Radiofrequency Ablation, Cryoablation, and Microwave Ablation for the Treatment of Small Renal Masses: Efficacy and Complications
Abstract
1. Introduction
2. Patient Selection
3. Study of the Lesion—Imaging Guide
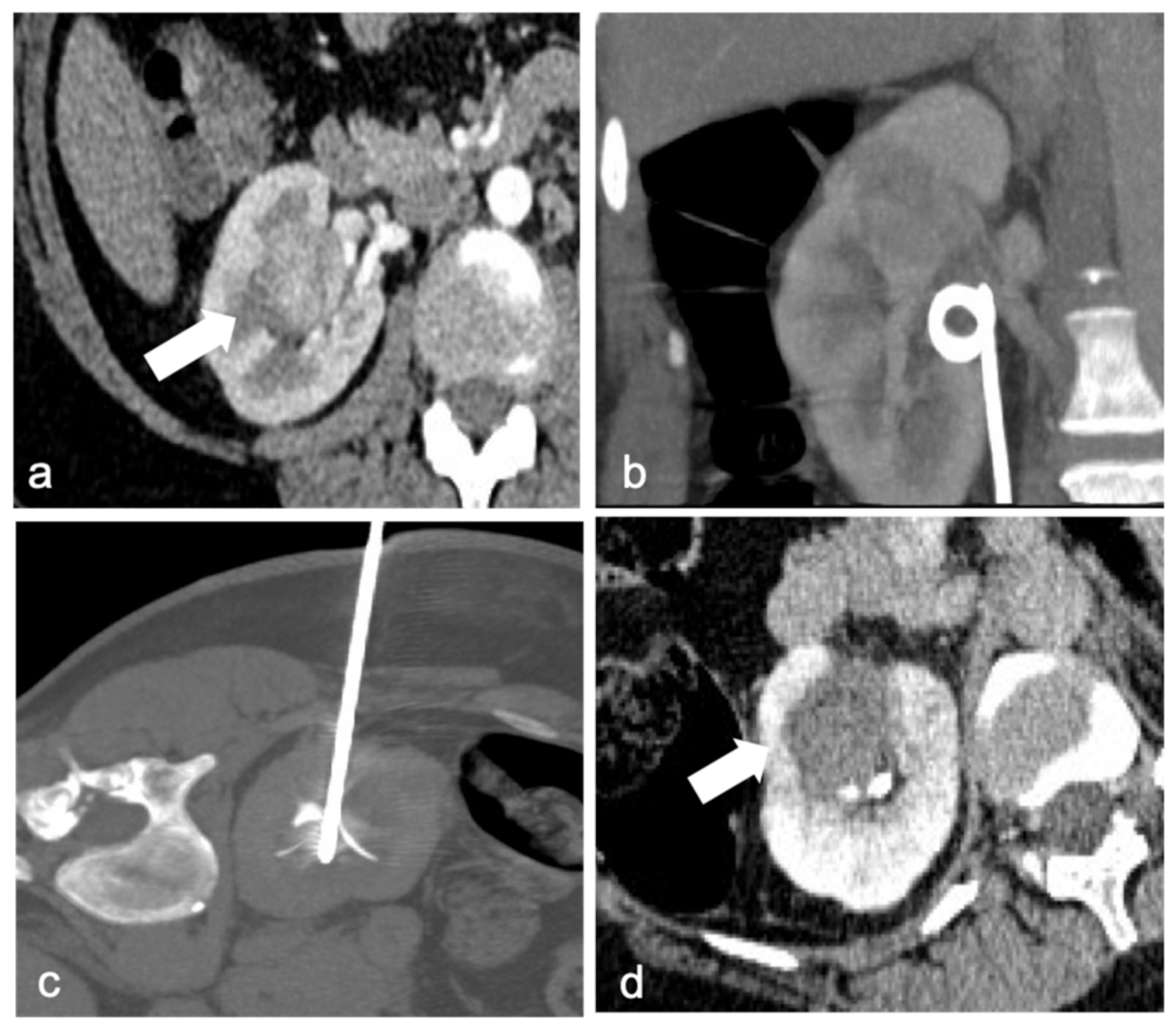
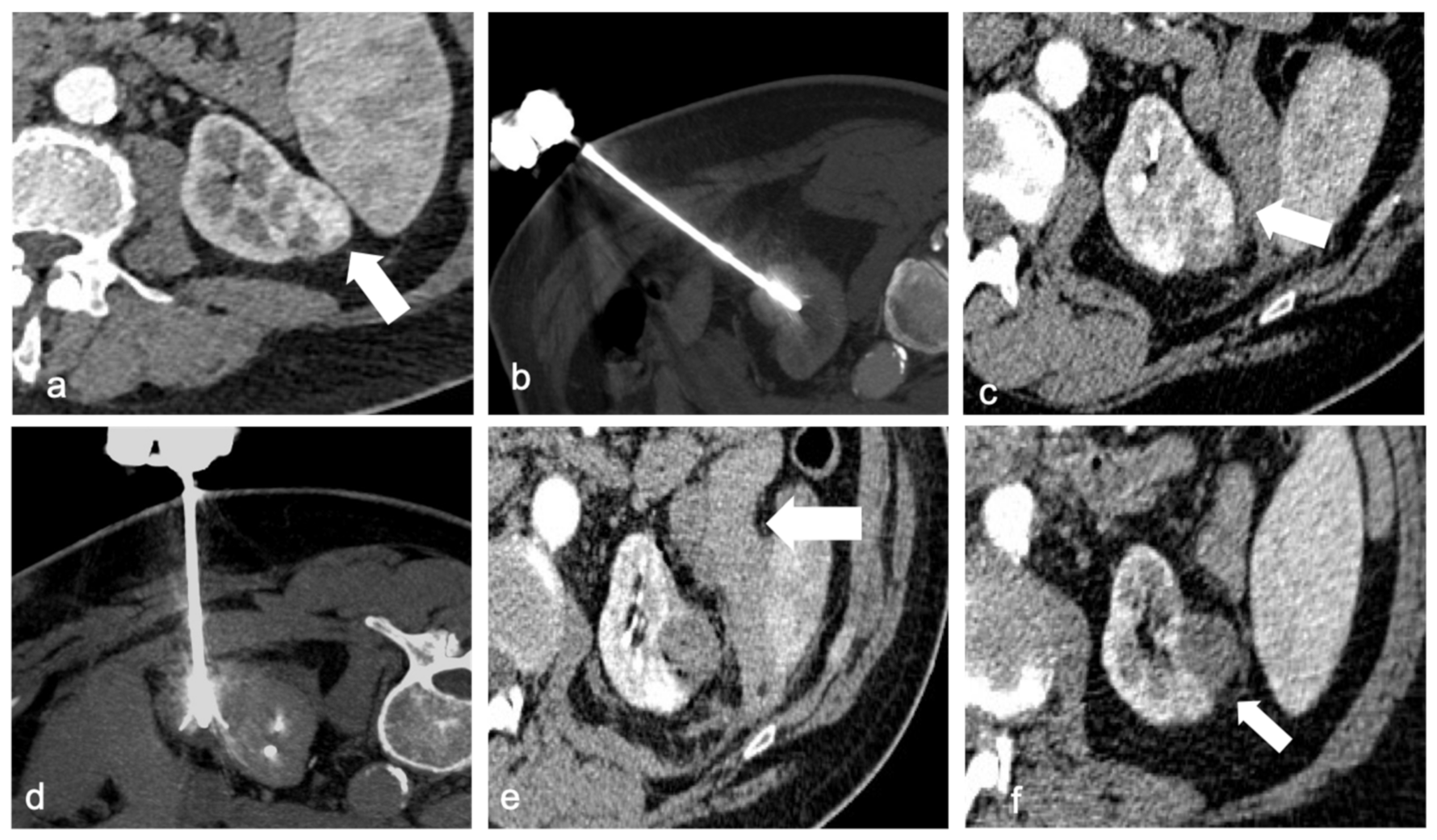
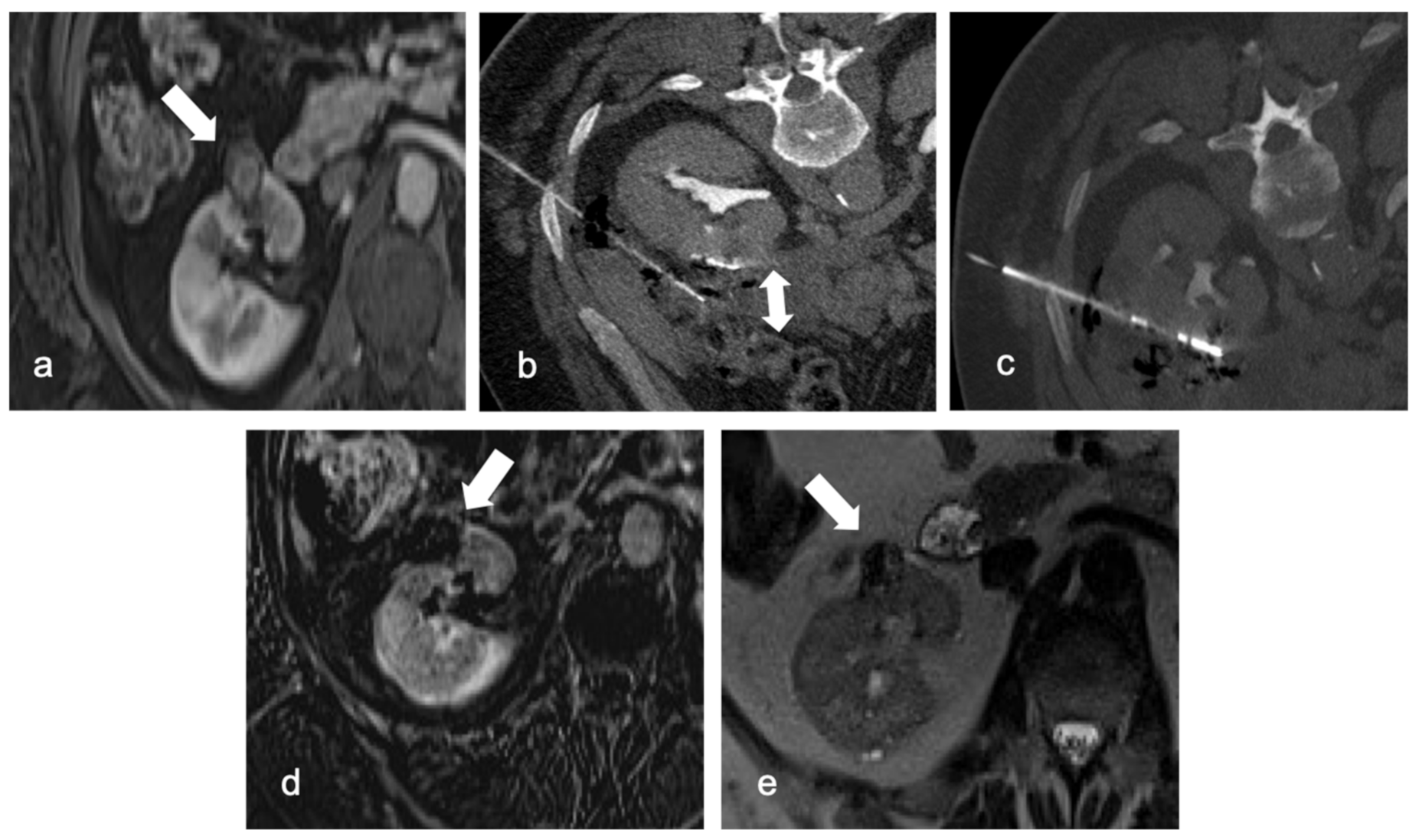
4. Ablation Techniques
4.1. Radiofrequence Ablation (RFA)
4.2. Cryoablation (CA)
4.3. Microwave Ablation (MWA)
5. Outcomes
5.1. Radiofrequency Ablation
5.2. Cryoablation
5.3. Microwave Ablation (MWA)
6. Complications
6.1. RFA
6.2. CA
6.3. MWA
7. Current Guidelines
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yin, X.; Cui, L.; Li, F.; Qi, S.; Yin, Z.; Gao, J. Radiofrequency Ablation Versus Partial Nephrectomy in Treating Small Renal Tumors: A Systematic Review and Meta-Analysis. Medicine 2015, 94, e2255. [Google Scholar] [CrossRef] [PubMed]
- Pierorazio, P.M.; Johnson, M.H.; Patel, H.D.; Sozio, S.M.; Sharma, R.; Iyoha, E.; Bass, E.B.; Allaf, M.E. Management of Renal Masses and Localized Renal Cancer: Systematic Review and Meta-Analysis. J. Urol. 2016, 196, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltran, A.; Carrasco, J.C.; Cheng, L.; Scarpelli, M.; Kirkali, Z.; Montironi, R. 2009 Update on the Classification of Renal Epithelial Tumors in Adults. Int. J. Urol. 2009, 16, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.H.; Devesa, S.S.; Warren, J.L.; Fraumeni, J.F. Rising Incidence of Renal Cell Cancer in the United States. JAMA 1999, 281, 1628–1631. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, G.; Shang, H.; Ding, B.; Sun, G.; Ouyang, W.; Liu, M.; Chen, Y.; Li, H.; Xu, H.; et al. Comparison of the oncological, perioperative and functional outcomes of partial nephrectomy versus radical nephrectomy for clinical T1b renal cell carcinoma: A systematic review and meta-analysis of retrospective studies. Asian J. Urol. 2021, 8, 117–125. [Google Scholar] [CrossRef]
- Badalato, G.M.; Kates, M.; Wisnivesky, J.P.; Choudhury, A.R.; McKiernan, J.M. Survival after partial and radical nephrectomy for the treatment of stage T1bN0M0 renal cell carcinoma (RCC) in the USA: A propensity scoring approach. BJU Int. 2012, 109, 1457–1462. [Google Scholar] [CrossRef]
- Campbell, S.C.; Novick, A.C.; Belldegrun, A.; Blute, M.L.; Chow, G.K.; Derweesh, I.H.; Faraday, M.M.; Kaouk, J.H.; Leveillee, R.J.; Matin, S.F.; et al. Guideline for Management of the Clinical T1 Renal Mass. J. Urol. 2009, 182, 1271–1279. [Google Scholar] [CrossRef]
- Shin, B.J.; Chick, J.F.B.; Stavropoulos, S.W. Contemporary Status of Percutaneous Ablation for the Small Renal Mass. Curr. Urol. Rep. 2016, 17, 23. [Google Scholar] [CrossRef]
- Escudier, B.; Porta, C.; Schmidinger, M.; Algaba, F.; Patard, J.J.; Khoo, V.; Eisen, T.; Horwich, A. ESMO Guidelines Working Group Renal Cell Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2014, 25 (Suppl. 3), iii49–iii56. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Lagerveld, B.W.; Keeley, F.; Lughezzani, G.; Sriprasad, S.; Barber, N.J.; Hansen, L.U.; Buffi, N.M.; Guazzoni, G.; van der Zee, J.A.; et al. Oncological Outcomes and Complication Rates after Laparoscopic-Assisted Cryoablation: A European Registry for Renal Cryoablation (EuRECA) Multi-Institutional Study. BJU Int. 2017, 119, 390–395. [Google Scholar] [CrossRef]
- Shapiro, D.D.; Abel, E.J. Predicting Aggressive Behavior in Small Renal Tumors Prior to Treatment. Ann. Transl. Med. 2018, 6, S132. [Google Scholar] [CrossRef]
- Smaldone, M.C.; Kutikov, A.; Egleston, B.L.; Canter, D.J.; Viterbo, R.; Chen, D.Y.T.; Jewett, M.A.; Greenberg, R.E.; Uzzo, R.G. Small Renal Masses Progressing to Metastases under Active Surveillance: A Systematic Review and Pooled Analysis. Cancer 2012, 118, 997–1006. [Google Scholar] [CrossRef]
- Pagnini, F.; Cervi, E.; Maestroni, U.; Agostini, A.; Borgheresi, A.; Piacentino, F.; Angileri, S.A.; Ierardi, A.M.; Floridi, C.; Carbone, M.; et al. Imaging Guided Percutaneous Renal Biopsy: Do It or Not? Acta Biomed. 2020, 91, 81–88. [Google Scholar] [CrossRef]
- Lim, A.; O’Neil, B.; Heilbrun, M.E.; Dechet, C.; Lowrance, W.T. The Contemporary Role of Renal Mass Biopsy in the Management of Small Renal Tumors. Front. Oncol. 2012, 2, 106. [Google Scholar] [CrossRef]
- Sutherland, E.L.; Choromanska, A.; Al-Katib, S.; Coffey, M. Outcomes of Ultrasound Guided Renal Mass Biopsies. J. Ultrasound 2018, 21, 99–104. [Google Scholar] [CrossRef]
- Gentili, F.; Bronico, I.; Maestroni, U.; Ziglioli, F.; Silini, E.M.; Buti, S.; de Filippo, M. Small Renal Masses (≤4 Cm): Differentiation of Oncocytoma from Renal Clear Cell Carcinoma Using Ratio of Lesion to Cortex Attenuation and Aorta–Lesion Attenuation Difference (ALAD) on Contrast-Enhanced CT. Radiol. Med. 2020, 125, 1280–1287. [Google Scholar] [CrossRef]
- Psutka, S.P.; Feldman, A.S.; McDougal, W.S.; McGovern, F.J.; Mueller, P.; Gervais, D.A. Long-Term Oncologic Outcomes after Radiofrequency Ablation for T1 Renal Cell Carcinoma. Eur. Urol. 2013, 63, 486–492. [Google Scholar] [CrossRef]
- Davis, K.; Kielar, A.; Jafari, K. Effectiveness of Ultrasound-Guided Radiofrequency Ablation in the Treatment of 36 Renal Cell Carcinoma Tumours Compared with Published Results of Using Computed Tomography Guidance. Can. Assoc. Radiol. J. 2012, 63, S23–S32. [Google Scholar] [CrossRef]
- De Filippo, M.; Puglisi, S.; D’Amuri, F.; Gentili, F.; Paladini, I.; Carrafiello, G.; Maestroni, U.; Del Rio, P.; Ziglioli, F.; Pagnini, F. CT-Guided Percutaneous Drainage of Abdominopelvic Collections: A Pictorial Essay. Radiol. Med. 2021, 126, 1561–1570. [Google Scholar] [CrossRef]
- Maybody, M. An Overview of Image-Guided Percutaneous Ablation of Renal Tumors. Semin. Interv. Radiol. 2010, 27, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Arzola, J.; Baughman, S.M.; Hernandez, J.; Bishoff, J.T. Computed Tomography-Guided, Resistance-Based, Percutaneous Radiofrequency Ablation of Renal Malignancies under Conscious Sedation at Two Years of Follow-Up. Urology 2006, 68, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Zagoria, R.J.; Traver, M.A.; Werle, D.M.; Perini, M.; Hayasaka, S.; Clark, P.E. Oncologic Efficacy of CT-Guided Percutaneous Radiofrequency Ablation of Renal Cell Carcinomas. AJR Am. J. Roentgenol. 2007, 189, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, B.K.; Park, J.J.; Kim, C.K. CT-Guided Radiofrequency Ablation of T1a Renal Cell Carcinoma in Korea: Mid-Term Outcomes. Korean J. Radiol. 2016, 17, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Levesque, V.M.; Shyn, P.B.; Tuncali, K.; Tatli, S.; Nawfel, R.D.; Olubiyi, O.; Silverman, S.G. Radiation Dose during CT-Guided Percutaneous Cryoablation of Renal Tumors: Effect of a Dose Reduction Protocol. Eur. J. Radiol. 2015, 84, 2218–2221. [Google Scholar] [CrossRef]
- Andersson, M.; Hashimi, F.; Lyrdal, D.; Lundstam, S.; Hellström, M. Improved Outcome with Combined US/CT Guidance as Compared to US Guidance in Percutaneous Radiofrequency Ablation of Small Renal Masses. Acta Radiol. 2015, 56, 1519–1526. [Google Scholar] [CrossRef]
- Mauri, G.; Gennaro, N.; De Beni, S.; Ierace, T.; Goldberg, S.N.; Rodari, M.; Solbiati, L.A. Real-Time US-18FDG-PET/CT Image Fusion for Guidance of Thermal Ablation of 18FDG-PET-Positive Liver Metastases: The Added Value of Contrast Enhancement. Cardiovasc. Interv. Radiol. 2019, 42, 60–68. [Google Scholar] [CrossRef]
- Split-Dose Technique for FDG PET/CT-Guided Percutaneous Ablation: A Method to Facilitate Lesion Targeting and to Provide Immediate Assessment of Treatment Effectiveness—PubMed. Available online: https://pubmed-ncbi-nlm-nih-gov.ezproxy.unibo.it/23564714/ (accessed on 17 November 2022).
- Gervais, D.A.; McGovern, F.J.; Arellano, R.S.; McDougal, W.S.; Mueller, P.R. Radiofrequency Ablation of Renal Cell Carcinoma: Part 1, Indications, Results, and Role in Patient Management over a 6-Year Period and Ablation of 100 Tumors. Am. J. Roentgenol. 2005, 185, 64–71. [Google Scholar] [CrossRef]
- Kwan, K.G.; Matsumoto, E.D. Radiofrequency Ablation and Cryoablation of Renal Tumours. Curr. Oncol. 2007, 14, 34–38. [Google Scholar] [CrossRef]
- De Filippo, M.; Ziglioli, F.; Russo, U.; Pagano, P.; Brunese, L.; Bertelli, E.; Pagnini, F.; Maestroni, U. Radiofrequency Ablation (RFA) of T1a Renal Cancer with Externally Cooled Multitined Expandable Electrodes. Radiol. Med. 2020, 125, 790–797. [Google Scholar] [CrossRef]
- McCarthy, C.J.; Gervais, D.A. Decision Making: Thermal Ablation Options for Small Renal Masses. Semin. Interv. Radiol. 2017, 34, 167–175. [Google Scholar] [CrossRef]
- Regier, M.; Chun, F. Thermal Ablation of Renal Tumors: Indications, Techniques and Results. Dtsch. Arztebl. Int. 2015, 112, 412–418. [Google Scholar] [CrossRef]
- Gervais, D.A.; Arellano, R.S.; McGovern, F.J.; McDougal, W.S.; Mueller, P.R. Radiofrequency ablation of renal cell carcinoma: Part 2, Lessons learned with ablation of 100 tumors. AJR Am. J. Roentgenol. 2005, 185, 72–80. [Google Scholar] [CrossRef]
- Varkarakis, I.M.; Allaf, M.E.; Inagaki, T.; Bhayani, S.B.; Chan, D.Y.; Su, L.-M.; Jarrett, T.W.; Kavoussi, L.R.; Solomon, S.B. Percutaneous radio frequency ablation of renal masses: Results at a 2-year mean followup. J. Urol. 2005, 174, 456–460. [Google Scholar] [CrossRef]
- Ziglioli, F.; De Filippo, M.; Cavalieri, D.M.; Pagnini, F.; Campobasso, D.; Guarino, G.; Maestroni, U. Percutaneous Radiofrequency Ablation (RFA) in Renal Cancer. How to Manage Challenging Masses. A Narrative Review. Acta Biomed. 2022, 93, e2022220. [Google Scholar] [CrossRef]
- Mauri, G.; Mistretta, F.A.; Bonomo, G.; Camisassi, N.; Conti, A.; Della Vigna, P.; Ferro, M.; Luzzago, S.; Maiettini, D.; Musi, G.; et al. Long-Term Follow-Up Outcomes after Percutaneous US/CT-Guided Radiofrequency Ablation for CT1a-b Renal Masses: Experience from Single High-Volume Referral Center. Cancers 2020, 12, 1183. [Google Scholar] [CrossRef]
- De Menezes, M.R.; Viana, P.C.C.; Yamanari, T.R.; Reis, L.O.; Nahas, W. Safety and Feasibility of Radiofrequency Ablation for Treatment of Bosniak IV Renal Cysts. Int. Braz. J. Urol. 2016, 42, 456–463. [Google Scholar] [CrossRef]
- Allen, B.C.; Remer, E.M. Percutaneous Cryoablation of Renal Tumors: Patient Selection, Technique, and Postprocedural Imaging. RadioGraphics 2010, 30, 887–900. [Google Scholar] [CrossRef]
- Gage, A.A.; Baust, J. Mechanisms of Tissue Injury in Cryosurgery. Cryobiology 1998, 37, 171–186. [Google Scholar] [CrossRef]
- Schmit, G.D.; Atwell, T.D.; Callstrom, M.R.; Farrell, M.A.; Leibovich, B.C.; Patterson, D.E.; Chow, G.K.; Blute, M.L.; Charboneau, J.W. Percutaneous Cryoablation of Renal Masses ≥3 Cm: Efficacy and Safety in Treatment of 108 Patients. J. Endourol. 2010, 24, 1255–1262. [Google Scholar] [CrossRef]
- Aboumarzouk, O.M.; Ismail, M.; Breen, D.J.; Van Strijen, M.; Garnon, J.; Lagerveld, B.; Nielsen, T.K.; Keeley, F.X., Jr. Laparoscopic vs. Percutaneous Cryotherapy for Renal Tumors: A Systematic Review and Meta-Analysis. J. Endourol. 2018, 32, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Krokidis, M.E.; Kitrou, P.; Spiliopoulos, S.; Karnabatidis, D.; Katsanos, K. Image-Guided Minimally Invasive Treatment for Small Renal Cell Carcinoma. Insights Imaging 2018, 9, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Nonboe, L.L.; Nielsen, T.K.; Høyer, S.; Graumann, O.; Frøkiær, J.; Borre, M. Arterial Clamping Increases Central Renal Cryoablation Efficacy: An Animal Study. Technol. Cancer Res. Treat. 2017, 16, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Microwave Ablation: Principles and Applications | RadioGraphics. Available online: https://pubs.rsna.org/doi/10.1148/rg.25si055501 (accessed on 1 September 2022).
- Yu, J.; Liang, P.; Yu, X.; Liu, F.; Chen, L.; Wang, Y. A Comparison of Microwave Ablation and Bipolar Radiofrequency Ablation Both with an Internally Cooled Probe: Results in Ex Vivo and in Vivo Porcine Livers. Eur. J. Radiol. 2011, 79, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Gahan, J. New Technologies in Tumor Ablation. Curr. Opin. Urol. 2016, 26, 248–253. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Cheng, Z.-G.; Liu, F.-Y.; Li, Q.; He, G.; Luo, Y.; Yu, X.-L.; Han, Z.-Y.; Liang, P. A Multicenter 10-Year Oncologic Outcome of Ultrasound-Guided Percutaneous Microwave Ablation of Clinical T1 Renal Cell Carcinoma: Will It Stand the Test of Time? Eur. Radiol. 2022, 32, 89–100. [Google Scholar] [CrossRef]
- Klapperich, M.E.; Abel, E.J.; Ziemlewicz, T.J.; Best, S.; Lubner, M.G.; Nakada, S.Y.; Hinshaw, J.L.; Brace, C.L.; Lee, F.T.; Wells, S.A. Effect of Tumor Complexity and Technique on Efficacy and Complications after Percutaneous Microwave Ablation of Stage T1a Renal Cell Carcinoma: A Single-Center, Retrospective Study. Radiology 2017, 284, 272–280. [Google Scholar] [CrossRef]
- Piccioni, F.; Poli, A.; Templeton, L.C.; Templeton, T.W.; Rispoli, M.; Vetrugno, L.; Santonastaso, D.; Valenza, F. Anesthesia for Percutaneous Radiofrequency Tumor Ablation (PRFA): A Review of Current Practice and Techniques. Local Reg. Anesth. 2019, 12, 127–137. [Google Scholar] [CrossRef]
- Russo, U.; Maestroni, U.; Papapietro, R.V.; Trunfio, V.; Ziglioli, F.; Ferretti, S.; Brunese, L.; Carrafiello, G.; De Filippo, M. Imaging after Radiofrequency Ablation of Renal Tumors. Future Oncol. 2018, 14, 2915–2922. [Google Scholar] [CrossRef]
- Talenfeld, A.D.; Gennarelli, R.L.; Elkin, E.B.; Atoria, C.L.; Durack, J.C.; Huang, W.C.; Kwan, S.W. Percutaneous Ablation Versus Partial and Radical Nephrectomy for T1a Renal Cancer: A Population-Based Analysis. Ann. Intern. Med. 2018, 169, 69–77. [Google Scholar] [CrossRef]
- Johnson, B.A.; Sorokin, I.; Cadeddu, J.A. Ten-Year Outcomes of Renal Tumor Radio Frequency Ablation. J. Urol. 2019, 201, 251–258. [Google Scholar] [CrossRef]
- Andrews, J.R.; Atwell, T.; Schmit, G.; Lohse, C.M.; Kurup, A.N.; Weisbrod, A.; Callstrom, M.R.; Cheville, J.C.; Boorjian, S.A.; Leibovich, B.C.; et al. Oncologic Outcomes Following Partial Nephrectomy and Percutaneous Ablation for CT1 Renal Masses. Eur. Urol. 2019, 76, 244–251. [Google Scholar] [CrossRef]
- Atwell, T.D.; Carter, R.E.; Schmit, G.D.; Carr, C.M.; Boorjian, S.A.; Curry, T.B.; Thompson, R.H.; Kurup, A.N.; Weisbrod, A.J.; Chow, G.K.; et al. Complications Following 573 Percutaneous Renal Radiofrequency and Cryoablation Procedures. J. Vasc. Interv. Radiol. 2012, 23, 48–54. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Puliti, A.; Angileri, S.A.; Petrillo, M.; Duka, E.; Floridi, C.; Lecchi, M.; Carrafiello, G. Microwave Ablation of Malignant Renal Tumours: Intermediate-Term Results and Usefulness of RENAL and MRENAL Scores for Predicting Outcomes and Complications. Med. Oncol. 2017, 34, 97. [Google Scholar] [CrossRef]
- Martin, J.; Athreya, S. Meta-Analysis of Cryoablation versus Microwave Ablation for Small Renal Masses: Is There a Difference in Outcome? Diagn. Interv. Radiol. 2013, 19, 501–507. [Google Scholar] [CrossRef]
- Zhou, W.; Arellano, R.S. Thermal Ablation of T1c Renal Cell Carcinoma: A Comparative Assessment of Technical Performance, Procedural Outcome, and Safety of Microwave Ablation, Radiofrequency Ablation, and Cryoablation. J. Vasc. Interv. Radiol. 2018, 29, 943–951. [Google Scholar] [CrossRef]
- Simon, C.J.; Dupuy, D.E.; Mayo-Smith, W.W. Microwave Ablation: Principles and Applications. Radiographics 2005, 25 (Suppl. 1), S69–S83. [Google Scholar] [CrossRef]
- Manekk, R.S.; Gharde, P.; Gattani, R.; Lamture, Y. Surgical Complications and Its Grading: A Literature Review. Cureus 2022, 14, e24963. [Google Scholar] [CrossRef]
- Miyamoto, S.; Nakao, J.; Higashino, T.; Yoshimoto, S.; Hayashi, R.; Sakuraba, M. Clavien-Dindo Classification for Grading Complications after Total Pharyngolaryngectomy and Free Jejunum Transfer. PLoS ONE 2019, 14, e0222570. [Google Scholar] [CrossRef]
- Rivero, J.R.; De La Cerda, J.; Wang, H.; Liss, M.A.; Farrell, A.M.; Rodriguez, R.; Suri, R.; Kaushik, D. Partial Nephrectomy versus Thermal Ablation for Clinical Stage T1 Renal Masses: Systematic Review and Meta-Analysis of More than 3,900 Patients. J. Vasc. Interv. Radiol. 2018, 29, 18–29. [Google Scholar] [CrossRef]
- Weizer, A.Z.; Raj, G.V.; O’Connell, M.; Robertson, C.N.; Nelson, R.C.; Polascik, T.J. Complications after Percutaneous Radiofrequency Ablation of Renal Tumors. Urology 2005, 66, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.T.; Gill, I.S.; Hsu, T.H.S.; Meraney, A.M.; Skacel, M.; Brainard, J.A.; Remer, E.M. Effect of Intentional Cryo-Injury to the Renal Collecting System. J. Urol. 2003, 170, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A.; Wheeler, K.M.; Mithqal, A.; Patel, M.S.; Brace, C.L.; Schenkman, N.S. Percutaneous Microwave Ablation of T1a and T1b Renal Cell Carcinoma: Short-Term Efficacy and Complications with Emphasis on Tumor Complexity and Single Session Treatment. Abdom. Radiol. 2016, 41, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Moreland, A.J.; Ziemlewicz, T.J.; Best, S.L.; Hinshaw, J.L.; Lubner, M.G.; Alexander, M.L.; Brace, C.L.; Kitchin, D.R.; Hedican, S.P.; Nakada, S.Y.; et al. High-Powered Microwave Ablation of T1a Renal Cell Carcinoma: Safety and Initial Clinical Evaluation. J. Endourol. 2014, 28, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Renal Mass and Localized Renal Cancer: AUA Guideline—PubMed. Available online: https://pubmed-ncbi-nlm-nih-gov.ezproxy.unibo.it/28479239/ (accessed on 17 November 2022).
- Finelli, A.; Ismaila, N.; Bro, B.; Durack, J.; Eggener, S.; Evans, A.; Gill, I.; Graham, D.; Huang, W.; Jewett, M.A.S.; et al. Management of Small Renal Masses: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Comparison of Partial Nephrectomy and Percutaneous Ablation for CT1 Renal Masses—European Urology. Available online: https://www.europeanurology.com/article/S0302-2838(14)00673-3/fulltext (accessed on 14 November 2022).
- Filippiadis, D.; Mauri, G.; Marra, P.; Charalampopoulos, G.; Gennaro, N.; De Cobelli, F. Percutaneous Ablation Techniques for Renal Cell Carcinoma: Current Status and Future Trends. Int. J. Hyperth. 2019, 36, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU Guidelines on Renal Cell Carcinoma: 2014 Update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Bhayani, S.; Bro, W.P.; Chang, S.S.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; Fishman, M.; et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 804–834. [Google Scholar] [CrossRef]
- Atwell, T.D.; Vlaminck, J.J.; Boorjian, S.A.; Kurup, A.N.; Callstrom, M.R.; Weisbrod, A.J.; Lohse, C.M.; Hartman, W.R.; Stockland, A.H.; Leibovich, B.C.; et al. Percutaneous Cryoablation of Stage T1b Renal Cell Carcinoma: Technique Considerations, Safety, and Local Tumor Control. J. Vasc. Interv. Radiol. 2015, 26, 792–799. [Google Scholar] [CrossRef]
- Lyrdal, D.; Andersson, M.; Hellström, M.; Sternal, J.; Lundstam, S. Ultrasound-Guided Percutaneous Radiofrequency Ablation of Small Renal Tumors: Clinical Results and Radiological Evolution during Follow-Up. Acta Radiol. 2010, 51, 808–818. [Google Scholar] [CrossRef]
| Ablation Technique | Advantages | Disadvantages |
|---|---|---|
| RFA (heat-based) | Lower bleeding rate Shorter procedural time than CA | Lower success rate if diameter of the lesion >3 cm No real-time visualization of ablation zone |
| CA (cold-based) | Real time visualization of ablation zone (ice-ball) Treat bigger lesions (>3 cm) Less painful than heat-based ablations | Requires longer procedural time Higher bleeding risk |
| MW (heat-based) | No heat sink effect Shorter procedural time than RFA and CA Treat bigger lesions (>3 cm) | More painful for the patient No real-time visualization of ablation zone More dangerous in hilar lesions |
| Immediate Post-Ablation | Follow-Up | |
|---|---|---|
| CT |
|
|
| MRI |
|
|
| Grade I | Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic, or radiological interventions Allowed therapeutic regimens are: drugs as antiemetics, antipyretics, analgetics, diuretics, and electrolytes and physiotherapy. This grade also includes wound infections opened at the bedside. |
| Grade II | Requiring pharmacological treatment other than such allowed for grade I complications. Blood transfusions and parenteral nutrition are also included. |
| Grade III | Requiring surgical, endoscopic, or radiological intervention |
| IIIa | Intervention not requiring general anesthesia |
| IIIb | Intervention under general anesthesia |
| Grade IV | Life threatening complication (including stroke and subarrachnoidal bleeding, but excluding TIA) requiring IC/ICU management |
| IVa | Single organ disfunction (including dialysis) |
| IVb | Multiorgan disfunction |
| Grade V | Death |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertolotti, L.; Bazzocchi, M.V.; Iemma, E.; Pagnini, F.; Ziglioli, F.; Maestroni, U.; Patera, A.; Natale, M.P.; Martini, C.; De Filippo, M. Radiofrequency Ablation, Cryoablation, and Microwave Ablation for the Treatment of Small Renal Masses: Efficacy and Complications. Diagnostics 2023, 13, 388. https://doi.org/10.3390/diagnostics13030388
Bertolotti L, Bazzocchi MV, Iemma E, Pagnini F, Ziglioli F, Maestroni U, Patera A, Natale MP, Martini C, De Filippo M. Radiofrequency Ablation, Cryoablation, and Microwave Ablation for the Treatment of Small Renal Masses: Efficacy and Complications. Diagnostics. 2023; 13(3):388. https://doi.org/10.3390/diagnostics13030388
Chicago/Turabian StyleBertolotti, Lorenzo, Maria Vittoria Bazzocchi, Enrico Iemma, Francesco Pagnini, Francesco Ziglioli, Umberto Maestroni, Annalisa Patera, Matteo Pio Natale, Chiara Martini, and Massimo De Filippo. 2023. "Radiofrequency Ablation, Cryoablation, and Microwave Ablation for the Treatment of Small Renal Masses: Efficacy and Complications" Diagnostics 13, no. 3: 388. https://doi.org/10.3390/diagnostics13030388
APA StyleBertolotti, L., Bazzocchi, M. V., Iemma, E., Pagnini, F., Ziglioli, F., Maestroni, U., Patera, A., Natale, M. P., Martini, C., & De Filippo, M. (2023). Radiofrequency Ablation, Cryoablation, and Microwave Ablation for the Treatment of Small Renal Masses: Efficacy and Complications. Diagnostics, 13(3), 388. https://doi.org/10.3390/diagnostics13030388









