Abstract
Dependence behaviors are common in patients with medication-overuse headache (MOH). This prospective study aimed to characterize dependence behaviors in MOH by using Leeds dependence questionnaire (LDQ), and to determine the clinical utility of LDQ in the diagnosis of MOH. In total, 563 consecutive chronic migraine (CM) patients (451F/112M, mean age 41.7 ± 12.0 years) were recruited, including 320 with MOH (56.8%) (254F/66M, mean age 42.3 ± 11.6 years). LDQ scores were positively correlated with the monthly frequency of acute medication use (Spearman’s rho = 0.680, p < 0.001). When compared with patients without, those with MOH scored higher on LDQ (13.0 ± 7.6 vs. 3.9 ± 5.1, p < 0.001). By using a receiver operating characteristics curve, the cutoff value of LDQ was determined at 7 (sensitivity = 77.5%, specificity = 77.4%, area under curve = 0.85) for a diagnosis of MOH. An LDQ score of ≥7 was predictive of MOH (odds ratio = 11.80, 95% confidence interval = 7.87–17.67, p < 0.001). In conclusion, the presence of MOH in patients with CM is associated with more severe dependence behaviors. An LDQ score of ≥7 is useful in the detection of MOH in CM patients.
1. Introduction
Medication-overuse headache (MOH) is characterized by a paradoxical increase in headache frequency and severity following regular overuse of acute medications for a long period of time [1]. MOH typically evolves from a pre-existing primary headache disorder [2]. Withdrawal of the overused acute medications may lead to headache improvement and gradual reversion to the underlying primary headache disorder [1]. The prevalence of MOH is 0.5–2.6% in the general population [3], which is consistent in different parts of the world, including Taiwan [4,5,6]. MOH is frequently seen in the middle adulthood [7], which consists of the most productive years. It is the third most prevalent, and the sixth leading cause of disease-related disability among all neurological disorders, according to Global Burden of Disease Study 2015 by the World Health Organization [8]. Despite the tremendous impact on the quality of the life of patients suffering from MOH and the consequent enormous socioeconomic burden [3,8], this condition remains under-recognized and under-treated.
The majority of MOH patients have migraine as the underlying primary headache disorder [9]. Migraine is associated with an increased risk for developing psychiatric comorbidities, such as depression, anxiety disorders, etc., which are also believed to be involved in migraine chronification and development of MOH [10,11]. Interestingly, it was also reported that about two thirds of MOH patients could fulfill the criteria for substance dependence in the fourth edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [12,13,14]. Clinically, misuse or even abuse of psychoactive substances other than analgesics was reported to be present in a significant proportion of MOH patients or even in their family [15]. Supportive evidence could be derived from neuroimaging studies. It was found that MOH patients had persistent hypometabolism in the orbitofrontal cortex (OFC) on fluorodeoxyglucose positron emission tomography, which persisted after detoxification [16]. Furthermore, CM patients with MO had decreased gray matter volume in the OFC, which was predictive of treatment response [17]. In fact, structural or functional alterations in the OFC are important features of substance use disorders, and are related to drive, compulsive repetitive behaviors, or even relapse [18,19]. Therefore, MOH seems to bear resemblance to substance use disorders from clinical and pathophysiological perspectives. The above findings are suggestive of the presence of impairment in the reward circuit in MOH patients, as seen in those with substance use disorders. However, more clinical evidence supporting such a hypothesis is needed.
Dependence behaviors in MOH are gaining increasing attention is recent years and are of clinical interest and importance for diagnostic and prognostic purposes. It was found that chronic daily headache (CDH) patients with medication overuse (MO) had more severe dependence behaviors compared with patients with episodic migraine, episodic cluster headache, or episodic tension-type headache (TTH) [20]. Moreover, it was demonstrated that the severity of dependence behaviors was not only correlated with medication overuse (MO) in the general population [21] but was also predictive of the prognosis of detoxification in primary MOH [22,23]. However, many prior studies involved comparisons between MOH patients and patients with episodic headache disorders or even healthy controls, and data on direct comparisons between CM patients with and without MOH are scarce. Furthermore, the Severity of Dependence Scale appeared to be more widely used in the studies of MOH. Whether the Leeds Dependence Questionnaire (LDQ), another neuropsychological instrument commonly used in the measurement of dependence behaviors in patients with substance use disorders [24], could also be useful in MOH patients remains to be elucidated.
The present study intended to characterize behaviors of dependence in CM patients with and without coexisting MOH by using the LDQ and to determine the potential use of the LDQ in the screening of MOH in patients with CM.
2. Methods
2.1. Patients
This was a prospective study involving newly diagnosed CM patients with and without a concomitant diagnosis of MOH. Patients were enrolled consecutively at their first visit to the Headache Clinic of Taipei Veterans General Hospital, a tertiary medical center for both veterans and civilians in Taiwan. The initial evaluation consisted of questionnaire-based interviews by headache specialists. The diagnoses of CM and MOH were made according to the diagnostic criteria of the Third Edition of the International Classification of Headache Disorders (ICHD) (ICHD-3) [25]. Patients were included if they (a) were willing to participate in the study, (b) were aged between 20 and 65 years, and (c) fulfilled the ICHD-3 criteria for CM. The exclusion criteria included (a) an acute headache disorder (within one month of headache onset), (b) a secondary headache disorder, and (c) difficulties completing the history taking or the questionnaire-based interview. The study protocols were approved by the Institutional Review Board of Taipei Veterans General Hospital (protocol number 2018-07-020BC, date of approval: 25 July 2018; protocol number 2019-07-002CC, date of approval: 12 July 2019). All of the patients gave written informed consent before entering the study.
2.2. Questionnaire-Based Interviews
The questionnaire was designed to collect demographics, clinical profiles, and headache characteristics, as well as to screen for psychological disturbances and behaviors of dependence. Headache-related disabilities were assessed with the Migraine Disability Assessment Scale (MIDAS) [26]. Symptoms of depression and anxiety were assessed by using the Hospital Anxiety and Depression Scale (HADS), including anxiety (HADS-A) and depression subscales (HADS-D) [27]. The Pittsburg Sleep Quality Index (PSQI) was used to evaluate the severity of sleep disturbances [28]. Dependence behaviors were screened by using the LDQ [29], with some modifications for the use in headache disorders [20]. The scores of these instruments were verified during the face-to-face interviews by headache specialists.
The modified version of LDQ consists of ten questions [20], each which is to be rated on a scale of 0 to 3 (0 = never, 1 = sometimes, 2 = often, 3 = nearly always) (Table 1). The total score ranges from 0 to 30. The questions are as follows: 1. Do you find yourself thinking about when you will next be able to take analgesics? 2. Is taking analgesics more important than anything else you might do during the day? 3. Do you feel your need for analgesics is too strong to control? 4. Do you plan your days around taking analgesics? 5. Do you take analgesic in a particular way in order to increase the effect it gives you? 6. Do you take analgesics morning, afternoon and evening? 7. Do you feel you have to carry on taking analgesics once you have started? 8. Is getting the effect you want more important than the particular analgesic you use? 9. Do you want to take more analgesics when the effect starts to wear off? 10. Do you find it difficult to cope with life without analgesics? This questionnaire was translated into traditional Chinese for its use in Taiwan following standard protocols for cross-cultural research: translation, back translation, and bilingual expert panel evaluation.

Table 1.
Clinical characteristics in chronic migraine patients with and without medication-overuse headache.
2.3. Statistical Analysis
Data were expressed as mean ± standard deviations (SD) or number (n) (percentage). Continuous variables were compared by using Student’s t test, or Mann–Whitney U test for non-normally distributed variables. Categorical variables were compared by using chi-square test. Correlations between LDQ scores and clinical parameters, including age, CM duration, frequencies of headache and analgesic use, etc., were evaluated by Spearman’s rank correlation coefficient, as many of the data were not normally distributed. The optimum cut-off score of the LDS for detecting patients with coexisting MOH was determined by using a receiver operating characteristics (ROC) curve, in conjunction with Youden’s J statistics. The areas under the ROC curves (AUC) were calculated to evaluate the discriminative performance of these instruments. In general, an AUC of 0.7 to 0.8 indicates acceptable, 0.8 to 0.9 excellent, and >0.9 outstanding accuracy of a diagnostic test [29]. Logistic regression modeling was carried out to examine the association between the cut-off score of the LDQ and the diagnosis of MOH, and to estimate the odds ratios (ORs) and the 95% confidence intervals (CIs). Statistical analysis was carried out by using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA). Statistical significance was defined as a two-sided p of <0.05.
3. Results
3.1. Demographics and Clinical Characteristics
In total, 563 consecutive CM patients (451F/112M, mean age 41.7 ± 12.0 years) were recruited, including 320 with MOH (56.8%) (254F/66M, mean age 42.3 ± 11.6 years) (Table 1). When compared with patients without MOH, those with MOH were less likely to have a bachelor’s degree or higher (43.4% vs. 56.0%, p = 0.003), although they had a longer duration of CM (90.4 ± 100.6 vs. 60.4 ± 112.4 months, p < 0.001), greater average intensity (7.0 ± 1.9 vs. 6.2 ± 1.8, p < 0.001), many more days per month with acute medication use (19.4 ± 7.8 vs. 3.7 ± 5.3 days/month, p < 0.001), greater disabilities (MIDAS 66.2 ± 77.4 vs. 42.5 ± 51.3, p < 0.001), and poorer sleep quality (PSQI 11.9 ± 4.4 vs. 11.1 ± 4.2, p = 0.030). Furthermore, there was a trend toward an earlier onset of migraine in patients with MOH (20.7 ± 9.2 vs. 22.8 ± 10.8, p = 0.065). On the other hand, the age, gender distribution, marital status, employment status, the presence of migraine aura, the number of monthly headache days, and scores on the HADS-A, HADS-D and HADS-T were comparable between these two groups.
3.2. Dependence Behavior
Among the entire study population, the LDQ score was positively correlated with the number of days per month with acute medication use (Spearman’s rho = 0.680, p < 0.001) and CM duration (Spearman’s rho = 0.304, p < 0.001), although there was only a weak correlation with the number of MHDs (Spearman’s rho = 0.095, p = 0.024). Moreover, there were also correlations between LDQ scores and the scores of MIDAS (Spearman’s rho = 0.260, p < 0.001), PSQI (Spearman’s rho = 0.256, p < 0.001), HADS-D (Spearman’s rho = 0.193, p < 0.001), and HADS-A (Spearman’s rho = 0.120, p = 0.004).
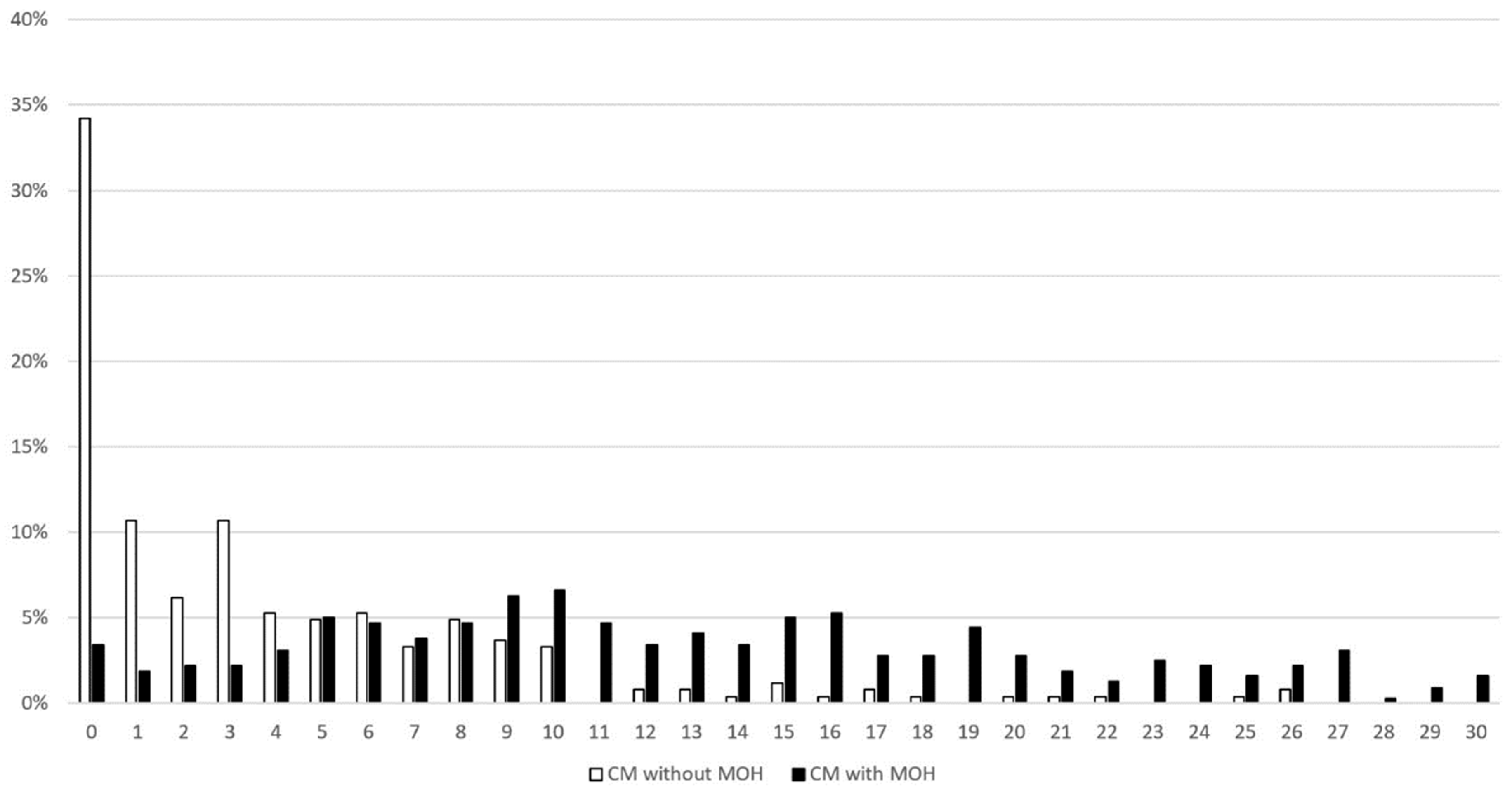
When compared with patients without, those with MOH scored higher in the LDQ (13.0 ± 7.6 vs. 3.9 ± 5.1, p < 0.001) (Table 2). In fact, the between-group difference was significant for every single question included in the instrument. The mode of the LDQ for patients with MOH was 10 (Figure 1), which was reported by 6.6% of patients, whereas it was 0 for those without MOH, which was reported by 34.2%.

Table 2.
Dependence behaviors in patients with and without medication-overuse headache (MOH).
Figure 1.
Distribution of LDQ scores in patients with and without MOH. Abbreviations: LDQ = Leeds Dependence Questionnaire, MOH = medication-overuse headache.
3.3. Clinical Utility of LDQ
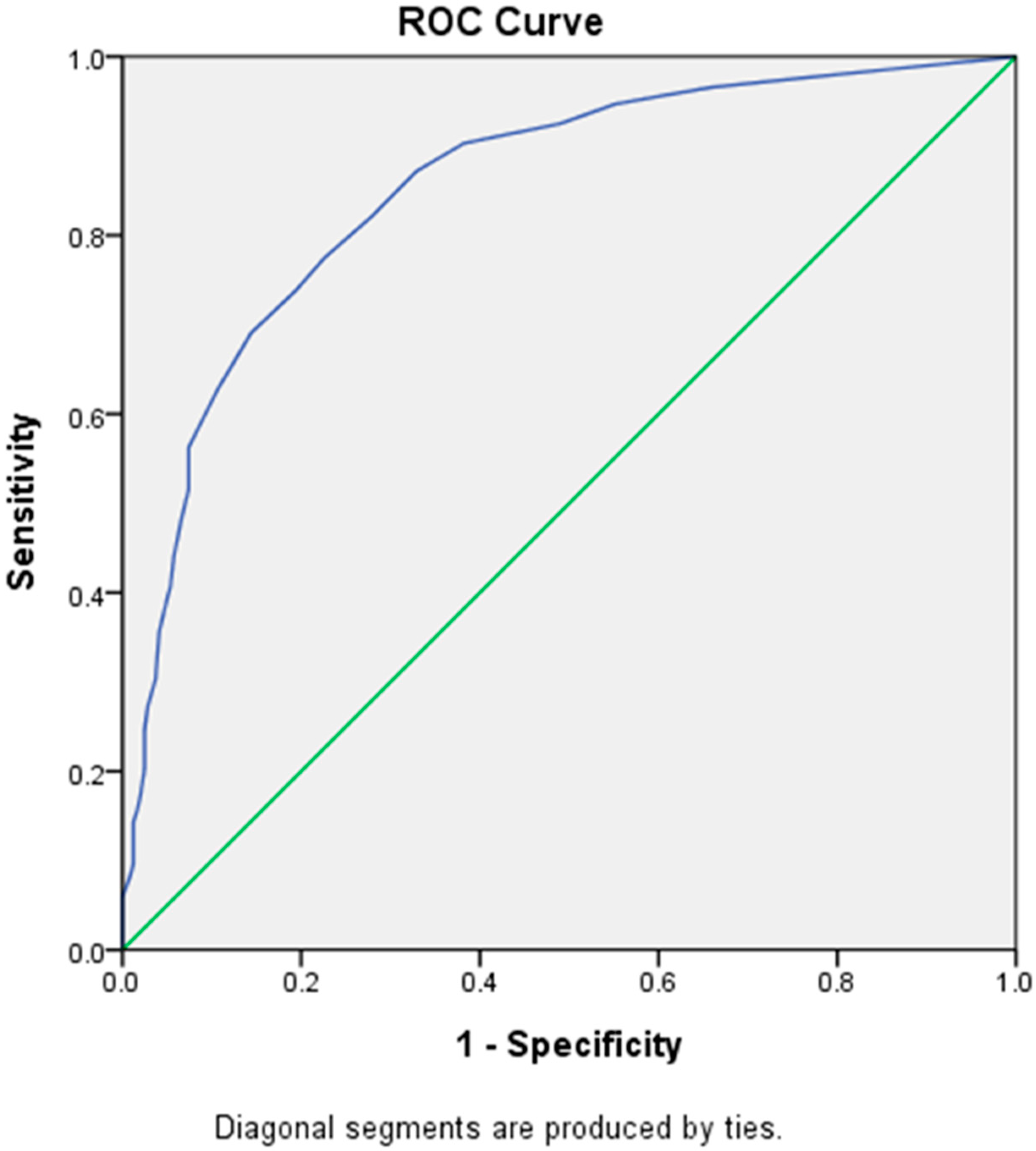
By using the ROC curve, the cut-off score of LDQ for a diagnosis of MOH was determined at 7, with a sensitivity of 77.5% and a specificity of 77.4% (Youden’s J index = 0.55) (Figure 2), which belonged to the category of excellent diagnostic accuracy (AUC = 0.85 [Asymptotic 95% Confidence Interval = 0.82–0.88]). However, a cut-off score of 4 (sensitivity = 90.3%, specificity = 61.7%) or 5 (sensitivity = 87.2%, specificity = 67.1%) could be more appropriate for screening purposes because of higher sensitivities.
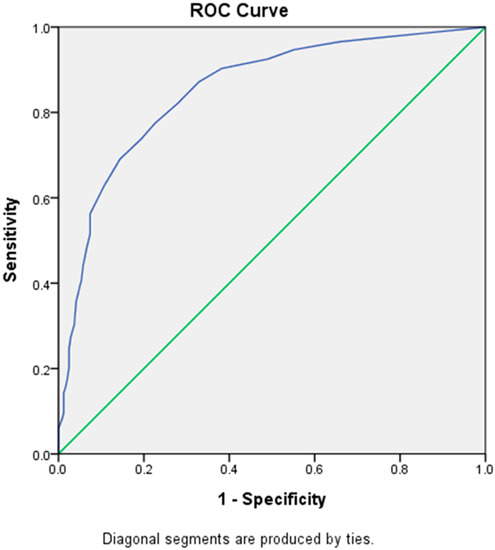
Figure 2.
Receiver Operating Characteristic (ROC) curve of LDQ scores predictive of MOH. Abbreviations: LDQ = Leeds Dependence Questionnaire, MOH = medication-overuse headache.
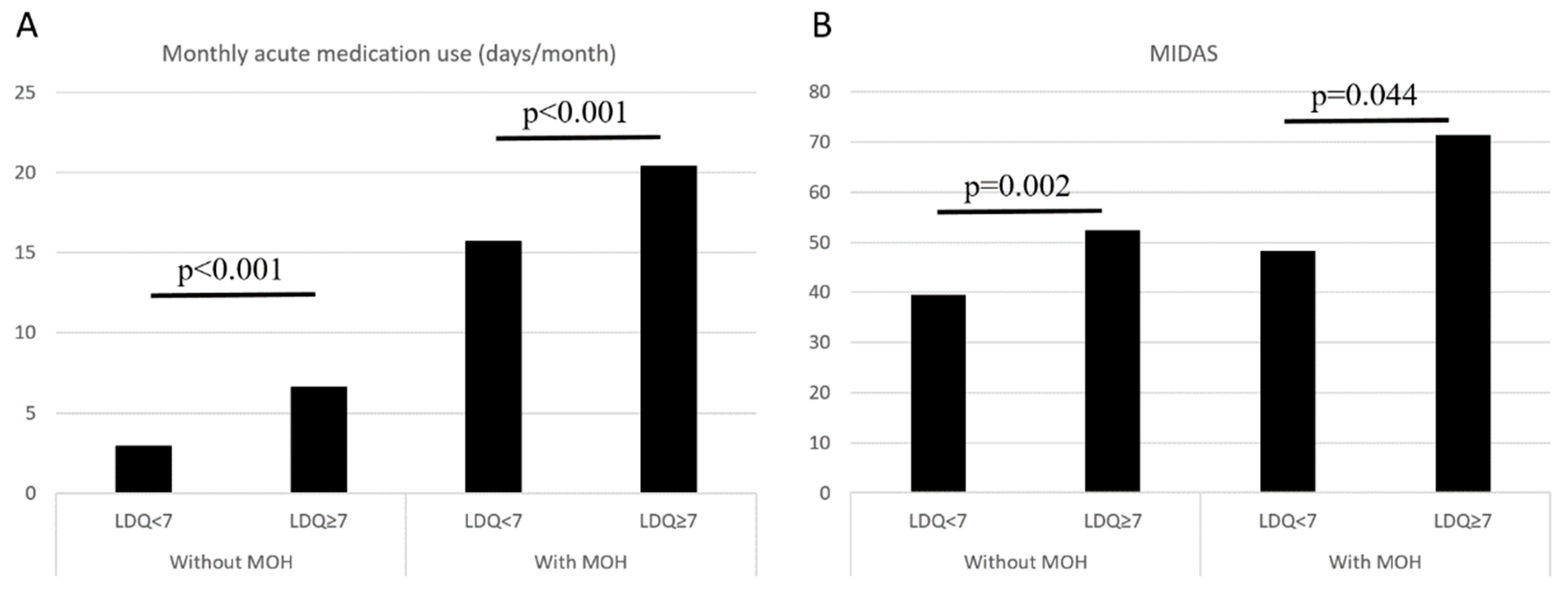
In the entire study population (n = 563), an LDQ score of ≥7 was associated with the presence of MOH (OR = 11.77, 95% CI = 7.90–17.55, p < 0.001), and the findings were consistent (OR = 11.80, 95% CI = 7.87–17.67, p < 0.001) after controlling for demographics (age, sex, education level, marital status, and employment status). In patients without MOH (n = 243), an LDQ score of ≥7 was associated with more days per month with acute medication use (6.6 ± 7.4 vs. 2.9 ± 4.1 days/month, p < 0.001) and greater disabilities (MIDAS 52.4 ± 42.9 vs. 39.6 ± 53.2, p < 0.001). The findings were similar in patients with MOH (n = 320) (Monthly analgesic use 20.4 ± 7.6 vs. 15.7 ± 7.5, p = 0.002; MIDAS 71.4 ± 82.0 vs. 48.3 ± 55.8, p = 0.044) (Figure 3).
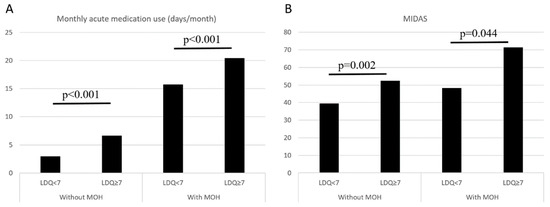
Figure 3.
Comparisons on monthly acute medication use (A) and MIDAS scores (B) between patients with LDQ < 7 and LDQ ≥ 7 stratified by the presence of MOH. Abbreviations: LDQ = Leeds Dependence Questionnaire, MIDAS = Migraine Disability Assessment Scale, MOH = medication-overuse headache.
4. Discussion
In the current study, it was found that CM patients with coexisting MOH had a lower education level, a longer duration of CM, greater headache intensities and disabilities, and poorer sleep quality when compared with those without. More importantly, the presence of MOH was associated with more severe dependence behaviors, as evidenced by higher scores on the LDQ, and the LDQ score was highly correlated with the frequency of acute medication use. Furthermore, a cut-off score of LDQ ≥ 7 was useful in the diagnosis of MOH among CM patients, and it was 4 or 5 for screening purposes. An LDQ score of ≥7 was associated with an increased risk of MOH by 10 folds and was associated with more acute medication use and greater disabilities regardless of the presence of MOH. The LDQ appeared to be a powerful instrument in the screening of dependence behaviors in CM patients. Moreover, a cut-off score of ≥7 on the LDQ should alert the treating physician of the possibility of coexisting MOH. However, whether the treatment response to preventive medications could be different needs to be further studied.
One of the most important strengths of the current study was the sample size. More than 550 CM patients with MO were recruited consecutively, which could be one of the largest studies carried out in the clinical setting so far. A large sample size could reduce selection bias and could give more accurate estimates. Second, direct comparisons were made between CM patients with and without MOH. In comparison, many prior reports compared MOH with episodic migraine, cluster headache, TTH, or even healthy controls [20,21,23,30], and it is uncertain whether these findings were pertinent to MOH or coexisting headache disorders. Therefore, the findings of the present study are more relevant to MOH rather than the underlying CM. Third, the present study was carried out in the clinical setting and involved CM patients. Even though there were studies evaluating the potential use of the Severity of Dependence Scale as a screening instrument for MOH, these studies were population-based and chronic TTH was the predominant headache diagnosis [21,30]. Therefore, the findings of the current study could have more practical impacts for clinicians treating headache patients.
Some of the clinical manifestations of substance use disorders are shared by MOH patients, particularly dependence behaviors [20,21]. It was reported that the need for analgesics, as measured by the LDQ, in CDH patients with daily use of acute medications was comparable to that for drugs of abuse in substance use disorders involving heroin, cocaine, alcohol, cannabis derivatives, and amphetamine [20]. In the current study, the severity of dependence behaviors, as measured by LDQ scores, was strongly correlated with monthly acute medication use. The finding was in keeping with prior reports and suggested the LDQ could be useful in measuring the severity of dependence behaviors in MOH patients. Moreover, the LDQ was useful in the diagnosis or even screening of MOH in CM patients. In the literature, there was a screening tool consisting of two questions, namely “do you take a treatment for attacks more than 10 days per month” and “is this intake on a regular basis” [31], and it was concluded that the tool was sensitive and specific for MOH based on revised criteria of the Second Edition of ICHD [32]. However, it provided only qualitative rather than quantitative information, and could not reflect the severity of the condition. On the other hand, in prior studies, it was also demonstrated that a score of ≥5 on the Severity of Dependence Scale was useful not only in the screening of MO in patients with primary chronic headaches, but also in detecting dependence defined by DSM-IV among MOH patients [14,21,23]. Although the LDQ and the Severity of Dependence Scale were both developed as measures for dependence behaviors [24,33], the LDQ seemed to be under-utilized in clinical studies of headache disorders. Further studies involving direct comparisons on the diagnostic or screening performances of these two instruments would help clarify the role of LDQ in the clinical evaluation and management of MOH.
The severity of dependence behaviors could be an important factor associated with the prognosis of MOH patients. However, whether the LDQ could be predictive of treatment outcome remains to be explored. It was shown that patients with more severe dependence behaviors, as measured by the Severity of Dependence Scale, had a poorer prognosis of detoxification [22], which highlights the importance of screening for dependence behaviors for patients with CDH, including CM. In fact, MOH patients who scored ≥7 on the LDQ in the present study had more frequent acute medication use, which could a risk factor for migraine chronification and relapse of MOH after withdrawal of acute medications [34,35]. However, as a cross-sectional study, the question whether higher LDQ scores could be associated with a less favorable outcome or a higher relapse rate after detoxification could not be answered. Interestingly, it was also found that even in patients without MOH, an LDQ score of ≥7 was associated with more days per month with acute medication use and greater disabilities, when compared with an LDQ score of <7. Whether such patients are at an increased risk of developing MOH in the future deserves further study.
There were some limitations. In particular, the external and internal validity of the findings of the present study could be important concerns. First of all, the study participants were recruited from the headache clinic of a tertiary medical center, which could potentially limit the generalizability of the findings. However, the present study involved a relatively large sample size, which could potentially reduce selection bias. Furthermore, a formal referral system is yet to be developed in our country, and most of patients came in directly without referral. Second, although the prospective nature reduced the risk of recall bias, the cross-sectional design could only give information for an association. Further studies of a longitudinal design are needed to determine whether LDQ scores could be associated treatment outcomes in these patients. Third, these were self-administered instruments, and the reliability could be a concern. However, the responses to the questions in the instruments were checked at face-to-face interviews, and the findings should be reliable.
In conclusions, the presence of MOH in CM patients was associated with dependence behavior. Moreover, the LDQ was useful in the diagnosis or even screening of MOH in patients with CM. Further studies in independent clinical samples are needed.
Author Contributions
Conceptualization: Y.-F.W. and S.-J.W.; methodology: Y.-F.W. and S.-J.W.; data curation: Y.-F.W., Y.-H.L., S.-P.C., K.-L.L. and S.-J.W.; formal analysis: Y.-F.W., Y.-S.T. and C.-C.Y.; writing—original draft preparation: Y.-F.W. and S.-J.W., writing—review and editing Y.-F.W. and S.-J.W.; supervision: S.-J.W.; funding acquisition: Y.-F.W. and S.-J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded in part by Taiwan Ministry of Science and Technology [MOST 109-2314-B-075 -054 and MOST 110-2314-B-075 -041 -MY3 (to Y.F.W.), and MOST 104-2314-B-010-015-MY2, MOST 106-2321-B-010-009, MOST 107-2321-B-010-001, MOST 108-2321-B-010-014 -MY2, 108-2321-B-010 001, MOST 108-2314-B-010-023-MY3, and MOST-110-2321-B-010-005 (to S.J.W.)]; and Taipei Veterans General Hospital [V108C-092, V109C-096, V110C-111, and V111C-161 (to YFW)]; this work was also supported by the Brain Research Center, National Yang Ming Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study protocols were approved by the Institutional Review Board of Taipei Veterans General Hospital (protocol numbers 2018-07-020BC, 2019-07-002CC).
Informed Consent Statement
All of the patients gave written informed consent before entering the study.
Data Availability Statement
Data are available from the authors on reasonable requests.
Acknowledgments
The authors would like to thank the patients for their help.
Conflicts of Interest
Y.-F.W. has received honoraria as a speaker from Taiwan branches of Allergan/AbbVie, Boehringer Ingelheim, Chugai, Eli Lilly, Novartis, Pfizer, Sanofi, UCB, and Viatris, Orient EuroPharma, and Teva. He has received research grants from the Taiwan Ministry of Science and Technology, and Taipei Veterans General Hospital. SJW has served on the advisory boards of Daiichi-Sankyo, Eli Lilly and Novartis; has received honoraria as a moderator from Allergan/AbbVie, Pfizer, Eli Lilly, Biogen and Eisai and has been the PI in trials sponsored by Eli Lilly, Novartis, and Allergan/AbbVie. He has received research grants from the Taiwan Minister of Technology and Science (MOST), Brain Research Center, National Yang Ming Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan, Taipei Veterans General Hospital, Taiwan Headache Society and Taiwan branches of Eli Lilly, Novartis, and Pfizer.
References
- Diener, H.C.; Dodick, D.; Evers, S.; Holle, D.; Jensen, R.H.; Lipton, R.B.; Porreca, F.; Silberstein, S.; Schwedt, T. Pathophysiology, prevention, and treatment of medication overuse headache. Lancet Neurol. 2019, 18, 891–902. [Google Scholar] [CrossRef]
- Katsarava, Z.; Muessig, M.; Dzagnidze, A.; Fritsche, G.; Diener, H.C.; Limmroth, V. Medication overuse headache: Rates and predictors for relapse in a 4-year prospective study. Cephalalgia 2005, 25, 12–15. [Google Scholar] [CrossRef]
- Chen, P.K.; Wang, S.J. Medication Overuse and Medication Overuse Headache: Risk Factors, Comorbidities, Associated Burdens and Nonpharmacologic and Pharmacologic Treatment Approaches. Curr. Pain Headache Rep. 2019, 23, 60. [Google Scholar] [CrossRef]
- Wang, S.J.; Fuh, J.L.; Lu, S.R.; Liu, C.Y.; Hsu, L.C.; Wang, P.N.; Liu, H.C. Chronic daily headache in Chinese elderly: Prevalence, risk factors, and biannual follow-up. Neurology 2000, 54, 314–319. [Google Scholar] [CrossRef]
- Lu, S.R.; Fuh, J.L.; Chen, W.T.; Juang, K.D.; Wang, S.J. Chronic daily headache in Taipei, Taiwan: Prevalence, follow-up and outcome predictors. Cephalalgia 2001, 21, 980–986. [Google Scholar] [CrossRef]
- Wang, S.J.; Fuh, J.L.; Lu, S.R.; Juang, K.D. Chronic daily headache in adolescents: Prevalence, impact, and medication overuse. Neurology 2006, 66, 193–197. [Google Scholar] [CrossRef]
- Vandenbussche, N.; Laterza, D.; Lisicki, M.; Lloyd, J.; Lupi, C.; Tischler, H.; Toom, K.; Vandervorst, F.; Quintana, S.; Paemeleire, K.; et al. Medication-overuse headache: A widely recognized entity amidst ongoing debate. J. Headache Pain 2018, 19, 50. [Google Scholar] [CrossRef]
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Diener, H.C.; Holle, D.; Solbach, K.; Gaul, C. Medication-overuse headache: Risk factors, pathophysiology and management. Nat. Rev. Neurol. 2016, 12, 575–583. [Google Scholar] [CrossRef]
- Minen, M.T.; Begasse De Dhaem, O.; Kroon Van Diest, A.; Powers, S.; Schwedt, T.J.; Lipton, R.; Silbersweig, D. Migraine and its psychiatric comorbidities. J. Neurol. Neurosurg. Psychiatry 2016, 87, 741–749. [Google Scholar] [CrossRef]
- Dresler, T.; Caratozzolo, S.; Guldolf, K.; Huhn, J.I.; Loiacono, C.; Niiberg-Pikksoot, T.; Puma, M.; Sforza, G.; Tobia, A.; Ornello, R.; et al. Understanding the nature of psychiatric comorbidity in migraine: A systematic review focused on interactions and treatment implications. J. Headache Pain 2019, 20, 51. [Google Scholar] [CrossRef]
- Fuh, J.L.; Wang, S.J.; Lu, S.R.; Juang, K.D. Does medication overuse headache represent a behavior of dependence? Pain 2005, 119, 49–55. [Google Scholar] [CrossRef]
- Radat, F.; Creac'h, C.; Guegan-Massardier, E.; Mick, G.; Guy, N.; Fabre, N.; Giraud, P.; Nachit-Ouinekh, F.; Lanteri-Minet, M. Behavioral dependence in patients with medication overuse headache: A cross-sectional study in consulting patients using the DSM-IV criteria. Headache 2008, 48, 1026–1036. [Google Scholar] [CrossRef]
- Bottiroli, S.; Galli, F.; Ballante, E.; Pazzi, S.; Sances, G.; Guaschino, E.; Allena, M.; Tassorelli, C. Validity of the Severity of Dependence Scale for detecting dependence behaviours in chronic migraine with medication overuse. Cephalalgia 2022, 42, 209–217. [Google Scholar] [CrossRef]
- Radat, F.; Creac'h, C.; Swendsen, J.D.; Lafittau, M.; Irachabal, S.; Dousset, V.; Henry, P. Psychiatric comorbidity in the evolution from migraine to medication overuse headache. Cephalalgia 2005, 25, 519–522. [Google Scholar] [CrossRef]
- Fumal, A.; Laureys, S.; Di Clemente, L.; Boly, M.; Bohotin, V.; Vandenheede, M.; Coppola, G.; Salmon, E.; Kupers, R.; Schoenen, J. Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain 2006, 129 Pt 2, 543–550. [Google Scholar] [CrossRef]
- Lai, T.H.; Chou, K.H.; Fuh, J.L.; Lee, P.L.; Kung, Y.C.; Lin, C.P.; Wang, S.J. Gray matter changes related to medication overuse in patients with chronic migraine. Cephalalgia 2016, 36, 1324–1333. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb. Cortex 2000, 10, 318–325. [Google Scholar] [CrossRef]
- Schoenbaum, G.; Shaham, Y. The role of orbitofrontal cortex in drug addiction: A review of preclinical studies. Biol. Psychiatry 2008, 63, 256–262. [Google Scholar] [CrossRef]
- Ferrari, A.; Cicero, A.F.; Bertolini, A.; Leone, S.; Pasciullo, G.; Sternieri, E. Need for analgesics/drugs of abuse: A comparison between headache patients and addicts by the Leeds Dependence Questionnaire (LDQ). Cephalalgia 2006, 26, 187–193. [Google Scholar] [CrossRef]
- Grande, R.B.; Aaseth, K.; Saltyte Benth, J.; Gulbrandsen, P.; Russell, M.B.; Lundqvist, C. The Severity of Dependence Scale detects people with medication overuse: The Akershus study of chronic headache. J. Neurol. Neurosurg. Psychiatry 2009, 80, 784–789. [Google Scholar] [CrossRef]
- Lundqvist, C.; Grande, R.B.; Aaseth, K.; Russell, M.B. Dependence scores predict prognosis of medication overuse headache: A prospective cohort from the Akershus study of chronic headache. Pain 2012, 153, 682–686. [Google Scholar] [CrossRef]
- Lundqvist, C.; Gossop, M.; Russell, M.B.; Straand, J.; Kristoffersen, E.S. Severity of Analgesic Dependence and Medication-overuse Headache. J. Addict. Med. 2019, 13, 346–353. [Google Scholar] [CrossRef]
- Raistrick, D.; Bradshaw, J.; Tober, G.; Weiner, J.; Allison, J.; Healey, C. Development of the Leeds Dependence Questionnaire (LDQ): A questionnaire to measure alcohol and opiate dependence in the context of a treatment evaluation package. Addiction 1994, 89, 563–572. [Google Scholar] [CrossRef]
- Anonymous; Anonymous. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Stewart, W.F.; Lipton, R.B.; Kolodner, K.B.; Sawyer, J.; Lee, C.; Liberman, J.N. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000, 88, 41–52. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Lundqvist, C.; Benth, J.S.; Grande, R.B.; Aaseth, K.; Russell, M.B. An adapted Severity of Dependence Scale is valid for the detection of medication overuse: The Akershus study of chronic headache. Eur. J. Neurol. 2011, 18, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Dousset, V.; Maud, M.; Legoff, M.; Radat, F.; Brochet, B.; Dartigues, J.F.; Kurth, T. Probable medications overuse headaches: Validation of a brief easy-to-use screening tool in a headache centre. J. Headache Pain 2013, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Olesen, J.; Bousser, M.G.; Diener, H.C.; Dodick, D.; First, M.; Goadsby, P.J.; Gobel, H.; Lainez, M.J.; Lance, J.W.; et al. The International Classification of Headache Disorders, 2nd Edition (ICHD-II)—Revision of criteria for 8.2 Medication-overuse headache. Cephalalgia 2005, 25, 460–465. [Google Scholar] [CrossRef]
- Gossop, M.; Darke, S.; Griffiths, P.; Hando, J.; Powis, B.; Hall, W.; Strang, J. The Severity of Dependence Scale (SDS): Psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 1995, 90, 607–614. [Google Scholar] [CrossRef]
- May, A.; Schulte, L.H. Chronic migraine: Risk factors, mechanisms and treatment. Nat. Rev. Neurol. 2016, 12, 455–464. [Google Scholar] [CrossRef]
- Sances, G.; Ghiotto, N.; Galli, F.; Guaschino, E.; Rezzani, C.; Guidetti, V.; Nappi, G. Risk factors in medication-overuse headache: A 1-year follow-up study (care II protocol). Cephalalgia 2010, 30, 329–336. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).