the musculocutaneous nerve? |
| 8. Does the median nerve receive branches from the posterior cord? |
| Variant | Yes— Male | Yes— Female | Yes— Total |
|---|---|---|---|
| Classic M pattern | 11/38 (29%) | 18/38 (47%) | 29/76 (38%) |
| Common trunk of MBC + MAC | 1/38 (3%) | 3/38 (8%) | 4/76 (5%) |
| MAC from IT or ulnar n. | 2/36 (6%) | 0/33 (0%) | 2/69 (3%) |
| MPN from MC + LC | 4/23 (18%) | 3/21 (14%) | 7/44 (15.9%) |
| MPN from LC | 1/23 (4%) | 5/21 (24%) | 6/44 (14%) |
| Thoracodorsal n. from axillary n. | 6/25 (24%) | 2/22 (9%) | 8/47 (17%) |
| Ulnar n. rec bb. from LC | 4/38 (11%) | 2/38 (5%) | 6/76 (8%) |
| Musculocutaneous n. bb to median n. | 0/38 (0%) | 4/37 (11%) | 4/75 (5%) |
| PC gives bb. to median n. | 0/38 (0%) | 1/38 (3%) | 1/76 (1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, N.T.; Smith, H.F. Clinically Relevant Anatomical Variations in the Brachial Plexus. Diagnostics 2023, 13, 830. https://doi.org/10.3390/diagnostics13050830
Patel NT, Smith HF. Clinically Relevant Anatomical Variations in the Brachial Plexus. Diagnostics. 2023; 13(5):830. https://doi.org/10.3390/diagnostics13050830
Chicago/Turabian StylePatel, Niki T., and Heather F. Smith. 2023. "Clinically Relevant Anatomical Variations in the Brachial Plexus" Diagnostics 13, no. 5: 830. https://doi.org/10.3390/diagnostics13050830
APA StylePatel, N. T., & Smith, H. F. (2023). Clinically Relevant Anatomical Variations in the Brachial Plexus. Diagnostics, 13(5), 830. https://doi.org/10.3390/diagnostics13050830







