Is the Level of Contrast Enhancement on Contrast-Enhanced Mammography (CEM) Associated with the Presence and Biological Aggressiveness of Breast Cancer?
Abstract
1. Introduction
2. Methods and Materials
2.1. Patient Population
2.2. Contrast-Enhanced Mammography
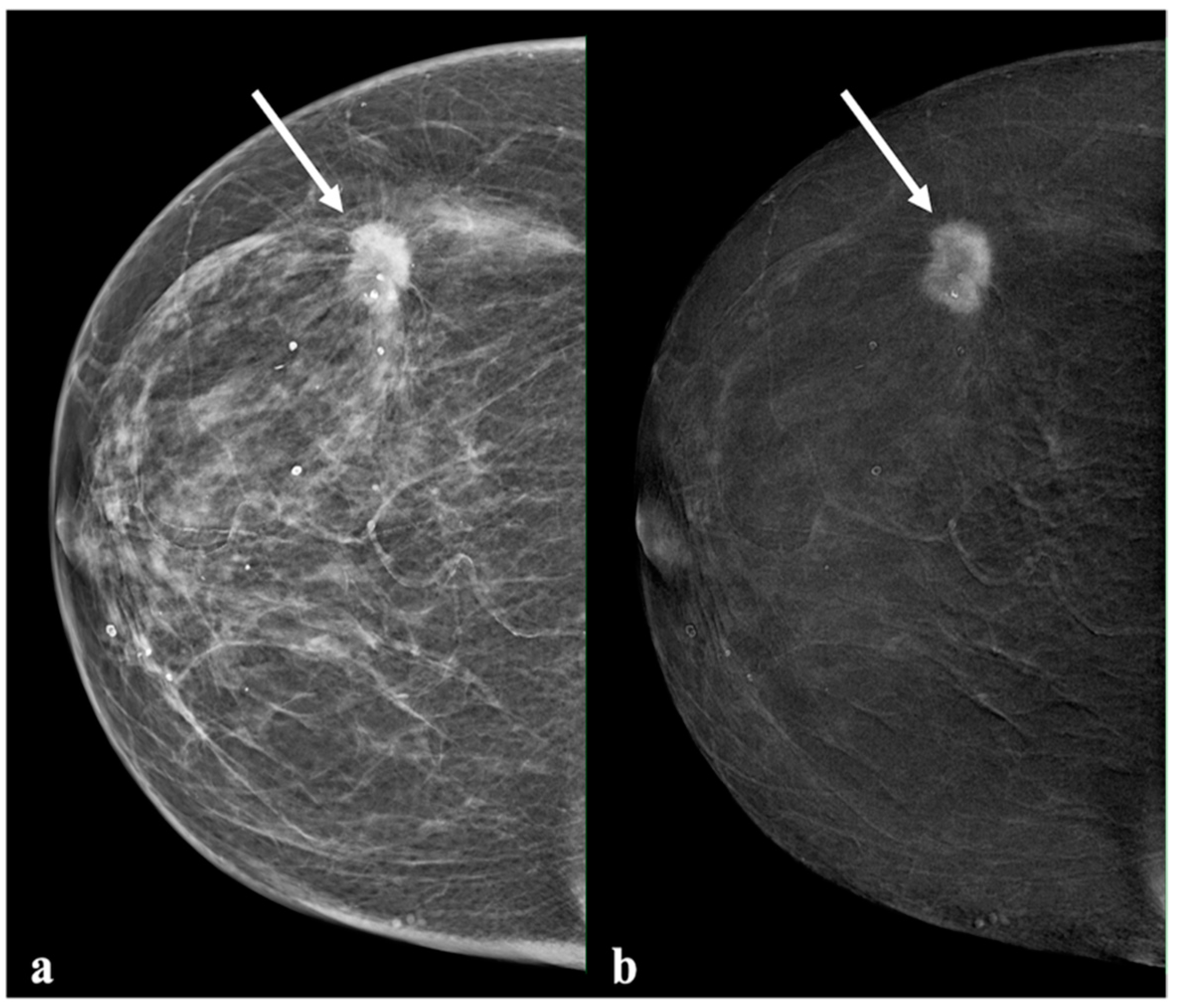
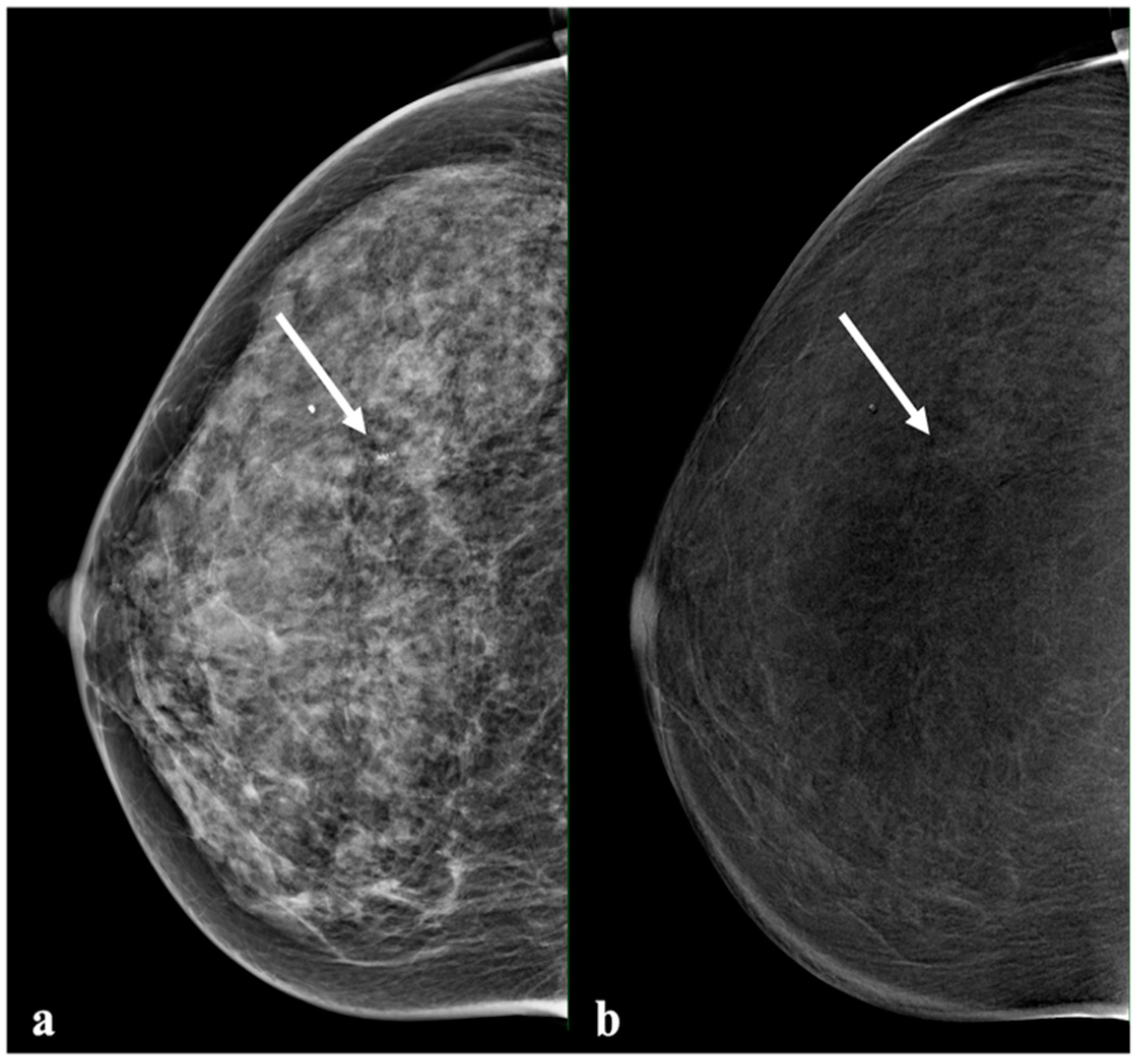
2.3. Image Analysis
2.4. Histology and Molecular Analysis
2.5. Statistical Analysis
3. Results
3.1. Lesion Characteristics
3.2. Diagnostic Performance and Inter-Reader Agreement
3.3. Comparison of Enhancement Intensity, Histological Results, and Molecular Subtype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melnikow, J.; Fenton, J.J.; Whitlock, E.P.; Miglioretti, D.L.; Weyrich, M.S.; Thompson, J.H.; Shah, K. Supplemental Screening for Breast Cancer in Women with Dense Breasts: A Systematic Review for the U.S. Preventive Service Task Force. In U.S. Preventive Services Task Force Evidence Syntheses, Formerly Systematic Evidence Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2016. [Google Scholar]
- Tagliafico, A.S.; Calabrese, M.; Mariscotti, G.; Durando, M.; Tosto, S.; Monetti, F.; Airaldi, S.; Bignotti, B.; Nori, J.; Bagni, A.; et al. Adjunct Screening with Tomosynthesis or Ultrasound in Women with Mammography-Negative Dense Breasts: Interim Report of a Prospective Comparative Trial. J. Clin. Oncol. 2016, 34, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Mizzi, D.; Allely, C.; Zarb, F.; Kelly, J.; Hogg, P.; McEntee, M.; England, A.; Mercer, C. Examining the Effectiveness of Supplementary Imaging Modalities for Breast Cancer Screening in Women with Dense Breasts: A Systematic Review and Meta-Analysis. Eur. J. Radiol. 2022, 154, 110416. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Dromain, C.; Thibault, F.; Diekmann, F.; Fallenberg, E.M.; Jong, R.A.; Koomen, M.; Hendrick, R.E.; Tardivon, A.; Toledano, A. Dual-Energy Contrast-Enhanced Digital Mammography: Initial Clinical Results of a Multireader, Multicase Study. Breast Cancer Res. 2012, 14, R94. [Google Scholar] [CrossRef]
- Zanardo, M.; Cozzi, A.; Trimboli, R.M.; Labaj, O.; Monti, C.B.; Schiaffino, S.; Carbonaro, L.A.; Sardanelli, F. Technique, Protocols and Adverse Reactions for Contrast-Enhanced Spectral Mammography (CESM): A Systematic Review. Insights Imaging 2019, 10, 76. [Google Scholar] [CrossRef]
- Patel, B.K.; Lobbes, M.B.I.; Lewin, J. Contrast Enhanced Spectral Mammography: A Review. Semin. Ultrasound CT MRI 2018, 39, 70–79. [Google Scholar] [CrossRef]
- Ghaderi, K.F.; Phillips, J.; Perry, H.; Lotfi, P.; Mehta, T.S. Contrast-Enhanced Mammography: Current Applications and Future Directions. Radiographics 2019, 39, 1907–1920. [Google Scholar] [CrossRef]
- Pötsch, N.; Vatteroni, G.; Clauser, P.; Helbich, T.H.; Baltzer, P.A.T. Contrast-Enhanced Mammography versus Contrast-Enhanced Breast MRI: A Systematic Review and Meta-Analysis. Radiology 2022, 305, 94–103. [Google Scholar] [CrossRef]
- Dietzel, M.; Baltzer, P.A.T. How to Use the Kaiser Score as a Clinical Decision Rule for Diagnosis in Multiparametric Breast MRI: A Pictorial Essay. Insights Imaging 2018, 9, 325–335. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.J.; Hwangbo, L.; Suh, H.B.; Kim, S.; Choo, K.S.; Nam, K.J.; Kang, T. Kinetic Heterogeneity of Breast Cancer Determined Using Computer-Aided Diagnosis of Preoperative MRI Scans: Relationship to Distant Metastasis-Free Survival. Radiology 2020, 295, 517–526. [Google Scholar] [CrossRef]
- Rudnicki, W.; Heinze, S.; Popiela, T.; Kojs, Z.; Luczynska, E. Quantitative Assessment of Contrast Enhancement on Contrast Enhancement Spectral Mammography (CESM) and Comparison with Qualitative Assessment. Anticancer Res. 2020, 40, 2925–2932. [Google Scholar] [CrossRef]
- Boy, F.N.S.; Goksu, K.; Tasdelen, I. Association between Lesion Enhancement and Breast Cancer in Contrast-Enhanced Spectral Mammography. Acta Radiol. 2023, 64, 74–79. [Google Scholar] [CrossRef]
- Bennani-Baiti, B.; Baltzer, P.A. MR Imaging for Diagnosis of Malignancy in Mammographic Microcalcifications: A Systematic Review and Meta-Analysis. Radiology 2017, 283, 692–701. [Google Scholar] [CrossRef]
- Fueger, B.J.; Clauser, P.; Kapetas, P.; Pötsch, N.; Helbich, T.H.; Baltzer, P.A.T. Can Supplementary Contrast-Enhanced MRI of the Breast Avoid Needle Biopsies in Suspicious Microcalcifications Seen on Mammography? A Systematic Review and Meta-Analysis. Breast 2021, 56, 53–60. [Google Scholar] [CrossRef]
- Łuczyńska, E.; Niemiec, J.; Hendrick, E.; Heinze, S.; Jaszczyński, J.; Jakubowicz, J.; Sas-Korczyńska, B.; Rys, J. Degree of Enhancement on Contrast Enhanced Spectral Mammography (CESM) and Lesion Type on Mammography (MG): Comparison Based on Histological Results. Med. Sci. Monit. 2016, 22, 3886–3893. [Google Scholar] [CrossRef]
- Deng, C.-Y.; Juan, Y.-H.; Cheung, Y.-C.; Lin, Y.-C.; Lo, Y.-F.; Lin, G.; Chen, S.-C.; Ng, S.-H. Quantitative Analysis of Enhanced Malignant and Benign Lesions on Contrast-Enhanced Spectral Mammography. Br. J. Radiol. 2018, 91, 20170605. [Google Scholar] [CrossRef]
- Bae, K.T. Intravenous Contrast Medium Administration and Scan Timing at CT: Considerations and Approaches. Radiology 2010, 256, 32–61. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Clauser, P.; Helbich, T.H.; Kapetas, P.; Pinker, K.; Bernathova, M.; Woitek, R.; Kaneider, A.; Baltzer, P.A.T. Breast Lesion Detection and Characterization with Contrast-Enhanced Magnetic Resonance Imaging: Prospective Randomized Intraindividual Comparison of Gadoterate Meglumine (0.15 Mmol/Kg) and Gadobenate Dimeglumine (0.075 Mmol/Kg) at 3T. J. Magn. Reson. Imaging 2019, 49, 1157–1165. [Google Scholar] [CrossRef]
- Kuhl, C.K. Current Status of Breast MR Imaging Part 2. Clinical Applications. Radiology 2007, 244, 672–691. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Vuong, D.; Simpson, P.T.; Green, B.; Cummings, M.C.; Lakhani, S.R. Molecular Classification of Breast Cancer. Virchows Arch. 2014, 465, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Mehta, T.S.; Brook, A.; Du, L.H.; Legare, K.; Phillips, J. Enhancement Type at Contrast-Enhanced Mammography and Association with Malignancy. Radiology 2022, 305, 299–306. [Google Scholar] [CrossRef]
- Lee, C.H.; Phillips, J.; Sung, J.S.; Lewin, J.M.; Newell, M.S. CONTRAST ENHANCED MAMMOGRAPHY (CEM) (A Supplement to ACR BI-RADS® Mammography 2013); American College of Radiology: Reston, VA, USA, 2022. [Google Scholar]
- Phillips, J.; Achibiri, J.U.; Kim, G.; Quintana, L.M.; Mehta, R.J.; Mehta, T.S. Characterization of True and False Positive Findings on Contrast-Enhanced Mammography. Acad. Radiol. 2022, 29, 1672–1681. [Google Scholar] [CrossRef]
- Bicchierai, G.; Amato, F.; Vanzi, B.; De Benedetto, D.; Boeri, C.; Vanzi, E.; Di Naro, F.; Bianchi, S.; Cirone, D.; Cozzi, D.; et al. Which Clinical, Radiological, Histological, and Molecular Parameters Are Associated with the Absence of Enhancement of Known Breast Cancers with Contrast Enhanced Digital Mammography (CEDM)? Breast 2020, 54, 15–24. [Google Scholar] [CrossRef]
- Cozzi, A.; Magni, V.; Zanardo, M.; Schiaffino, S.; Sardanelli, F. Contrast-Enhanced Mammography: A Systematic Review and Meta-Analysis of Diagnostic Performance. Radiology 2022, 302, 568–581. [Google Scholar] [CrossRef]
- Rudnicki, W.; Heinze, S.; Piegza, T.; Pawlak, M.; Kojs, Z.; Łuczyńska, E. Correlation Between Enhancement Intensity in Contrast Enhancement Spectral Mammography and Types of Kinetic Curves in Magnetic Resonance Imaging. Med. Sci. Monit. 2020, 26, e920742. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Bozzini, A.C.; Palma, S.; Montesano, M.; Pesapane, F.; Ferrari, F.; Dominelli, V.; Rotili, A.; Meneghetti, L.; Frassoni, S.; et al. A Score to Predict the Malignancy of a Breast Lesion Based on Different Contrast Enhancement Patterns in Contrast-Enhanced Spectral Mammography. Cancers 2022, 14, 4337. [Google Scholar] [CrossRef]
- Luczynska, E.; Piegza, T.; Szpor, J.; Heinze, S.; Popiela, T.; Kargol, J.; Rudnicki, W. Contrast-Enhanced Mammography (CEM) Capability to Distinguish Molecular Breast Cancer Subtypes. Biomedicines 2022, 10, 2384. [Google Scholar] [CrossRef] [PubMed]
- Vasselli, F.; Fabi, A.; Ferranti, F.R.; Barba, M.; Botti, C.; Vidiri, A.; Tommasin, S. How Dual-Energy Contrast-Enhanced Spectral Mammography Can Provide Useful Clinical Information about Prognostic Factors in Breast Cancer Patients: A Systematic Review of Literature. Front. Oncol. 2022, 12, 859838. [Google Scholar] [CrossRef]
- El Khouli, R.H.; Macura, K.J.; Kamel, I.R.; Bluemke, D.A.; Jacobs, M.A. The Effects of Applying Breast Compression in Dynamic Contrast Material-Enhanced MR Imaging. Radiology 2014, 272, 79–90. [Google Scholar] [CrossRef] [PubMed]


| Average of Enhancement Intensity Grade (%) | |||||||
|---|---|---|---|---|---|---|---|
| Subtype | n (%) | Absent | Subtle | Moderate | Distinct | ||
| Benign | 63/156 (40%) | 34.0% | 37.9% | 15.7% | 12.4% | ||
| Adenosis, sclerosing adenosis | 6/63 (9%) | ||||||
| Fibroadenoma, fibroepithelial hyperplasia | 11/63 (17%) | ||||||
| Benign epithelial proliferation | 14/63 (22%) | ||||||
| Benign breast tissue, Pseudoangiomatous stromal hyperplasia | 3/63 (5%) | ||||||
| Inflammation | 17/63 (27%) | ||||||
| B3 | 12 /63 (19%) | 38.9% | 44.5% | 11.1% | 5.5% | ||
| Atypical lobular hyperplasia | 4/12 (33%) | ||||||
| Papillary lesion | 5/12 (42%) | ||||||
| Flat epithelial atypia | 3/12 (25%) | ||||||
| Malignant | 93/156 (60%) | ||||||
| DCIS | 11/93 (11.8%) | 36.4% | 36.4% | 18.2% | 9.1% | ||
| Luminal A type | 3/11 (27.3%) | ||||||
| Luminal B type | 6/11 (54.5%) | ||||||
| HER2 type | 2/11 (18.2%) | ||||||
| Triple-negative | 0/ 11 (0%) | ||||||
| Invasive carcinoma | 82/93 (88.2%) | 1.2% | 9.3% | 27.7% | 61.8% | ||
| Invasive ductal carcinoma | 71/93 (76.3%) | ||||||
| Luminal A type | 14/71 (19.7%) | ||||||
| Luminal B type | 35/71 (49.3%) | ||||||
| HER2 type | 5/71 (7%) | ||||||
| Triple-negative | 17/71 (24%) | ||||||
| Invasive lobular carcinoma | 7/93 (7.5%) | ||||||
| Luminal A type | 1/7 (14.3%) | ||||||
| Luminal B type | 6/7 (85.7%) | ||||||
| Mucinous carcinoma | 1/93 (1%) | ||||||
| Luminal A | 1/1 (1%) | ||||||
| Papillary carcinoma | 3/93 (3.2%) | ||||||
| Luminal B type | 2/3 (66.7%) | ||||||
| Triple-negative | 1/3 (33.3%) | ||||||
| Sensitivity | 95% CI | Specificity | 95% CI | PPV% | NPV% | +LR | −LR | |
|---|---|---|---|---|---|---|---|---|
| Reader 1 | ||||||||
| >0 | 98.9 | 94.1–100 | 18.8 | 10.1–30.5 | 63.6 | 84.6 | 1.2 | 0.06 |
| >1 | 86.9 | 78.3–93.1 | 65.6 | 52.7–77.1 | 78.4 | 86.9 | 2.5 | 0.20 |
| >2 | 56.5 | 45.8–66.8 | 84.4 | 73.1–92.2 | 83.9 | 56.4 | 3.6 | 052 |
| Reader 2 | ||||||||
| >0 | 92.4 | 84.9–96.9 | 51.6 | 38.7–64.2 | 73.3 | 80.0 | 1.9 | 0.15 |
| >1 | 76.1 | 66.1–84.4 | 81.3 | 69.5–89.9 | 85.4 | 70.3 | 1.06 | 0.29 |
| >2 | 47.8 | 37.3–58.5 | 95.3 | 86.9–99.0 | 93.6 | 55.0 | 10.2 | 0.55 |
| Reader 3 | ||||||||
| >0 | 94.6 | 87.8–98.2 | 35.9 | 24.3–48.9 | 68.7 | 82.1 | 1.5 | 0.15 |
| >1 | 85.9 | 77.0–92.3 | 76.7 | 64.3–86.2 | 84.0 | 77.4 | 3.7 | 0.18 |
| >2 | 64.2 | 53.5–73.9 | 87.5 | 76.8–94.4 | 88.1 | 61.8 | 5.2 | 0.41 |
| All readers | 95.4 | 84.9–100 | 35.4 | 10.1–64.2 | 68.5 | 82.2 | 1.5 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzogi, A.; Baltzer, P.A.T.; Kapetas, P.; Milos, R.I.; Bernathova, M.; Helbich, T.H.; Clauser, P. Is the Level of Contrast Enhancement on Contrast-Enhanced Mammography (CEM) Associated with the Presence and Biological Aggressiveness of Breast Cancer? Diagnostics 2023, 13, 754. https://doi.org/10.3390/diagnostics13040754
Marzogi A, Baltzer PAT, Kapetas P, Milos RI, Bernathova M, Helbich TH, Clauser P. Is the Level of Contrast Enhancement on Contrast-Enhanced Mammography (CEM) Associated with the Presence and Biological Aggressiveness of Breast Cancer? Diagnostics. 2023; 13(4):754. https://doi.org/10.3390/diagnostics13040754
Chicago/Turabian StyleMarzogi, Alaa, Pascal A. T. Baltzer, Panagiotis Kapetas, Ruxandra I. Milos, Maria Bernathova, Thomas H. Helbich, and Paola Clauser. 2023. "Is the Level of Contrast Enhancement on Contrast-Enhanced Mammography (CEM) Associated with the Presence and Biological Aggressiveness of Breast Cancer?" Diagnostics 13, no. 4: 754. https://doi.org/10.3390/diagnostics13040754
APA StyleMarzogi, A., Baltzer, P. A. T., Kapetas, P., Milos, R. I., Bernathova, M., Helbich, T. H., & Clauser, P. (2023). Is the Level of Contrast Enhancement on Contrast-Enhanced Mammography (CEM) Associated with the Presence and Biological Aggressiveness of Breast Cancer? Diagnostics, 13(4), 754. https://doi.org/10.3390/diagnostics13040754






