Assessment of Cross-Reactivity of Chimeric Trypanosoma cruzi Antigens with Crithidia sp. LVH-60A: Implications for Accurate Diagnostics
Abstract
:1. Introduction
2. Materials and Methods
2.1. IBMP Chimeric Antigen Preparation
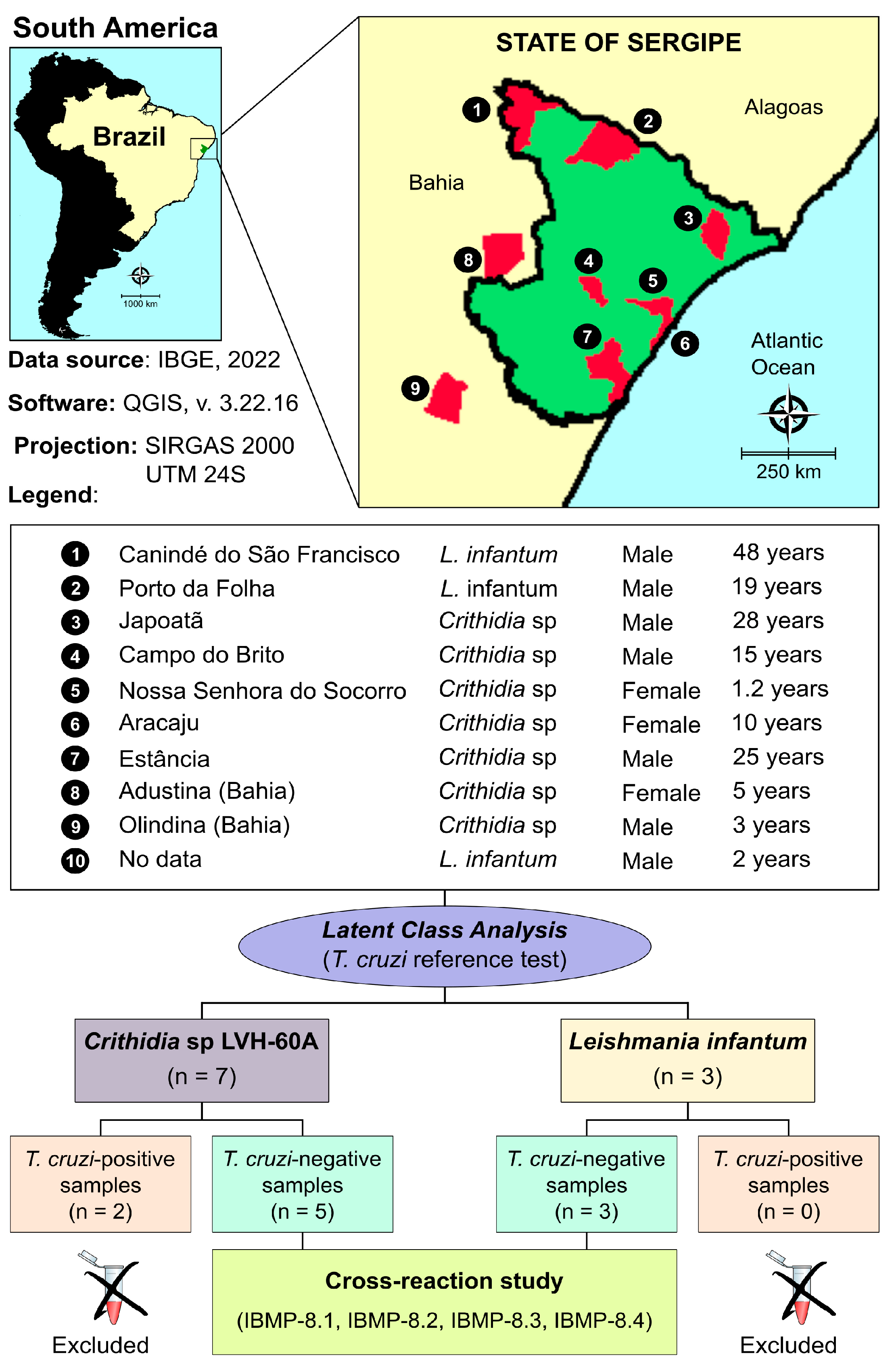
2.2. Sample Collection
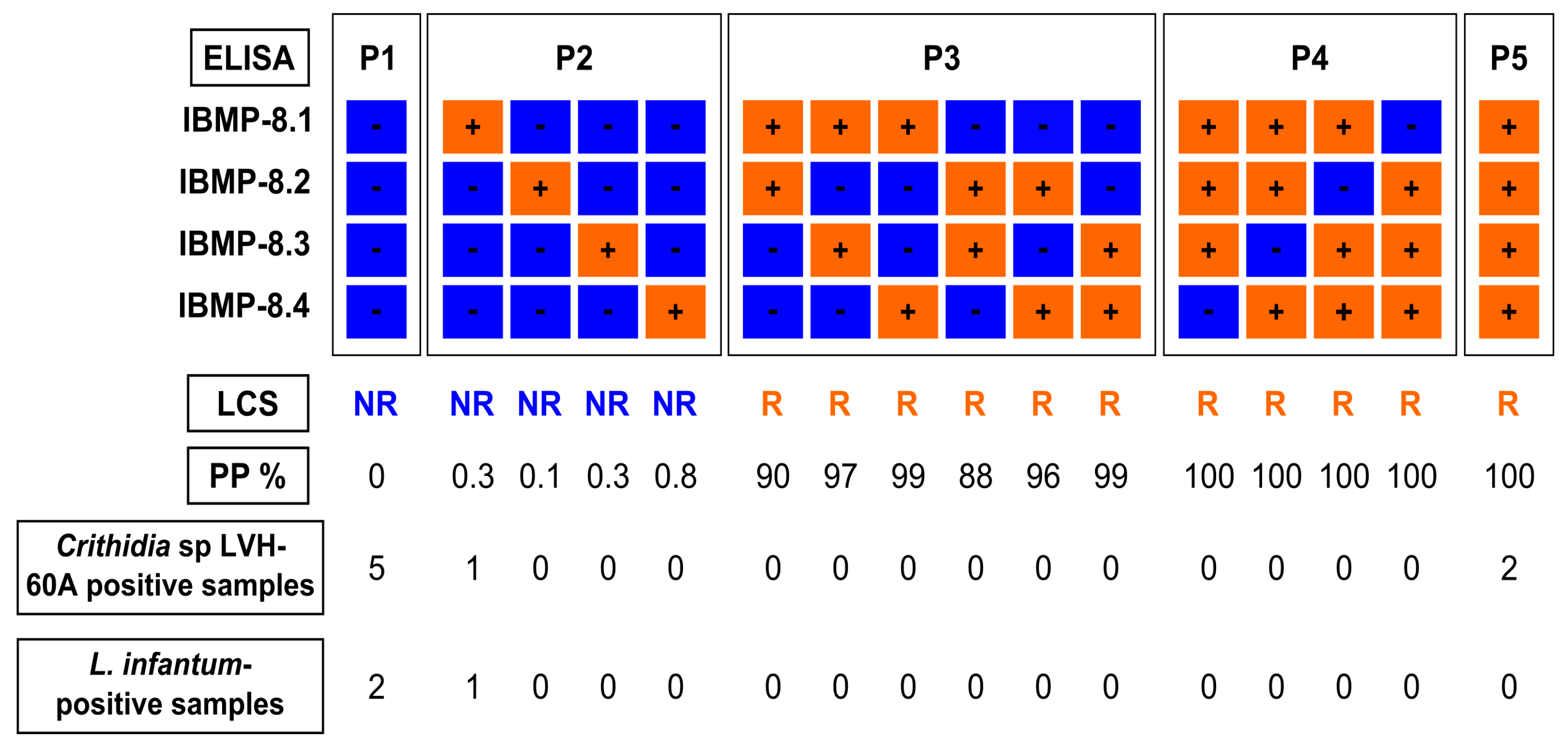
2.3. Laboratory Assays (IBMP-Indirect ELISA)
2.4. Latent Class Analysis (LCA) as a Reference Test
2.5. Data Analysis
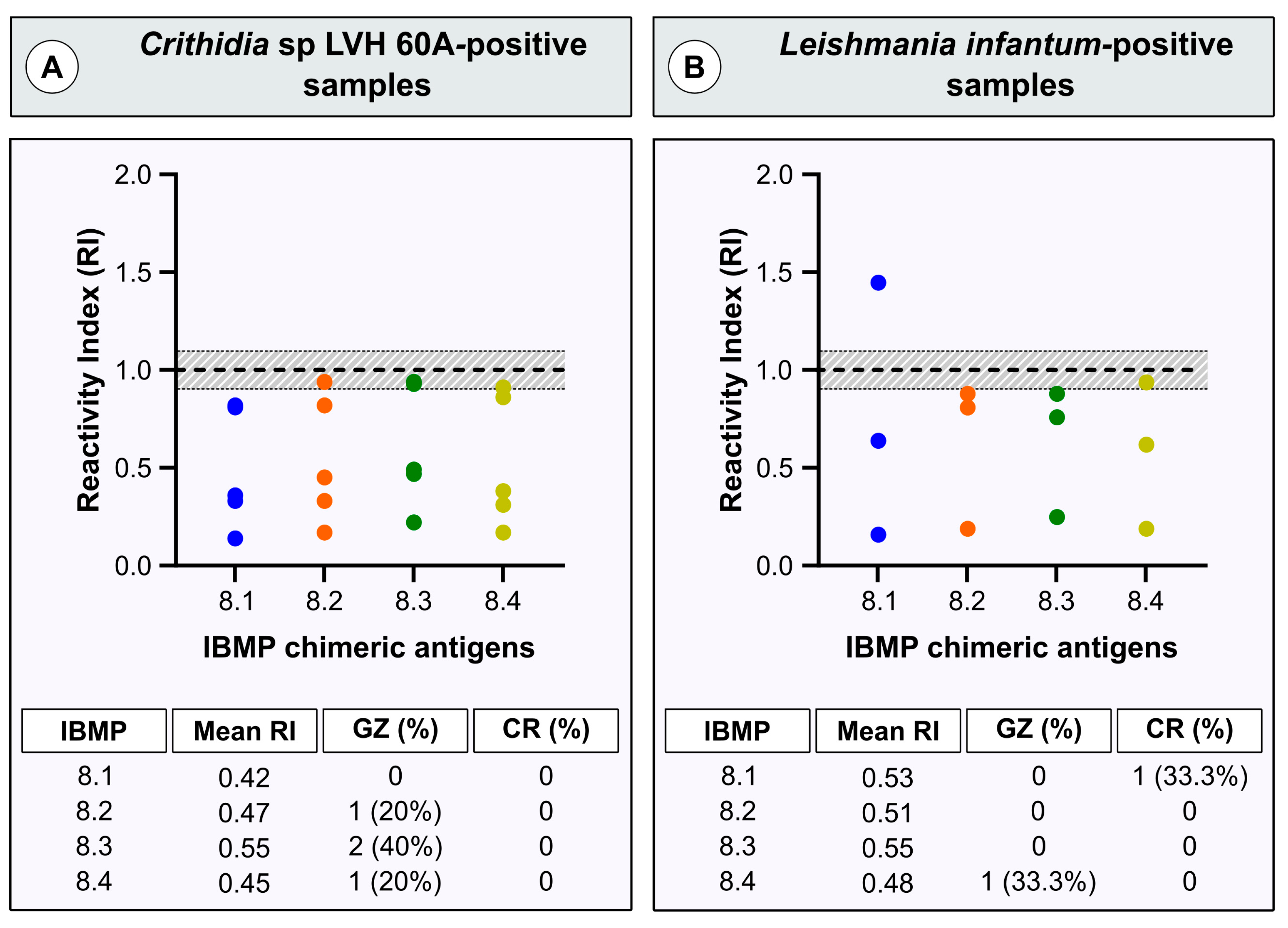
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chagas, C. Nova Tripanomíase Humana. Estudos Sobre Morfologia e o Ciclo Evolutivo do Schizotrypanum cruzi, n. Gen., n. Sp., Agente Etiológico da Nova Entidade Mórbida do Homem. Mem. Inst. Oswaldo. Cruz. 1909, 1, 159–218. [Google Scholar] [CrossRef]
- WHO. World Health Organization Chagas Disease in Latin America: An Epidemiological Update Based on 2010 Estimates. Wkly. Epidemiol. Rec. 2015, 90, 33–43. [Google Scholar]
- World Health Organization Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 20 October 2023).
- Coura, J.R.; Viñas, P.A. Chagas Disease: A New Worldwide Challenge. Nature 2010, 465, S6–S7. [Google Scholar] [CrossRef]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef]
- López-García, A.; Gilabert, J.A. Oral Transmission of Chagas Disease from a One Health Approach: A Systematic Review. Trop. Med. Int. Health 2023, 28, 689–698. [Google Scholar] [CrossRef]
- Chancey, R.J.; Edwards, M.S.; Montgomery, S.P. Congenital Chagas Disease. Pediatr. Rev. 2023, 44, 213–221. [Google Scholar] [CrossRef]
- Wendel, S. Transfusion Transmitted Chagas Disease: Is It Really under Control? Acta Trop. 2010, 115, 28–34. [Google Scholar] [CrossRef]
- Radisic, M.V.; Repetto, S.A. American Trypanosomiasis (Chagas Disease) in Solid Organ Transplantation. Transpl. Infect. Dis. 2020, 22, e13429. [Google Scholar] [CrossRef]
- Hofflin, J.M.; Sadler, R.H.; Araujo, F.G.; Page, W.E.; Remington, J.S. Laboratory-Acquired Chagas Disease. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 437–440. [Google Scholar] [CrossRef]
- Rassi, A.J.; Rassi, A.J.; Marcondes de Rezende, J. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. North Am. 2012, 26, 275–291. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Beaton, A.; Acquatella, H.; Bern, C.; Bolger, A.F.; Echeverría, L.E.; Dutra, W.O.; Gascon, J.; Morillo, C.A.; Oliveira-Filho, J.; et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement from the American Heart Association. Circulation 2018, 138, e169–e209. [Google Scholar] [CrossRef]
- Santos, F.L.N.; Souza, W.V.; Barros, M.S.; Nakazawa, M.; Krieger, M.A.; Gomes, Y.M. Chronic Chagas Disease Diagnosis: A Comparative Performance of Commercial Enzyme Immunoassay Tests. Am. J. Trop. Med. Hyg. 2016, 94, 1034–1039. [Google Scholar] [CrossRef]
- Gomes, Y.M.; Lorena, V.M.B.; Luquetti, A.O. Diagnosis of Chagas Disease: What Has Been Achieved? What Remains to Be Done with Regard to Diagnosis and Follow up Studies? Mem. Inst. Oswaldo. Cruz. 2009, 104, 115–121. [Google Scholar] [CrossRef]
- Dias, J.C.P.; Ramos, A.N., Jr.; Gontijo, E.D.; Luquetti, A.; Shikanai-Yasuda, M.A.; Coura, J.R.; Torres, R.M.; Melo, J.R.d.C.; de Almeida, E.A.; de Oliveira, W., Jr.; et al. 2 nd Brazilian Consensus on Chagas Disease, 2015. Rev. Soc. Bras. Med. Trop. 2016, 49, 3–60. [Google Scholar] [CrossRef]
- Daltro, R.T.; Santos, E.F.; Silva, Â.A.O.; Freitas, N.E.M.; Leony, L.M.; Vasconcelos, L.C.M.; Luquetti, A.O.; Celedon, P.A.F.; Zanchin, N.I.T.; Regis-Silva, C.G.; et al. Western Blot Using Trypanosoma cruzi Chimeric Recombinant Proteins for the Serodiagnosis of Chronic Chagas Disease: A Proof-of-Concept Study. PLoS Negl. Trop. Dis. 2022, 16, e0010944. [Google Scholar] [CrossRef]
- Silva, E.D.; Silva, Â.A.O.; Santos, E.F.; Leony, L.M.; Freitas, N.E.M.; Daltro, R.T.; Ferreira, A.G.P.; Diniz, R.L.; Bernardo, A.R.; Luquetti, A.O.; et al. Development of a New Lateral Flow Assay Based on IBMP-8.1 and IBMP-8.4 Chimeric Antigens to Diagnose Chagas Disease. Biomed. Res. Int. 2020, 2020, 1803515. [Google Scholar] [CrossRef]
- Teixeira-Carvalho, A.; Campos, F.M.F.; Geiger, S.M.; Rocha, R.D.R.; de Araújo, F.F.; Vitelli-Avelar, D.M.; Andrade, M.C.; Araújo, M.S.S.; Lemos, E.M.; de Freitas Carneiro Proietti, A.B.; et al. FC-TRIPLEX Chagas/Leish IgG1: A Multiplexed Flow Cytometry Method for Differential Serological Diagnosis of Chagas Disease and Leishmaniasis. PLoS ONE 2015, 10, e0122938. [Google Scholar] [CrossRef]
- Luz, J.G.G.; Souto, D.E.P.; Machado-Assis, G.F.; de Lana, M.; Luz, R.C.S.; Martins-Filho, O.A.; Damos, F.S.; Martins, H.R. Applicability of a Novel Immunoassay Based on Surface Plasmon Resonance for the Diagnosis of Chagas Disease. Clin. Chim. Acta 2016, 454, 39–45. [Google Scholar] [CrossRef]
- Vega Benedetti, A.F.; Cimino, R.O.; Cajal, P.S.; Juarez, M.D.V.; Villalpando, C.A.; Gil, J.F.; Marcipar, I.S.; Krolewiecki, A.J.; Nasser, J.R. Performance of Different Trypanosoma cruzi Antigens in the Diagnosis of Chagas Disease in Patients with American Cutaneous Leishmaniasis from a Co-Endemic Region in Argentina. Trop. Med. Int. Health 2013, 18, 1103–1109. [Google Scholar] [CrossRef]
- Gomes, Y.M.; Pereira, V.R.; Nakazawa, M.; Rosa, D.S.; Barros, M.D.; Ferreira, A.G.; Silva, E.D.; Ogatta, S.F.; Krieger, M.A.; Goldenberg, S.; et al. Serodiagnosis of Chronic Chagas Infection by Using EIE-Recombinant-Chagas-Biomanguinhos Kit. Mem. Inst. Oswaldo. Cruz. 2001, 96, 497–501. [Google Scholar] [CrossRef]
- Daltro, R.T.; Leony, L.M.; Freitas, N.E.M.; Silva, Â.A.O.; Santos, E.F.; Del-Rei, R.P.; Brito, M.E.F.; Brandão-Filho, S.P.; Gomes, Y.M.; Silva, M.S.; et al. Cross-Reactivity Using Chimeric Trypanosoma cruzi Antigens: Diagnostic Performance in Settings Co-Endemic for Chagas Disease and American Cutaneous or Visceral Leishmaniasis. J. Clin. Microbiol. 2019, 57, e00762-19. [Google Scholar] [CrossRef]
- Bartholomeu, D.C.; Teixeira, S.M.R.; Cruz, A.K. Genomics and Functional Genomics in Leishmania and Trypanosoma cruzi: Statuses, Challenges and Perspectives. Mem. Inst. Oswaldo. Cruz. 2021, 116, e200634. [Google Scholar] [CrossRef]
- Teixeira, S.M.; de Paiva, R.M.C.; Kangussu-Marcolino, M.M.; Darocha, W.D. Trypanosomatid Comparative Genomics: Contributions to the Study of Parasite Biology and Different Parasitic Diseases. Genet. Mol. Biol. 2012, 35, 1–17. [Google Scholar] [CrossRef]
- Caballero, Z.C.; Sousa, O.E.; Marques, W.P.; Saez-Alquezar, A.; Umezawa, E.S. Evaluation of Serological Tests to Identify Trypanosoma cruzi Infection in Humans and Determine Cross-Reactivity with Trypanosoma rangeli and Leishmania spp. Clin. Vaccine Immunol. 2007, 14, 1045–1049. [Google Scholar] [CrossRef]
- Umezawa, E.S.; Bastos, S.F.; Coura, J.R.; Levin, M.J.; Gonzalez, A.; Rangel-Aldao, R.; Zingales, B.; Luquetti, A.O.; da Silveira, J.F. An Improved Serodiagnostic Test for Chagas’ Disease Employing a Mixture of Trypanosoma cruzi Recombinant Antigens. Transfusion 2003, 43, 91–97. [Google Scholar] [CrossRef]
- Telles, S.; Abate, T.; Slezynger, T.; Henriquez, D.A. Trypanosoma cruzi Ubiquitin as an Antigen in the Differential Diagnosis of Chagas Disease and Leishmaniasis. FEMS Immunol. Med. Microbiol. 2003, 37, 23–28. [Google Scholar] [CrossRef]
- Camussone, C.; Gonzalez, V.; Belluzo, M.S.; Pujato, N.; Ribone, M.E.; Lagier, C.M.; Marcipar, I.S. Comparison of Recombinant Trypanosoma cruzi Peptide Mixtures versus Multiepitope Chimeric Proteins as Sensitizing Antigens for Immunodiagnosis. Clin. Vaccine Immunol. 2009, 16, 899–905. [Google Scholar] [CrossRef]
- Peverengo, L.M.; Garcia, V.; Rodeles, L.M.; Mendicino, D.; Vicco, M.; Lagier, C.; Gonzalez, V.; Gugliotta, L.; Marcipar, I. Development and Assessment of an Improved Recombinant Multiepitope Antigen-Based Immunoassay to Diagnose Chronic Chagas Disease. Parasitology 2018, 145, 1594–1599. [Google Scholar] [CrossRef]
- Santos, F.L.N.; Celedon, P.A.F.; Zanchin, N.I.T.; Brasil, T.A.C.; Foti, L.; Souza, W.V.; Silva, E.D.; Gomes, Y.M.; Krieger, M.A. Performance Assessment of Four Chimeric Trypanosoma cruzi Antigens Based on Antigen-Antibody Detection for Diagnosis of Chronic Chagas Disease. PLoS ONE 2016, 11, e0161100. [Google Scholar] [CrossRef]
- Zrein, M.; Granjon, E.; Gueyffier, L.; Caillaudeau, J.; Liehl, P.; Pottel, H.; Cardoso, C.S.; Oliveira, C.D.L.; de Oliveira, L.C.; Lee, T.H.; et al. A Novel Antibody Surrogate Biomarker to Monitor Parasite Persistence in Trypanosoma cruzi-Infected Patients. PLoS Negl. Trop. Dis. 2018, 12, e0006226. [Google Scholar] [CrossRef]
- Santos, F.L.N.; Celedon, P.A.F.; Zanchin, N.I.T.; Souza, W.V.; Silva, E.D.; Foti, L.; Krieger, M.A.; Gomes, Y.M. Accuracy of Chimeric Proteins in the Serological Diagnosis of Chronic Chagas Disease—A Phase II Study. PLoS Negl. Trop. Dis. 2017, 11, e0005433. [Google Scholar] [CrossRef]
- Santos, F.L.N.; Campos, A.C.P.; Amorim, L.D.A.F.; Silva, E.D.; Zanchin, N.I.T.; Celedon, P.A.F.; Del-Rei, R.P.; Krieger, M.A.; Gomes, Y.M. Highly Accurate Chimeric Proteins for the Serological Diagnosis of Chronic Chagas Disease: A Latent Class Analysis. Am. J. Trop. Med. Hyg. 2018, 99, 1174–1179. [Google Scholar] [CrossRef]
- Maruyama, S.R.; Santana, A.K.M.; Takamiya, N.T.; Takahashi, T.Y.; Rogerio, L.A.; Oliveira, C.A.B.; Milanezi, C.M.; Trombela, V.A.; Cruz, A.K.; Jesus, A.R.; et al. Non-Leishmania Parasite in Fatal Visceral Leishmaniasis-like Disease, Brazil. Emerg. Infect. Dis. 2019, 25, 2088–2092. [Google Scholar] [CrossRef]
- Crithidia sp. LVH-60A. Available online: https://www.ncbi.nlm.nih.gov/datasets/taxonomy/3034688/ (accessed on 27 September 2023).
- Toledo, K. New Species of Parasite Is Identified in Fatal Case of Visceral Leishmaniasis. Available online: https://agencia.fapesp.br/new-species-of-parasite-is-identified-in-fatal-case-of-visceral-leishmaniasis/31581/ (accessed on 18 April 2023).
- World Health Organization. Second WHO Consultation on the Development of a WHO Reference Panel for the Control of Chagas Diagnostic Tests; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Brazil Government. Protocolo Clínico e Diretrizes Terapêuticas Doença de Chagas; Comissão Nacional de Incorporação de Tecnologias no SUS (CONITEC): Brasília, Brazil, 2018.
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 Guidelines for Reporting Diagnostic Accuracy Studies: Explanation and Elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- De Moraes, M.H.; Guarneri, A.A.; Girardi, F.P.; Rodrigues, J.B.; Eger, I.; Tyler, K.M.; Steindel, M.; Grisard, E.C. Different Serological Cross-Reactivity of Trypanosoma rangeli Forms in Trypanosoma cruzi-Infected Patients Sera. Parasit Vectors 2008, 1, 20. [Google Scholar] [CrossRef]
- Vergara-Meza, J.G.; Brilhante, A.F.; Valente, V.d.C.; Villalba-Alemán, E.; Ortiz, P.A.; Cosmiro de Oliveira, S.; Rodrigues Cavalcante, M.; Julião, G.R.; Gonçalves Pinto, M.C.; Valente, S.A.; et al. Trypanosoma cruzi and Trypanosoma rangeli in Acre, Brazilian Amazonia: Coinfection and Notable Genetic Diversity in an Outbreak of Orally Acquired Acute Chagas Disease in a Forest Community, Wild Reservoirs, and Vectors. Parasitologia 2022, 2, 350–365. [Google Scholar] [CrossRef]
- Desquesnes, M.; Bosseno, M.-F.; Brenière, S.F. Detection of Chagas Infections Using Trypanosoma evansi Crude Antigen Demonstrates High Cross-Reactions with Trypanosoma cruzi. Infect. Genet. Evol. 2007, 7, 457–462. [Google Scholar] [CrossRef]
- Malchiodi, E.L.; Chiaramonte, M.G.; Taranto, N.J.; Zwirner, N.W.; Margni, R.A. Cross-Reactivity Studies and Differential Serodiagnosis of Human Infections Caused by Trypanosoma cruzi and Leishmania spp; Use of Immunoblotting and ELISA with a Purified Antigen (Ag163B6). Clin. Exp. Immunol. 1994, 97, 417–423. [Google Scholar] [CrossRef]
- Frank, F.M.; Fernández, M.M.; Taranto, N.J.; Cajal, S.P.; Margni, R.A.; Castro, E.; Thomaz-Soccol, V.; Malchiodi, E.L. Characterization of Human Infection by Leishmania spp. in the Northwest of Argentina: Immune Response, Double Infection with Trypanosoma cruzi and Species of Leishmania Involved. Parasitology 2003, 126, 31–39. [Google Scholar] [CrossRef]
- Sánchez, B.; Monteón, V.; Reyes, P.A.; Espinoza, B. Standardization of Micro-Enzyme-Linked Immunosorbent Assay (ELISA) and Western Blot for Detection of Trypanosoma cruzi Antibodies Using Extracts from Mexican Strains as Antigens. Arch. Med. Res. 2001, 32, 382–388. [Google Scholar] [CrossRef]
- Alviarez, Y.; Lares, M.; Viettri, M.; Aguilar, C.M.; Herrera, L.; Ferrer, E. Standardization of a Direct Agglutination Test for the Immunodiagnosis of Chagas Disease. Biomedica 2014, 34, 308–317. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, E.F.; Daltro, R.T.; Regis-Silva, C.G.; Pavan, T.B.S.; de Oliveira, F.A.; da Silva, Â.M.; Almeida, R.P.; Gonçalves, N.L.S.; Sampaio, D.D.; Santos, F.N.; et al. Assessment of Cross-Reactivity of Chimeric Trypanosoma cruzi Antigens with Crithidia sp. LVH-60A: Implications for Accurate Diagnostics. Diagnostics 2023, 13, 3470. https://doi.org/10.3390/diagnostics13223470
Santos EF, Daltro RT, Regis-Silva CG, Pavan TBS, de Oliveira FA, da Silva ÂM, Almeida RP, Gonçalves NLS, Sampaio DD, Santos FN, et al. Assessment of Cross-Reactivity of Chimeric Trypanosoma cruzi Antigens with Crithidia sp. LVH-60A: Implications for Accurate Diagnostics. Diagnostics. 2023; 13(22):3470. https://doi.org/10.3390/diagnostics13223470
Chicago/Turabian StyleSantos, Emily F., Ramona T. Daltro, Carlos G. Regis-Silva, Tycha B. S. Pavan, Fabrícia A. de Oliveira, Ângela M. da Silva, Roque P. Almeida, Noilson L. S. Gonçalves, Daniel D. Sampaio, Faber N. Santos, and et al. 2023. "Assessment of Cross-Reactivity of Chimeric Trypanosoma cruzi Antigens with Crithidia sp. LVH-60A: Implications for Accurate Diagnostics" Diagnostics 13, no. 22: 3470. https://doi.org/10.3390/diagnostics13223470
APA StyleSantos, E. F., Daltro, R. T., Regis-Silva, C. G., Pavan, T. B. S., de Oliveira, F. A., da Silva, Â. M., Almeida, R. P., Gonçalves, N. L. S., Sampaio, D. D., Santos, F. N., Marchini, F. K., Celedon, P. A. F., Zanchin, N. I. T., & Santos, F. L. N. (2023). Assessment of Cross-Reactivity of Chimeric Trypanosoma cruzi Antigens with Crithidia sp. LVH-60A: Implications for Accurate Diagnostics. Diagnostics, 13(22), 3470. https://doi.org/10.3390/diagnostics13223470






