An Open-Source Graphical User Interface-Embedded Automated Electrocardiogram Quality Assessment: A Balanced Class Representation Approach
Abstract
:1. Introduction
2. Methodology
2.1. Training Datasets: CinC11 and CinC17
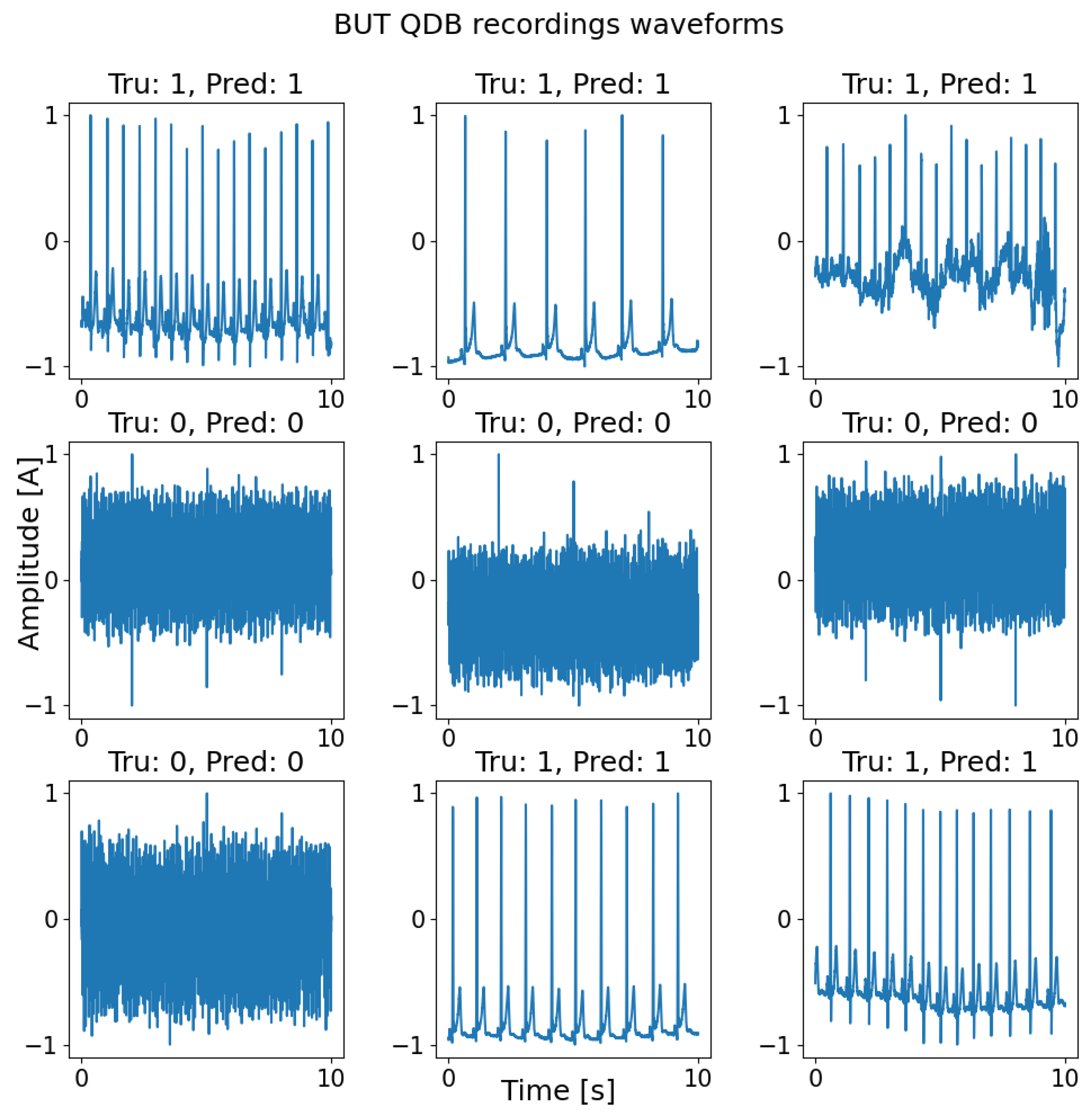
2.2. Testing Dataset: BUT QDB
2.3. Spectrogram Conversion
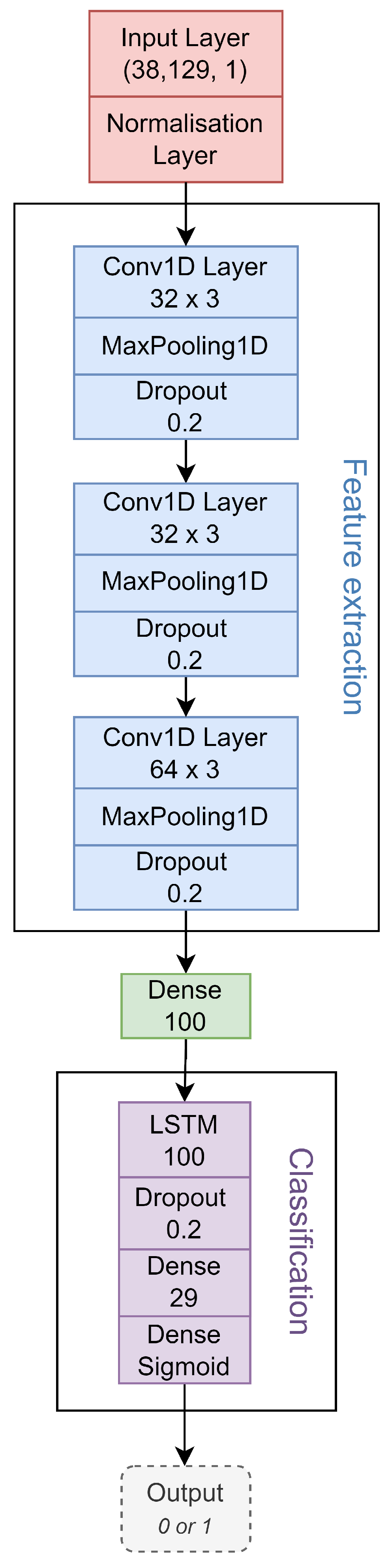
2.4. CNN-LSTM Model
3. Results
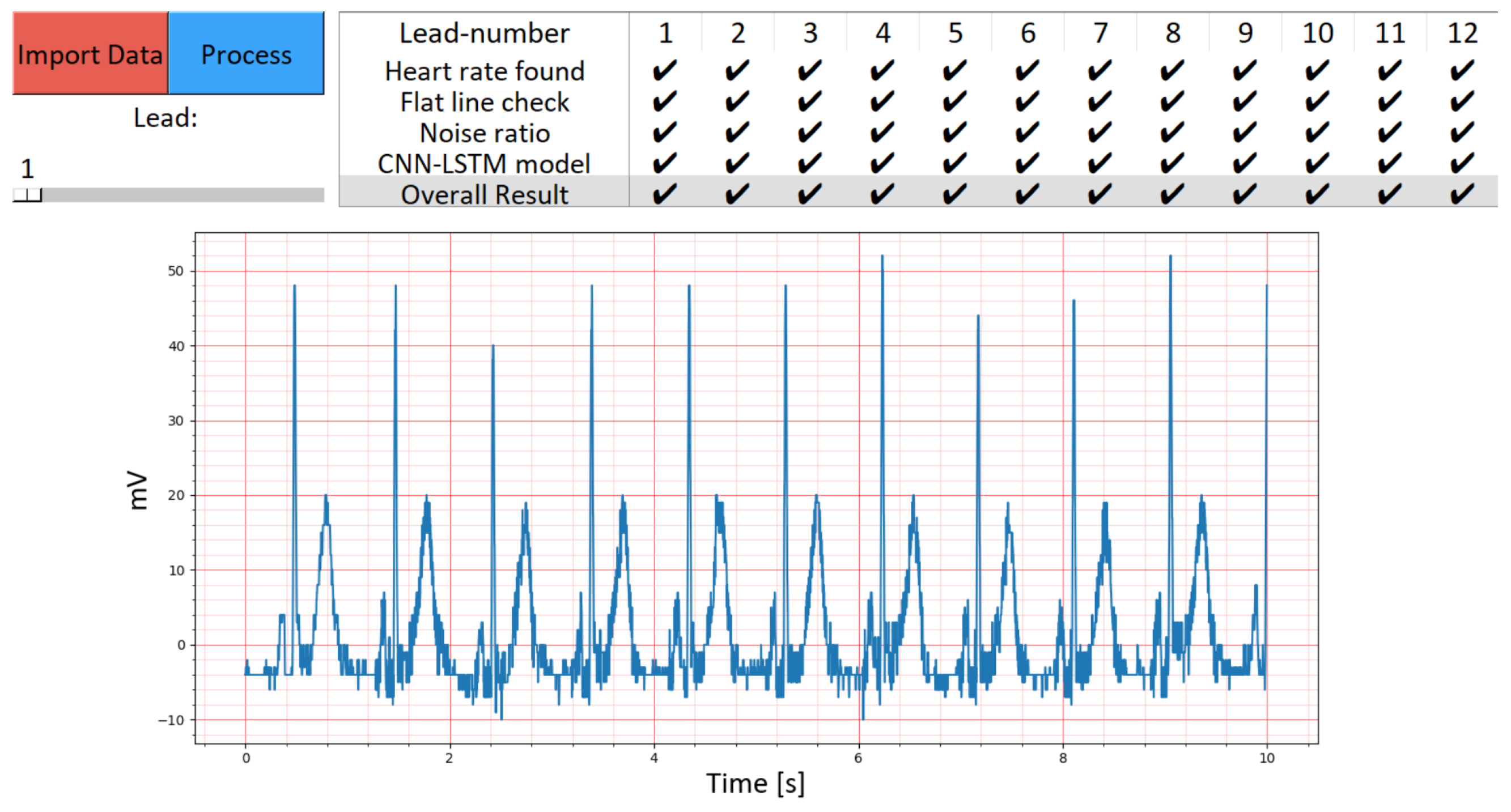
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization; Cardiovascular Diseases World Health Organization. Cardiovascular Diseases. Key Facts. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 16 November 2023).
- Nemati, R.; Ganjoo, M.; Jadidi, F.; Tanha, A.; Baghbani, R. Electrocardiography in early diagnosis of cardiovascular complications of COVID-19; a systematic literature review. Arch. Acad. Emerg. Med. 2021, 9, e10. [Google Scholar] [PubMed]
- Chung, C.T.; Lee, S.; King, E.; Liu, T.; Armoundas, A.A.; Bazoukis, G.; Tse, G. Clinical significance, challenges and limitations in using artificial intelligence for electrocardiography-based diagnosis. Int. J. Arrhythmia 2022, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Friedman, R.A.; Kligfield, P.; Levine, B.D.; Viskin, S.; Chaitman, B.R.; Okin, P.M.; Saul, J.P.; Salberg, L.; Van Hare, G.F.; et al. Assessment of the 12-Lead Electrocardiogram as a Screening Test for Detection of Cardiovascular Disease in Healthy General Populations of Young People (12–25 Years of Age) A Scientific Statement From the American Heart Association and the American College of Cardiology. J. Am. Coll. Cardiol. 2014, 64, 1479–1514. [Google Scholar] [PubMed]
- Kamga, P.; Mostafa, R.; Zafar, S. The use of wearable ECG devices in the clinical setting: A review. Curr. Emerg. Hosp. Med. Rep. 2022, 10, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Neri, L.; Oberdier, M.T.; van Abeelen, K.C.; Menghini, L.; Tumarkin, E.; Tripathi, H.; Jaipalli, S.; Orro, A.; Paolocci, N.; Gallelli, I.; et al. Electrocardiogram Monitoring Wearable Devices and Artificial-Intelligence-Enabled Diagnostic Capabilities: A Review. Sensors 2023, 23, 4805. [Google Scholar] [CrossRef]
- Breen, C.; Kelly, G.; Kernohan, W. ECG interpretation skill acquisition: A review of learning, teaching and assessment. J. Electrocardiol. 2022, 73, 125–128. [Google Scholar] [CrossRef]
- Krittanawong, C.; Rogers, A.J.; Johnson, K.W.; Wang, Z.; Turakhia, M.P.; Halperin, J.L.; Narayan, S.M. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat. Rev. Cardiol. 2021, 18, 75–91. [Google Scholar] [CrossRef]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G.; et al. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef]
- Lazarus, G.; Kirchner, H.; Siswanto, B.B. Prehospital tele-electrocardiographic triage improves the management of acute coronary syndrome in rural populations: A systematic review and meta-analysis. J. Telemed. Telecare 2022, 28, 632–641. [Google Scholar] [CrossRef]
- van der Bijl, K.; Elgendi, M.; Menon, C. Automatic ECG quality assessment techniques: A systematic review. Diagnostics 2022, 12, 2578. [Google Scholar] [CrossRef]
- Cook, D.A.; Oh, S.Y.; Pusic, M.V. Accuracy of physicians’ electrocardiogram interpretations: A systematic review and meta-analysis. JAMA Intern. Med. 2020, 180, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Pandiaraja, M.; Brimicombe, J.; Cowie, M.; Dymond, A.; Lindén, H.C.; Lip, G.Y.; Mant, J.; Williams, K.; Charlton, P.H.; Investigators, S. Screening for atrial fibrillation: Improving efficiency of manual review of handheld electrocardiograms. Eng. Proc. 2020, 2, 78. [Google Scholar] [PubMed]
- Siontis, K.C.; Noseworthy, P.A.; Attia, Z.I.; Friedman, P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 2021, 18, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Hong, S.; Zhang, R.; Du, S. Artificial-intelligence-enhanced mobile system for cardiovascular health management. Sensors 2021, 21, 773. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M.; Al-Ali, A.; Mohamed, A.; Ward, R. Improving remote health monitoring: A low-complexity ECG compression approach. Diagnostics 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Satija, U.; Ramkumar, B.; Manikandan, M.S. A new automated signal quality-aware ECG beat classification method for unsupervised ECG diagnosis environments. IEEE Sens. J. 2018, 19, 1. [Google Scholar] [CrossRef]
- Yao, X.; Rushlow, D.R.; Inselman, J.W.; McCoy, R.G.; Thacher, T.D.; Behnken, E.M.; Bernard, M.E.; Rosas, S.L.; Akfaly, A.; Misra, A.; et al. Artificial intelligence–enabled electrocardiograms for identification of patients with low ejection fraction: A pragmatic, randomized clinical trial. Nat. Med. 2021, 27, 815–819. [Google Scholar] [CrossRef]
- Elgendi, M.; Menon, C. Machine learning ranks ECG as an optimal wearable biosignal for assessing driving stress. IEEE Access 2020, 8, 34362–34374. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Z.; Ward, R.; Elgendi, M. Hypertension assessment via ECG and PPG signals: An evaluation using MIMIC database. Diagnostics 2018, 8, 65. [Google Scholar] [CrossRef]
- Avula, V.; Wu, K.C.; Carrick, R.T. Clinical applications, methodology, and scientific reporting of electrocardiogram deep-learning models: A systematic review. JACC Adv. 2023, 2, 100686. [Google Scholar] [CrossRef]
- Elgendi, M.; Mohamed, A.; Ward, R. Efficient ECG compression and QRS detection for e-health applications. Sci. Rep. 2017, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Moody, G.B.; Celi, L. Improving the quality of ECGs collected using mobile phones: The Physionet/Computing in Cardiology Challenge 2011. In Proceedings of the 2011 Computing in Cardiology (CinC), Hangzhou, China, 18–21 September 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 273–276. [Google Scholar]
- Kramer, L.; Menon, C.; Elgendi, M. ECGAssess: A Python-Based Toolbox to Assess ECG lead signal quality. Front. Digit. Health 2022, 4, 847555. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.D.; Liu, C.; Moody, B.; Li-wei, H.L.; Silva, I.; Li, Q.; Johnson, A.; Mark, R.G. AF classification from a short single lead ECG recording: The PhysioNet/computing in cardiology challenge 2017. In Proceedings of the 2017 Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Kapoor, S.; Narayanan, A. Leakage and the Reproducibility Crisis in ML-based Science. arXiv 2022, arXiv:2207.07048. [Google Scholar] [CrossRef]
- Kaufman, S.; Rosset, S.; Perlich, C. Leakage in data mining: Formulation, detection, and avoidance. ACM Trans. Knowl. Discov. Data (TKDD) 2012, 6, 1–21. [Google Scholar] [CrossRef]
- Nemcova, A.; Smisek, R.; Opravilová, K.; Vitek, M.; Smital, L.; Maršánová, L. Brno University of Technology ECG Quality Database (BUT QDB) (version 1.0.0). PhysioNet 2020, 101, e215–e220. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Özer, İ.; Efe, S.B.; Özbay, H. CNN/Bi-LSTM-based deep learning algorithm for classification of power quality disturbances by using spectrogram images. Int. Trans. Electr. Energy Syst. 2021, 31, e13204. [Google Scholar] [CrossRef]
- Hermawan, I.; Ma’sum, M.A.; Intan, P.R.D.; Jatmiko, W.; Wiweko, B.; Boediman, A.; Pradekso, B.K. Temporal feature and heuristics-based noise detection over classical machine learning for ECG signal quality assessment. In Proceedings of the 2019 International Workshop on Big Data and Information Security (IWBIS), Bali, Indonesia, 11 October 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–8. [Google Scholar] [CrossRef]
- Clifford, G.D.; Behar, J.; Li, Q.; Rezek, I. Signal Quality Indices and data fusion for determining clinical acceptability of electrocardiograms. Physiol. Meas. 2012, 33, 1419–1433. [Google Scholar] [CrossRef]
- Taji, B.; Chan, A.D.; Shirmohammadi, S. Classifying measured electrocardiogram signal quality using deep belief networks. In Proceedings of the 2017 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Turin, Italy, 22–25 May 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Yaghmaie, N.; Maddah-Ali, M.A.; Jelinek, H.F.; Mazrbanrad, F. Dynamic signal quality index for electrocardiograms. Physiol. Meas. 2018, 39, 105008. [Google Scholar] [CrossRef]
- Fu, F.; Xiang, W.; An, Y.; Liu, B.; Chen, X.; Zhu, S.; Li, J. Comparison of Machine Learning Algorithms for the Quality Assessment of Wearable ECG Signals Via Lenovo H3 Devices. J. Med. Biol. Eng. 2021, 41, 231–240. [Google Scholar] [CrossRef]
- Elgendi, M.; Howard, N.; Lovell, N.; Cichocki, A.; Brearley, M.; Abbott, D.; Adatia, I. A six-step framework on biomedical signal analysis for tackling noncommunicable diseases: Current and future perspectives. JMIR Biomed. Eng. 2016, 1, e6401. [Google Scholar] [CrossRef]

| Method | Year | Train Dataset | Test Dataset | Train Ratio | Test Data | Sensitivity | Specificity | Accuracy | F1-Score |
|---|---|---|---|---|---|---|---|---|---|
| Ac:UnAc | Ac:UnAc | (%) | (%) | (%) | (%) | ||||
| Proposed method | 2022 | CinC11 1 and CinC17 2 | BUT QDB 3 | 50:50 | 50:50 | 92.43 | 99.60 | 96.02 | 95.87 |
| Proposed method | 2022 | CinC11 1 and CinC17 2 | CinC11 1 and CinC17 | 50:50 | 50:50 | 98.52 | 95.52 | 97.03 | 97.09 |
| Proposed method | 2022 | CinC11 1 and CinC17 4 | BUT QDB 3 | 85:15 | 50:50 | 99.41 | 96.71 | 98.06 | 98.09 |
| Proposed method | 2022 | CinC11 1 and CinC17 4 | CinC11 1 and CinC17 | 85:15 | 85:15 | 99.74 | 83.80 | 97.27 | 98.40 |
| Proposed method | 2022 | CinC11 1,5 | CinC11 1,6 | 68:32 | 68:32 | 98.24 | 92.04 | 96.23 | 97.25 |
| Kramer et al. [24] | 2022 | CinC11 1,5 | CinC11 1,6 | 68:32 | 68:32 | 98.03 | 86.21 | 94.21 | 96.31 7 |
| Hermawan et al. [31] | 2019 | CinC11 | CinC11 | 70:30 | 70:30 | 85.00 | 86.00 | 85.60 | N/R |
| Clifford et al. [32] | 2012 | CinC11 and NSTDB | CinC11 and NSTDB | 50:50 | 50:50 | N/R | N/R | 95.80 | N/R |
| Taji et al. [33] | 2017 | CinC11 and NSTDB | CinC11 and NSTDB | 50:50 | 50:50 | 98.20 | 98.20 | 97.20 | 98.38 |
| Yaghmaie et al. [34] | 2017 | CinC11 and NSTDB and | CinC11 and NSTDB | 50:50 | 50:50 | 96.20 | 97.60 | 96.90 | N/R |
| MIT-BIH | |||||||||
| Fu et al. [35] | 2021 | Private | Private | 80:20 | 84:16 | 98.66 | 86.65 | 96.73 | N/R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgendi, M.; van der Bijl, K.; Menon, C. An Open-Source Graphical User Interface-Embedded Automated Electrocardiogram Quality Assessment: A Balanced Class Representation Approach. Diagnostics 2023, 13, 3479. https://doi.org/10.3390/diagnostics13223479
Elgendi M, van der Bijl K, Menon C. An Open-Source Graphical User Interface-Embedded Automated Electrocardiogram Quality Assessment: A Balanced Class Representation Approach. Diagnostics. 2023; 13(22):3479. https://doi.org/10.3390/diagnostics13223479
Chicago/Turabian StyleElgendi, Mohamed, Kirina van der Bijl, and Carlo Menon. 2023. "An Open-Source Graphical User Interface-Embedded Automated Electrocardiogram Quality Assessment: A Balanced Class Representation Approach" Diagnostics 13, no. 22: 3479. https://doi.org/10.3390/diagnostics13223479
APA StyleElgendi, M., van der Bijl, K., & Menon, C. (2023). An Open-Source Graphical User Interface-Embedded Automated Electrocardiogram Quality Assessment: A Balanced Class Representation Approach. Diagnostics, 13(22), 3479. https://doi.org/10.3390/diagnostics13223479


_Elgendi.png)




