An Updated Evaluation of Intrathecal IgG Synthesis Markers in Relation to Oligoclonal Bands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Biological Samples and Assays
2.3. Ethics Approval
2.4. Statistical Analysis
3. Results
3.1. CSF Analysis Measurements
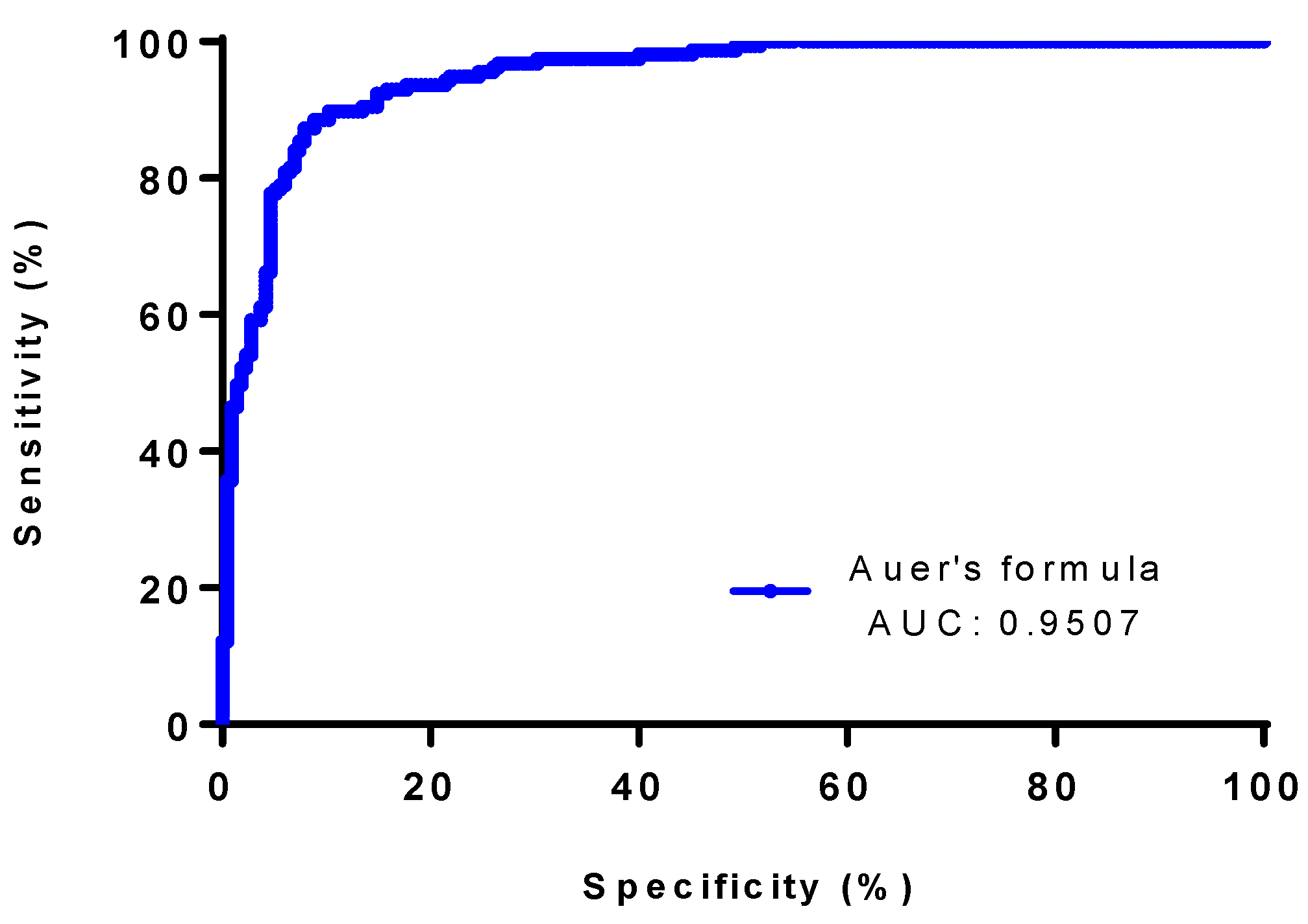
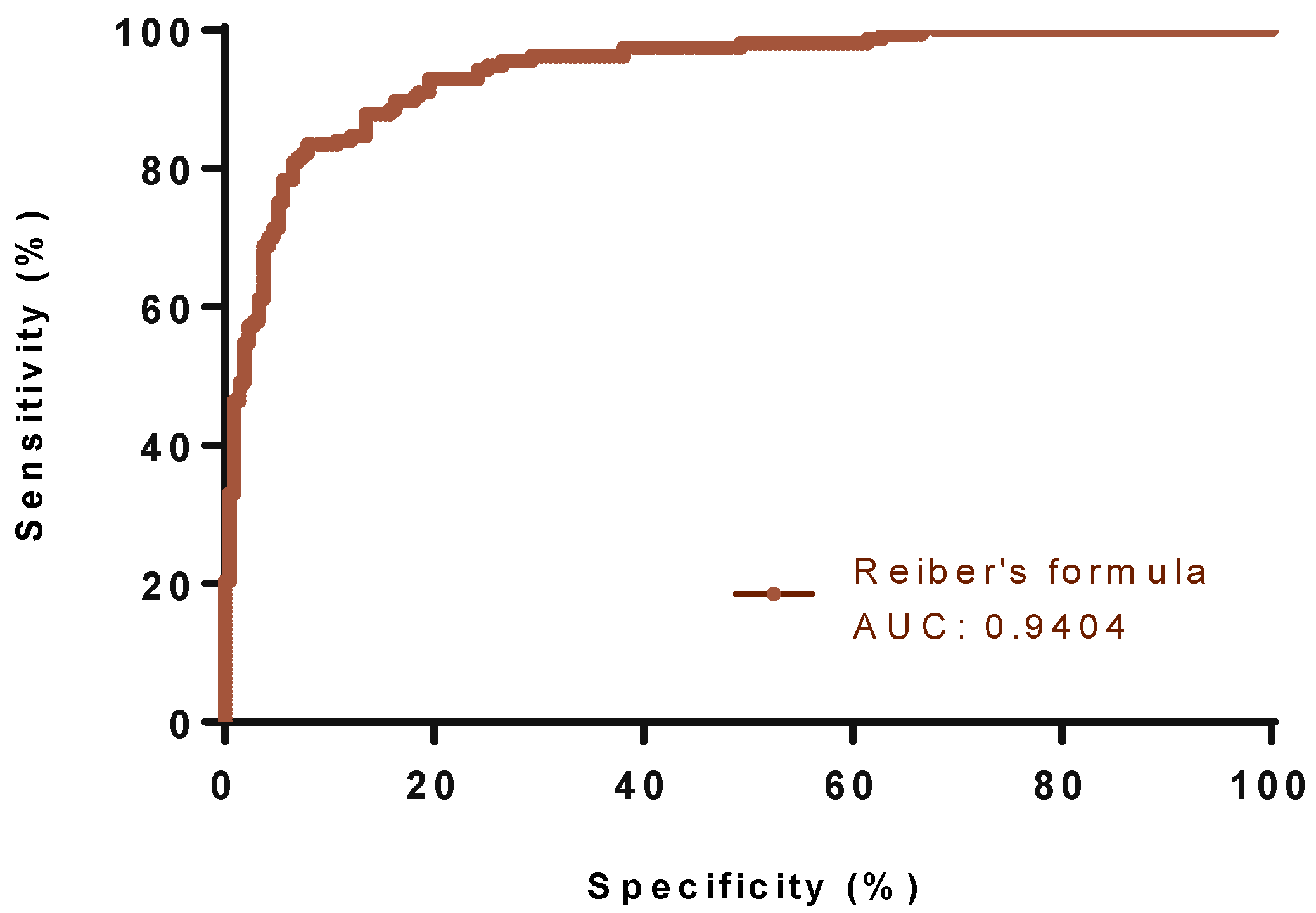
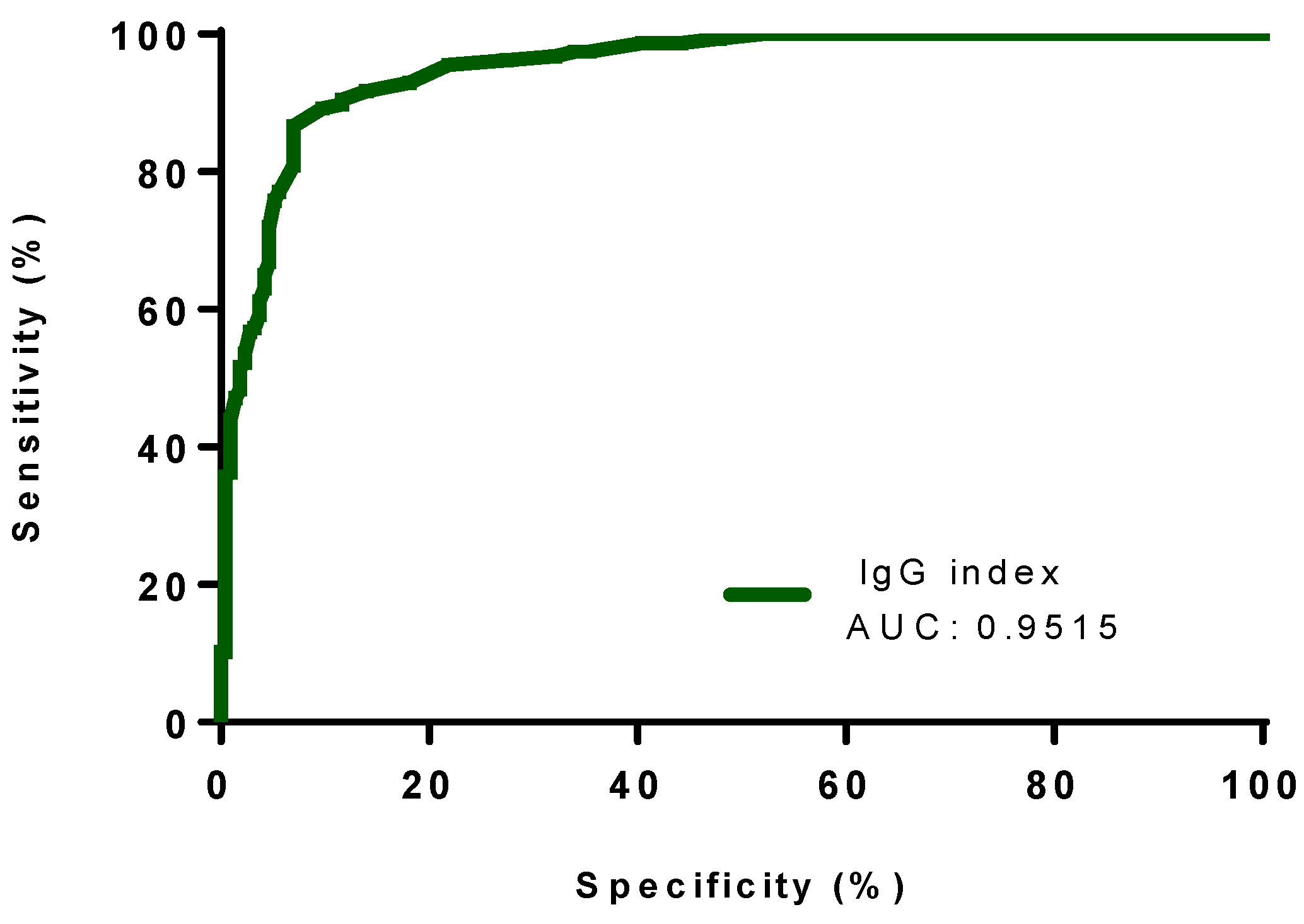
3.2. Classification Performance of Different Formulae, IgG Index, Auer and Reiber’s Formulae for the Presence of OCB Indicative of Intrathecal Synthesis
3.3. Accuracy Rates of Different Formulae, IgG Index, and Auer’s and Reiber’s Formulae for the Presence of OCB Indicative of Intrathecal Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Öhman, S.; Emerudh, J.; Fürsberg, P.; Henriksson, A.; von Schenck, H.; Vrethem, M. Comparison of seven formulae and isoelectric focusing for determination of intrathecally produced IgG in neurological diseases. Ann. Clin. Biochem. 1992, 29, 405–410. [Google Scholar] [CrossRef]
- Delpech, B.; Lichtblau, E. Etude quantitative des immunoglobuline et de l’albumine du liquid cephalo rachidien. Clin. Chim. Acta 1972, 37, 15–23. [Google Scholar] [CrossRef]
- Tibbling, G.; Link, H.; Ohman, S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand. J. Clin. Lab. Investig. 1977, 37, 385–390. [Google Scholar] [CrossRef]
- Reiber, H. Flow rate of cerebrospinal fluid (CSF)—A concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 1994, 2, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.S.; Thompson, E.J.; Deisenhammer, F.; Giovannoni, G.; Grimsley, G.; Keir, G.; Ohman, S.; Racke, M.K.; Sharief, M.; Sindic, C.J.; et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch. Neurol. 2005, 6, 865–870. [Google Scholar] [CrossRef]
- Gastaldi, M.; Zardini, E.; Leante, R.; Ruggieri, M.; Costa, G.; Cocco, E.; De Luca, G.; Cataldo, I.; Biagioli, T.; Ballerini, C.; et al. Cerebrospinal fluid analysis and the determination of oligoclonal bands. Neurol. Sci. 2017, 38, 217–224. [Google Scholar] [CrossRef]
- Luxton, R.W.; McLean, B.N.; Thompson, E.J. Isoelectric focusing versus quantitative measurements in the detection of intrathecal local synthesis of IgG. Clin. Chim. Acta 1990, 187, 297–308. [Google Scholar] [CrossRef]
- Auer, M.; Hegen, H.; Zeileis, A.; Deisenhammer, F. Quantitation of intrathecal immunoglobulin synthesis—A new empirical formula. Eur. J. Neurol. 2016, 4, 713–721. [Google Scholar] [CrossRef]
- Hopper, J.E.; Papagiannes, E. Evidence by radioimmunoassay that mitogenactivated human blood mononuclear cells secrete significant amounts of light chain Ig unassociated with heavy chain. Cell. Immunol. 1986, 101, 122–131. [Google Scholar] [CrossRef]
- Presslauer, S.; Milosavljevic, D.; Brucke, T.; Bayer, P.; Hubl, W. Elevated levels of kappa free light chains in CSF support the diagnosis of multiple sclerosis. J. Neurol. 2008, 255, 1508–1514. [Google Scholar] [CrossRef]
- Miller, D.H.; Weinshenker, B.G.; Filippi, M.; Banwell, B.L.; Cohen, J.A.; Freedman, M.S.; Galetta, S.L.; Hutchinson, M.; Johnson, R.T.; Kappos, L.; et al. Differential diagnosis of suspected multiple sclerosis: A consensus approach. Mult. Scler. 2008, 14, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Alvarez-Gemeno, J.; Bernardi, G.; Cogato, I.; Fredman, P.; Frederiksen, J.; Fredrikson, S.; Gallo, P.; Grimaldi, L.M.; Grønning, M. Cerebrospinal fluid in the diagnosis of multiple sclerosis: A consensous report. J. Neurol. Neurosurg. Psychiatry 1994, 57, 897–902. [Google Scholar] [CrossRef]
- Chicco, D.; Warrens, M.J.; Jurman, G. The Matthews correlation coefficient (MCC) is more informative than Cohen’s Kappa and Brier score in binary classification assessment. IEEE Access 2021, 9, 78368–78381. [Google Scholar] [CrossRef]
- McLean, B.N.; Luxton, R.W.; Thompson, E.J. A study of immunoglobulin G in the cerebrospinal fluid of 1007 patients with suspected neurological disease using iso-electric focusing and the log IgG-Index. Brain 1990, 113, 1269–1289. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; Sandberg-Wollheim, M.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Tintoré, M.; Rovira, A.; Río, J.; Tur, C.; Pelayo, R.; Nos, C.; Téllez, N.; Perkal, H.; Comabella, M.; Sastre-Garriga, J.; et al. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology 2008, 70, 1079–1083. [Google Scholar] [CrossRef]
- Kuhle, J.; Disanto, G.; Dobson, R.; Adiutori, R.; Bianchi, L.; Topping, J.; Bestwick, J.P.; Meier, U.-C.; Marta, M.; Costa, G.D.; et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicenter study. Mult. Scler. 2015, 8, 1013–1024. [Google Scholar]
- Csako, G. Isoelectric Focusing in Agarose Gel for Detection of Oligoclonal Bands in Cerebrospinal and Other Biological Fluids in Electrophoretic Separation of Proteins: Methods and Protocols. Methods Mol. Biol. 2012, 869, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H.; Felgenhauer, K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin. Chim. Acta 1987, 163, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Ohman, S.; Forsberg, P.; Nelson, N.; Vrethem, M. An improved formula for the judgement of intrathecally produced IgG in the presence of blood brain barrier damage. Clin. Chim. Acta 1989, 181, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Schuller, E.A.C.; Benabdallah, S.; Sagar, H.J.; Reboul, J.A.M.; Tempe, L.C. IgG synthesis within the central nervous system. Comparison of three formulas. Arch. Neurol. 1987, 44, 600–604. [Google Scholar] [CrossRef]
- Tourtellotte, W. What is multiple sclerosis? Laboratory criteria for diagnosis. In Multiple Sclerosis Research; Davidson, A.N., Humphrey, J.H., Liversedge, A.L., Eds.; Elsevier: New York, NY, USA, 1975; pp. 9–26. [Google Scholar]
- Reiber, H. Evaluation of IgG synthesis in the central nervous system. In Progress in Multiple Sclerosis Research; Bauer, H.J., Poser, S., Ritter, G., Eds.; Springer: Berlin, Germany, 1980; pp. 154–156. [Google Scholar]
- Berek, K.; Bsteh, G.; Auer, M.; Di Pauli, F.; Zinganell, A.; Berger, T.; Deisenhammer, F.; Hegen, H. Cerebrospinal Fluid Findings in 541 Patients with Clinically Isolated Syndrome and Multiple Sclerosis: A Monocentric Study. Front. Immunol. 2021, 12, 675307. [Google Scholar] [CrossRef]
- Keir, G.; Luxton, R.W.; Thompson, E.J. Isoelectricfocusing of cerebrospinal fluid immunoglobulin G: An annotated update. Ann. Clin. Biochem. 1990, 27, 436–443. [Google Scholar] [CrossRef]
- Link, H.; Huang, Y.M. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: An update on methodology and clinical usefulness. J. Neuroimmunol. 2006, 180, 17–28. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Bartos, A.; Egg, R.; Gilhus, N.E.; Giovannoni, G.; Rauer, S.; Sellebjerg, F. EFNS Task Force. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur. J. Neurol. 2006, 9, 913–922. [Google Scholar] [CrossRef]
- Reiber, H. Non-linear ventriculo—Lumbar protein gradients validate the diffusion-flow model for the blood-CSF barrier. Clin. Chim. Acta 2021, 513, 64–67. [Google Scholar] [CrossRef]
- Desplat-Jego, S.; Feuillet, L.; Pelletier, J.; Bernard, D.; Cherif, A.A.; Boucraut, J. Quantification of immunoglobulin free light chains in cerebrospinal fluid by nephelometry. J. Clin. Immunol. 2005, 25, 338–345. [Google Scholar] [CrossRef]
- Duranti, F.; Pieri, M.; Centonze, D.; Buttari, F.; Bernardini, S.; Dessi, M. Determination of κFLC and κ Index in cerebrospinal fluid: A valid alternative to assess intrathecal immunoglobulin synthesis. J. Neuroimmunol. 2013, 263, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Presslauer, S.; Milosavljevic, D.; Huebl, W.; Parigger, S.; Schneider-Koch, G.; Bruecke, T. Kappa free light chains: Diagnostic and prognostic relevance in MS and CIS. PLoS ONE 2014, 9, e89945. [Google Scholar] [CrossRef] [PubMed]
- Senel, M.; Mojib-Yezdani, F.; Braisch, U.; Bachhuber, F.; Lewerenz, J.; Ludolph, A.C.; Otto, M.; Tumani, H. CSF Free Light Chains as a Marker of Intrathecal Immunoglobulin Synthesis in Multiple Sclerosis: A Blood-CSF Barrier Related Evaluation in a Large Cohort. Front. Immunol. 2019, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Leurs, C.E.; Twaalfhoven, H.; Lissenberg-Witte, B.I.; van Pesch, V.; Dujmovic, I.; Drulovic, J.; Castellazzi, M.; Bellini, T.; Pugliatti, M.; Kuhle, J.; et al. Kappa free light chains is a valid tool in the diagnostics of MS: A large multicenter study. Mult. Scler. 2020, 8, 912–923. [Google Scholar] [CrossRef]
- Voortman, M.M.; Stojakovic, T.; Pirpamer, L.; Jehna, M.; Langkammer, C.; Scharnagl, H.; Reindl, M.; Ropele, S.; Seifert-Held, T.; Archelos, J.-J.; et al. Prognostic value of free light chains lambda and kappa in early multiple sclerosis. Mult. Scler. 2017, 23, 1496–1505. [Google Scholar] [CrossRef]
- Senel, M.; Tumani, H.; Lauda, F.; Presslauer, S.; Mojib-Yezdani, R.; Otto, M.; Brettschneider, J. Cerebrospinal fluid immunoglobulin kappa light chain in clinically isolated syndrome and multiple sclerosis. PLoS ONE 2014, 9, e88680. [Google Scholar] [CrossRef]
- Reiber, H.; Zeman, D.; Kušnierová, P.; Mundwiler, E.; Bernasconi, L. Diagnostic relevance of free light chains in cerebrospinal fluid—The hyperbolic reference range for reliable data interpretation in quotient diagrams. Clin. Chim. Acta 2019, 497, 153–162. [Google Scholar] [CrossRef]
- Hannich, M.J.; Abdullah, M.R.; Budde, K.; Petersmann, A.; Nauck, M.; Dressel, A.; Süße, M.A. New Laboratory Workflow Integrating the Free Light Chains Kappa Quotient into Routine CSF Analysis. Biomolecules 2022, 12, 1690. [Google Scholar] [CrossRef]
| Gender | No (%) |
|---|---|
| Female | 225 (60.5) |
| Male | 147 (39.5) |
| Age (mean ± SD) | 38 years (12) |
| Final Diagnosis | No (%) |
| RRMS | 204 (54.8) |
| CIS | 141(38.0) |
| PPMS | 19 (5.2) |
| RIS | 8 (2.0) |
| CSF Parameters | Median ± SD |
|---|---|
| White Blood Cell (/μL) | 2 (11.15) |
| Red Blood Cell (/μL) | 2 (190) |
| CSF IgG (mg/dL) | 39 (21.99) |
| QAlb | 4.48 (3.932) |
| QIgG | 3.08 (3.109) |
| OCBs | No (%) |
| Negative OCBs pattern 1 | 208 (55.9) |
| Positive OCBs pattern 2 | 155 (41.7) |
| Positive OCBs pattern 3 | 2 (0.5) |
| Negative OCBs pattern 4 | 7 (1.9) |
| Formula | Cut-Off | Sensitivity | Specificity | AUC (95% CI) | Std. Error | p Value | PPV (%) | NPV (%) | ACC | κ |
|---|---|---|---|---|---|---|---|---|---|---|
| IgG index | >0.65 | 0.89 (0.83–0.94) | 0.9023 (0.86–0.94) | 0.9515 (0.9312 to 0.9718) | 0.01 | <0.0001 | 86.96 | 91.94 | 0.898 | 0.792 |
| Reiber’s formula | >0.93 | 0.83 (0.76–0.88) | 0.92 (0.88–0.95) | 0.9404 (0.9171 to 0.9636) | 0.01 | <0.0001 | 87.33 | 88.29 | 0.885 | 0.762 |
| Auer’s formula | >0.88 | 0.68 (0.60–0.75) | 0.9535 (0.92–0.98) | 0.9507 (0.9302 to 0.9712) | 0.01 | <0.0001 | 91.59 | 81.02 | 0.839 | 0.658 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boufidou, F.; Vakrakou, A.G.; Anagnostouli, M.; Patas, K.; Paraskevas, G.; Chatzipanagiotou, S.; Stefanis, L.; Evangelopoulos, M.-E. An Updated Evaluation of Intrathecal IgG Synthesis Markers in Relation to Oligoclonal Bands. Diagnostics 2023, 13, 389. https://doi.org/10.3390/diagnostics13030389
Boufidou F, Vakrakou AG, Anagnostouli M, Patas K, Paraskevas G, Chatzipanagiotou S, Stefanis L, Evangelopoulos M-E. An Updated Evaluation of Intrathecal IgG Synthesis Markers in Relation to Oligoclonal Bands. Diagnostics. 2023; 13(3):389. https://doi.org/10.3390/diagnostics13030389
Chicago/Turabian StyleBoufidou, Fotini, Aigli G. Vakrakou, Maria Anagnostouli, Kostas Patas, Georgios Paraskevas, Stylianos Chatzipanagiotou, Leonidas Stefanis, and Maria-Eleftheria Evangelopoulos. 2023. "An Updated Evaluation of Intrathecal IgG Synthesis Markers in Relation to Oligoclonal Bands" Diagnostics 13, no. 3: 389. https://doi.org/10.3390/diagnostics13030389
APA StyleBoufidou, F., Vakrakou, A. G., Anagnostouli, M., Patas, K., Paraskevas, G., Chatzipanagiotou, S., Stefanis, L., & Evangelopoulos, M.-E. (2023). An Updated Evaluation of Intrathecal IgG Synthesis Markers in Relation to Oligoclonal Bands. Diagnostics, 13(3), 389. https://doi.org/10.3390/diagnostics13030389








