Evaluation of a Decision Support System Developed with Deep Learning Approach for Detecting Dental Caries with Cone-Beam Computed Tomography Imaging
Abstract
1. Introduction
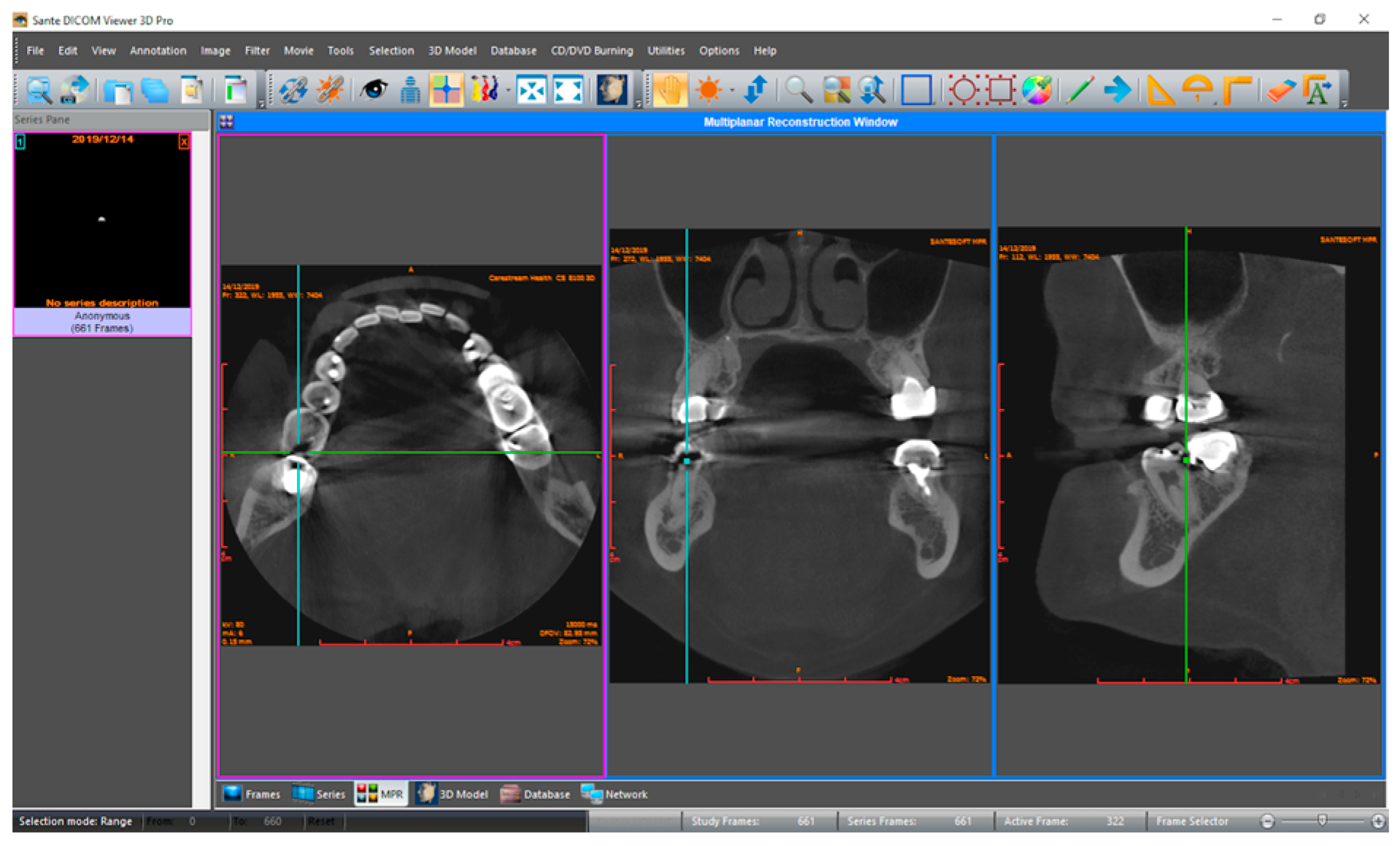
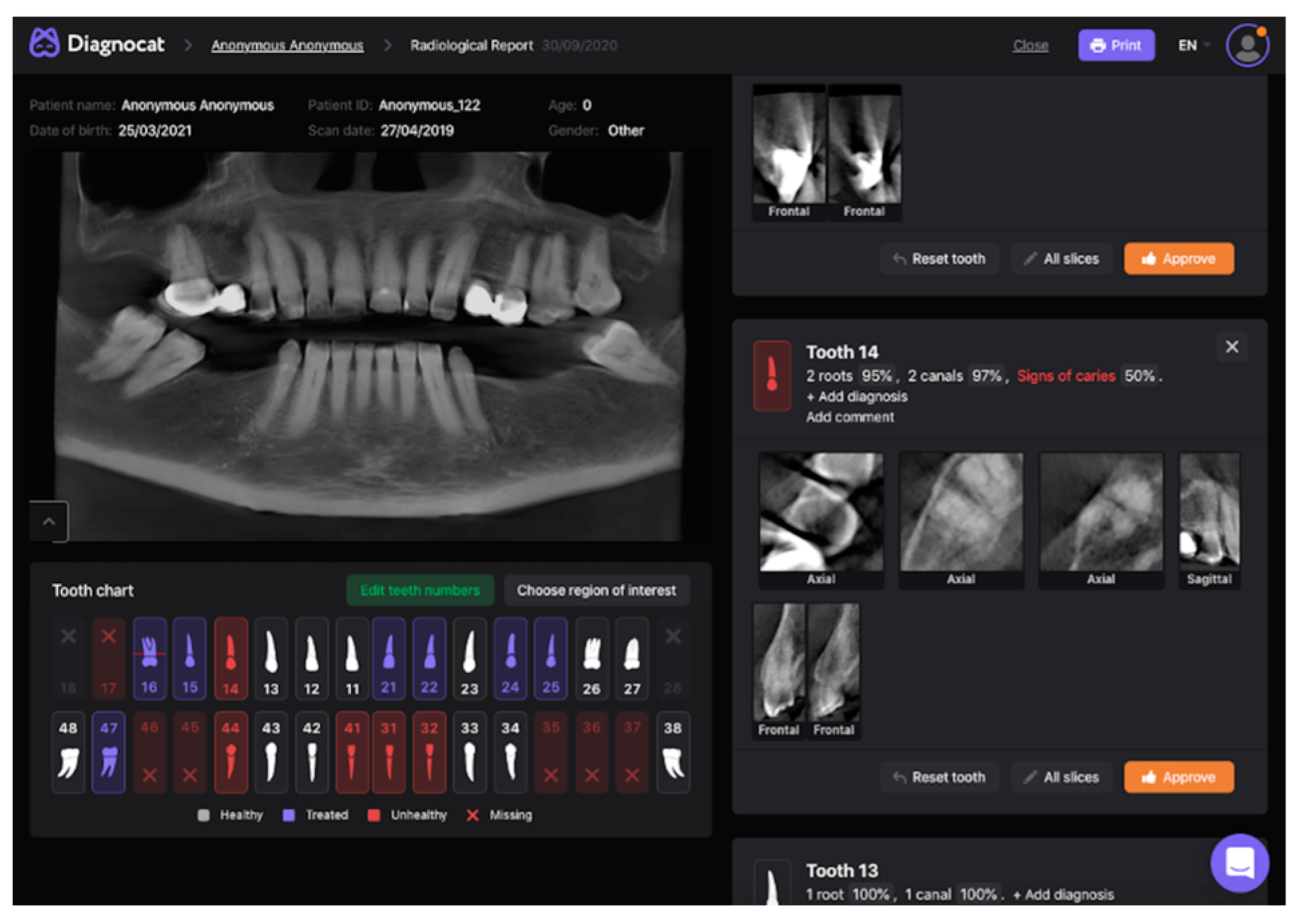
2. Materials and Methods
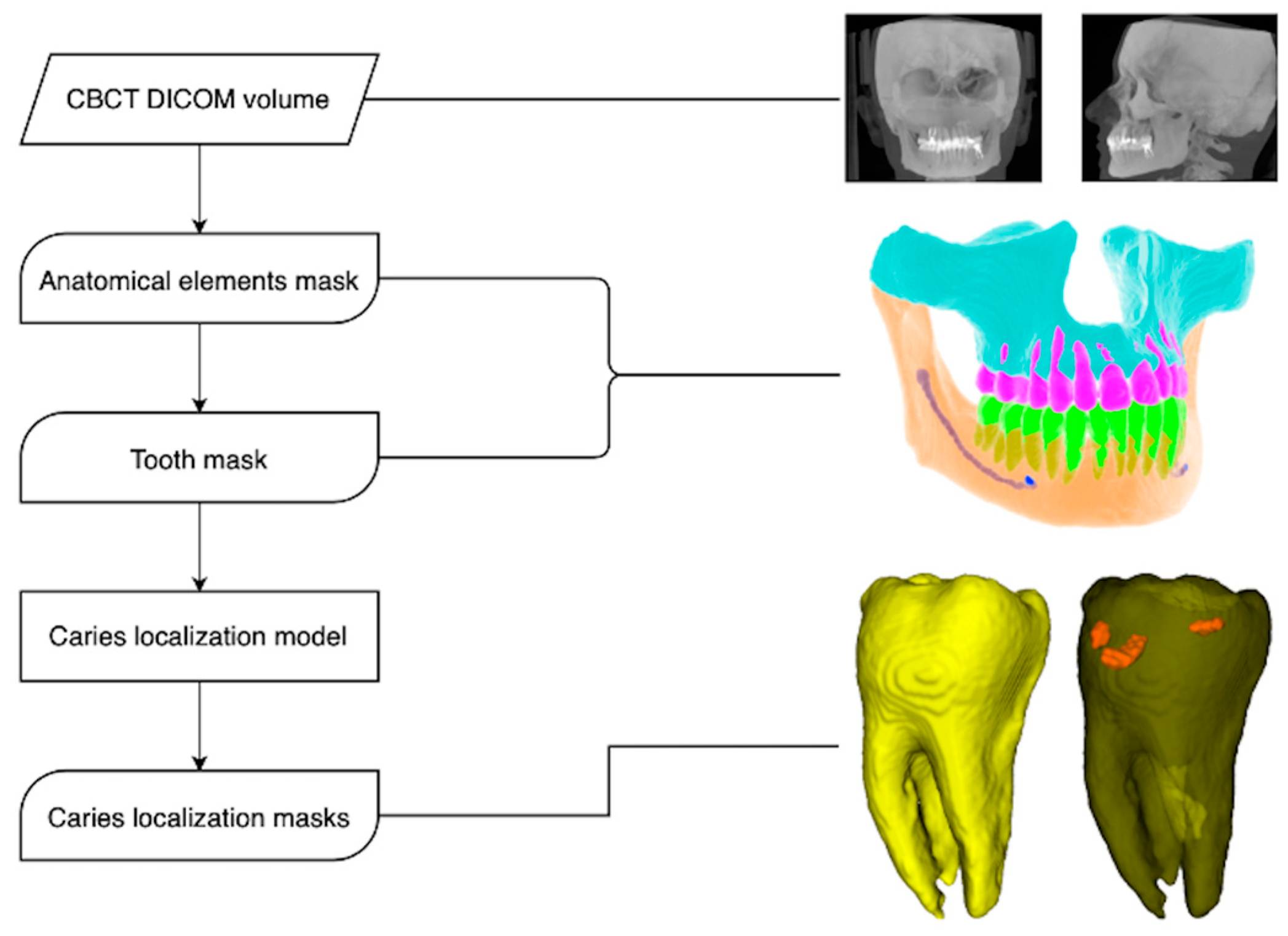
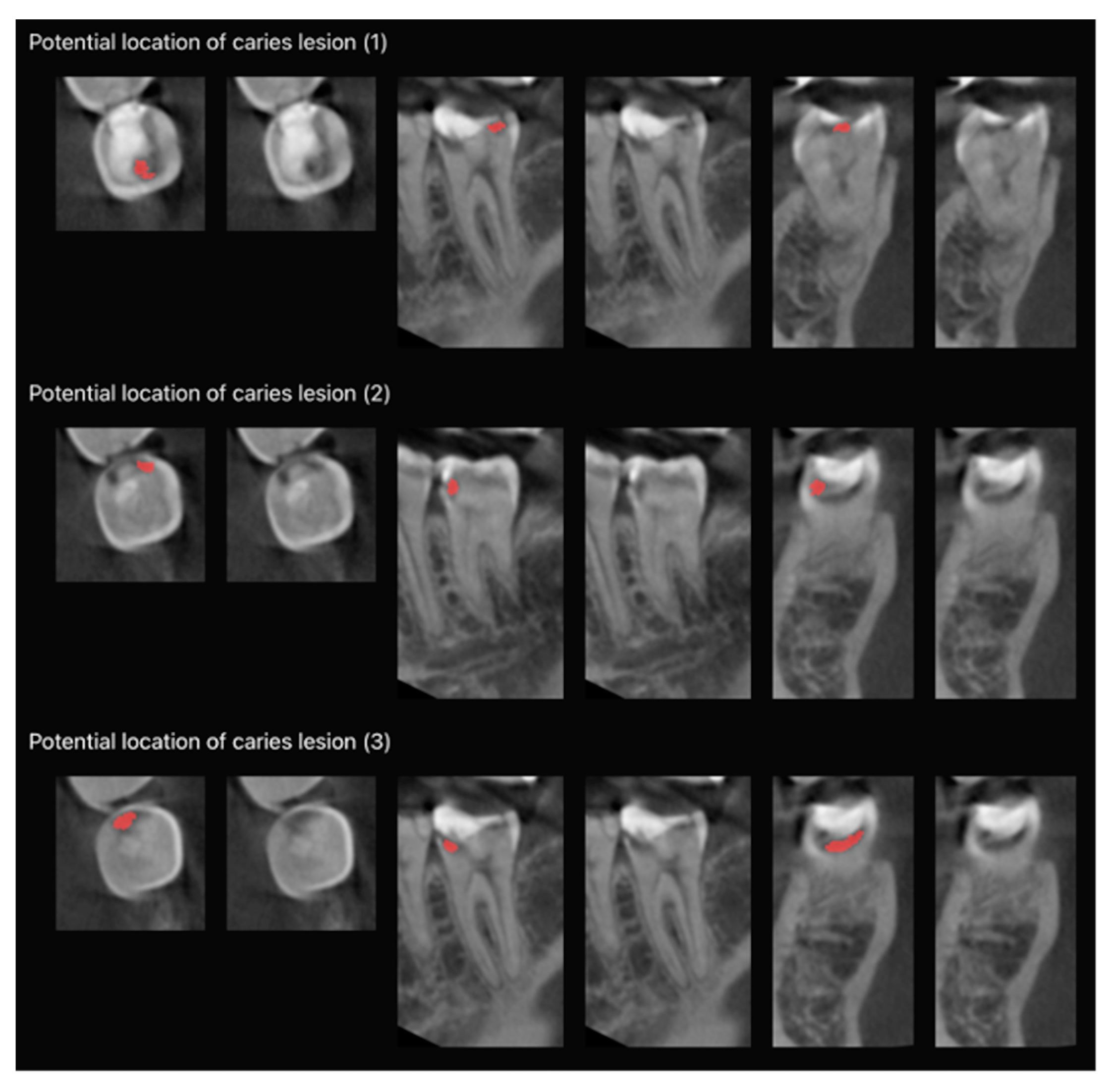
2.1. Model Pipeline
2.2. The Architecture of the Deep CNNs
2.3. Statistical Analysis
Specificity = TN⁄(TN + FP)
Accuracy = (TP + TN)⁄(TP + FP + TN + FP)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef]
- Towle, I.; Irish, J.D.; De Groote, I.; Fernee, C.; Loch, C. Dental caries in South African fossil hominins. S. Afr. J. Sci. 2021, 117, 3–4. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Vachirarojpisan, T.; Shinada, K.; Kawaguchi, Y.; Laungwechakan, P.; Somkote, T.; Detsomboonrat, P. Early childhood caries in children aged 6-19 months. Community Dent. Oral Epidemiol. 2004, 32, 133–142. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, J.; Chen, L.; Gan, N.; Yang, D. The Oral Microbiome in the Elderly With Dental Caries and Health. Front. Cell. Infect. Microbiol. 2019, 8, 442. [Google Scholar] [CrossRef]
- Usha, C.; Sathyanarayanan, R. Dental caries-A complete changeover (Part I). JCD 2009, 12, 46–54. [Google Scholar] [CrossRef]
- Mathur, V.P.; Dhillon, J.K. Dental Caries: A Disease Which Needs Attention. Indian J. Pediatr. 2018, 85, 202–206. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Yılmaz, H.; Keleş, S. Recent methods for diagnosis of dental caries in dentistry. Meandros Med. Dent. J. 2018, 19, 1–8. [Google Scholar] [CrossRef]
- Sukovic, P. Cone beam computed tomography in craniofacial imaging. Orthod. Craniofac. Res. 2003, 6, 31–36. [Google Scholar] [CrossRef]
- Price, J.B. Caries Detection with Dental Cone Beam Computed Tomography. In Detection and Assesment of Dental Caries: A Clinical Guide; Zandona, A.F., Longbottom, C., Eds.; Springer: Cham, Switzerland, 2019; pp. 127–138. [Google Scholar]
- Radiation Protection No 172. Cone beam CT for dental and maxillofacial radiology (Evidence-based guidelines). Available online: https://www.sedentexct.eu/files/radiation_protection_172.pdf (accessed on 10 October 2023).
- Bansal, G.J. Digital radiography. A comparison with modern conventional imaging. Postgrad. Med. J. 2006, 82, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, S. Digital Imaging in Dentistry: A Review. Contemp. Clin. Dent. 2017, 8, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, E.A. Clinical decision support systems: Perspectives in dentistry. J. Dent. Educ. 2004, 68, 589–597. [Google Scholar] [CrossRef]
- Musen, M.A.; Shahar, Y.; Shortliffe, E.H. Clinical Decision-Support Systems. In Medical Informatics; Health Informatics; Shortliffe, E.H., Perreault, L.E., Eds.; Springer: New York, NY, USA, 2001; pp. 573–609. [Google Scholar] [CrossRef]
- Sahota, N.; Lloyd, R.; Ramakrishna, A.; Mackay, J.A.; Prorok, J.C.; Weise-Kelly, L.; Navarro, T.; Wilczynski, N.L.; Haynes, R.B.; CCDSS Systematic Review Team. Computerized clinical decision support systems for acute care management: A decision-maker-researcher partnership systematic review of effects on process of care and patient outcomes. Implement. Sci. 2011, 6, 91. [Google Scholar] [CrossRef][Green Version]
- Jaspers, M.W.; Smeulers, M.; Vermeulen, H.; Peute, L.W. Effects of clinical decision-support systems on practitioner performance and patient outcomes: A synthesis of high-quality systematic review findings. J. Am. Med. Inform. Assoc. 2011, 18, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Giordano, R.; Lakhani, S.; Walker, D.M. A review of randomized controlled trials of medical record powered clinical decision support system to improve quality of diabetes care. Int. J. Med. Inform. 2016, 87, 91–100. [Google Scholar] [CrossRef]
- Pawloski, P.A.; Brooks, G.A.; Nielsen, M.E.; Olson-Bullis, B.A. A Systematic Review of Clinical Decision Support Systems for Clinical Oncology Practice. J. Natl. Compr. Canc. Netw. 2019, 17, 331–338. [Google Scholar] [CrossRef]
- Kahn, C.E., Jr. Artificial intelligence in radiology: Decision support systems. Radiographics 1994, 14, 849–861. [Google Scholar] [CrossRef]
- Syeda-Mahmood, T. Role of Big Data and Machine Learning in Diagnostic Decision Support in Radiology. J. Am. Coll. Radiol. 2018, 15, 569–576. [Google Scholar] [CrossRef]
- Kök, H.; İzgi, M.S.; Acılar, A.M. Evaluation of the Artificial Neural Network and Naive Bayes Models Trained with Vertebra Ratios for Growth and Development Determination. Turk. J. Orthod. 2020, 34, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.; Sing, S.; Yeong, W. A review on machine learning in 3D printing: Applications, potential, and challenges. Artif. Intell. Rev. 2021, 54, 63–94. [Google Scholar] [CrossRef]
- Burkov, A. The Hundred-Page Machine Learning Book; Andriy Burkov: Quebec City, QC, Canada, 2019; pp. 3–7. [Google Scholar]
- Zhu, X.; Goldberg, A.B. Introduction to Semi-Supervised Learning; Synthesis Lectures on Artificial Intelligence and Machine Learning (SLAIML); Springer: Berlin/Heidelberg, Germany, 2009; Volume 3, pp. 1–130. [Google Scholar]
- Montagnon, E.; Cerny, M.; Cadrin-Chênevert, A.; Hamilton, V.; Derennes, T.; Ilinca, A.; Vandenbroucke-Menu, F.; Turcotte, S.; Kadoury, S.; Tang, A. Deep learning workflow in radiology: A primer. Insights Imaging 2020, 11, 22. [Google Scholar] [CrossRef]
- Erickson, B.J.; Korfiatis, P.; Kline, T.L.; Akkus, Z.; Philbrick, K.; Weston, A.D. Deep Learning in Radiology: Does One Size Fit All? J. Am. Coll. Radiol. 2018, 15, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Sin, Ç.; Akkaya, N.; Aksoy, S.; Orhan, K.; Öz, U. A deep learning algorithm proposal to automatic pharyngeal airway detection and segmentation on CBCT images. Orthod. Craniofac. Res. 2021, 24, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Isman, O.; Aktan, A.M.; Ertas, E.T. Evaluating the effects of orthodontic materials, field of view, and artifact reduction mode on accuracy of CBCT-based caries detection. Clin. Oral Investig. 2020, 24, 2487–2496. [Google Scholar] [CrossRef]
- Kumar, T.P.; Sujatha, S.; Rakesh, N.; Shwetha, V. Applications of CBCT in Caries Detection and Endodontics-A Review. J. Dent. Res. 2019, 15, 71–76. [Google Scholar]
- Cebe, F.; Aktan, A.M.; Ozsevik, A.S.; Ciftci, M.E.; Surmelioglu, H.D. The effects of different restorative materials on the detection of approximal caries in cone-beam computed tomography scans with and without metal artifact reduction mode. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 392–400. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.H.; Jeong, S.N.; Choi, S.H. Detection and diagnosis of dental caries using a deep learning-based convolutional neural network algorithm. J. Dent. 2018, 77, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.H.; Hamamoto, K.; Paing, M.P. Deep Fusion Feature Extraction for Caries Detection on Dental Panoramic Radiographs. Appl. Sci. 2021, 11, 2005. [Google Scholar] [CrossRef]
- Devito, K.L.; de Souza Barbosa, F.; Felippe Filho, W.N. An artificial multilayer perceptron neural network for diagnosis of proximal dental caries. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 879–884. [Google Scholar] [CrossRef]
- Cantu, A.G.; Gehrung, S.; Krois, J.; Chaurasia, A.; Rossi, J.G.; Gaudin, R.; Elhennawy, K.; Schwendicke, F. Detecting caries lesions of different radiographic extension on bitewings using deep learning. J. Dent. 2020, 100, 103425. [Google Scholar] [CrossRef] [PubMed]
- Isensee, F.; Kickingereder, P.; Wick, W.; Bendszus, M.; Maier-Hein, K. Brain tumor segmentation and radiomics survival prediction: Contribution to the brats 2017 challenge. In Proceedings of the International MICCAI Brainlesion Workshop, BrainLes 2017, Quebec City, QC, Canada, 14 September 2017; pp. 287–297. [Google Scholar]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar]
- Esmaeili, F.; Johari, M.; Haddadi, P.; Vatankhah, M. Beam Hardening Artifacts: Comparison between Two Cone Beam Computed Tomography Scanners. J. Dent. Res. Dent. Clin. Dent. Prospects 2012, 6, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Schulze, R.; Heil, U.; Gross, D.; Bruellmann, D.D.; Dranischnikow, E.; Schwanecke, U.; Schoemer, E. Artefacts in CBCT: A review. Dentomaxillofac. Radiol. 2011, 40, 265–273. [Google Scholar] [CrossRef]
- Nagarajappa, A.K.; Dwivedi, N.; Tiwari, R. Artifacts: The downturn of CBCT image. J. Int. Soc. Prev. Community Dent. 2015, 5, 440–445. [Google Scholar] [CrossRef]
- Codari, M.; de Faria Vasconcelos, K.; Ferreira Pinheiro Nicolielo, L.; Haiter Neto, F.; Jacobs, R. Quantitative evaluation of metal artifacts using different CBCT devices, high-density materials and field of views. Clin. Oral Implants Res. 2017, 28, 1509–1514. [Google Scholar] [CrossRef]
- Panjnoush, M.; Kheirandish, Y.; Kashani, P.M.; Fakhar, H.B.; Younesi, F.; Mallahi, M. Effect of Exposure Parameters on Metal Artifacts in Cone Beam Computed Tomography. J. Dent. 2016, 13, 143–150. [Google Scholar]
- Candemil, A.P.; Salmon, B.; Freitas, D.Q.; Ambrosano, G.M.B.; Haiter-Neto, F.; Oliveira, M.L. Are metal artefact reduction algorithms effective to correct cone beam CT artefacts arising from the exomass? Dentomaxillofac. Radiol. 2019, 48, 20180290. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, P.M.; Oliveira, M.L.; Groppo, F.C.; Haiter-Neto, F.; Freitas, D.Q. Evaluation of metal artefact reduction in cone-beam computed tomography images of different dental materials. Clin. Oral Investig. 2018, 22, 419–423. [Google Scholar] [CrossRef]
- Vasconcelos, T.V.; Bechara, B.B.; McMahan, C.A.; Freitas, D.Q.; Noujeim, M. Evaluation of artifacts generated by zirconium implants in cone-beam computed tomography images. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yang, C.; Zhang, Z.; Li, H. Scatter artifacts removal using learning-based method for CBCT in IGRT system. IEEE Access 2018, 6, 78031–78037. [Google Scholar] [CrossRef]
- Young, S.M.; Lee, J.T.; Hodges, R.J.; Chang, T.L.; Elashoff, D.A.; White, S.C. A comparative study of high-resolution cone beam computed tomography and charge-coupled device sensors for detecting caries. Dentomaxillofac. Radiol. 2009, 38, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Kayipmaz, S.; Sezgin, Ö.S.; Saricaoğlu, S.T.; Çan, G. An in vitro comparison of diagnostic abilities of conventional radiography, storage phosphor, and cone beam computed tomography to determine occlusal and approximal caries. Eur. J. Radiol. 2011, 80, 478–482. [Google Scholar] [CrossRef]
- Krzyżostaniak, J.; Kulczyk, T.; Czarnecka, B.; Surdacka, A. A comparative study of the diagnostic accuracy of cone beam computed tomography and intraoral radiographic modalities for the detection of noncavitated caries. Clin. Oral Investig. 2015, 19, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Qu, X.M.; Li, G.; Zhang, Z.Y.; Ma, X.C. The detection accuracies for proximal caries by cone-beam computerized tomography, film, and phosphor plates. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, S.; Tavakkoli, M.A.; Karimi Vasigh, H.; Azizi, Z.; Zarrabian, T. Evaluation of Cone Beam Computed Tomography (CBCT) System: Comparison with Intraoral Periapical Radiography in Proximal Caries Detection. J. Dent. Res. Dent. Clin. Dent. Prospects. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Wenzel, A.; Hirsch, E.; Christensen, J.; Matzen, L.H.; Scaf, G.; Frydenberg, M. Detection of cavitated approximal surfaces using cone beam CT and intraoral receptors. Dentomaxillofac. Radiol. 2013, 42, 39458105. [Google Scholar] [CrossRef]
- Charuakkra, A.; Prapayasatok, S.; Janhom, A.; Pongsiriwet, S.; Verochana, K.; Mahasantipiya, P. Diagnostic performance of cone-beam computed tomography on detection of mechanically-created artificial secondary caries. Imaging Sci. Dent. 2011, 41, 143–150. [Google Scholar] [CrossRef]
- Sousa Melo, S.L.; Belem, M.D.F.; Prieto, L.T.; Tabchoury, C.P.M.; Haiter-Neto, F. Comparison of cone beam computed tomography and digital intraoral radiography performance in the detection of artificially induced recurrent caries-like lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Li, G.; Zhang, Z.; Ma, X. Detection accuracy of in vitro approximal caries by cone beam computed tomography images. Eur. J. Radiol. 2011, 79, e24–e27. [Google Scholar] [CrossRef]
- Cardoso, J.R.; Pereira, L.M.; Iversen, M.D.; Ramos, A.L. What is gold standard and what is ground truth? Dental Press J. Orthod. 2014, 19, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hong, D.; Zhang, D.; Huang, M.; Yu, H. Detecting Proximal Caries on Periapical Radiographs Using Convolutional Neural Networks with Different Training Strategies on Small Datasets. Diagnostics 2022, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Lee, C.; Da Silva, J.D.; Ohyama, H.; Roppongi, M.; Kihara, H.; Hatakeyama, W.; Ishikawa-Nagai, S.; Izumisawa, M. A comparison of diagnosis of early stage interproximal caries with bitewing radiographs and periapical images using consensus reference. Dentomaxillofac. Radiol. 2019, 48, 20170450. [Google Scholar] [CrossRef]
- Kallio-Pulkkinen, S.; Huumonen, S.; Haapea, M.; Liukkonen, E.; Sipola, A.; Tervonen, O.; Nieminen, M.T. Effect of display type, DICOM calibration and room illuminance in bitewing radiographs. Dentomaxillofac. Radiol. 2016, 45, 20150129. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.J.; Han, S.S.; Lee, C.; Choi, Y.J.; Jung, H.I.; Kim, Y.H. Application of panoramic radiography with a multilayer imaging program for detecting proximal caries: A preliminary clinical study. Dentomaxillofac. Radiol. 2020, 49, 20190467. [Google Scholar] [CrossRef] [PubMed]

| Kappa | Agreement |
|---|---|
| <0 | Less than change agreement |
| 0.01–0.20 | Slight agreement |
| 0.21–0.40 | Fair agreement |
| 0.41–0.60 | Moderate agreement |
| 0.61–0.80 | Substantial agreement |
| 0.81–0.99 | Almost perfect agreement |
| 1 | Perfect agreement |
| Cohen’s Kappa | Weighted Kappa | |||||||
|---|---|---|---|---|---|---|---|---|
| Five-Point Scale | Binary Scale | Five-Point Scale | Binary Scale | |||||
| Unaided | Aided | Unaided | Aided | Unaided | Aided | Unaided | Aided | |
| Observer 1 | 0.820 | 0.903 | 0.926 | 0.939 | 0.938 | 0.958 | 0.926 | 0.939 |
| Observer 2 | 0.903 | 0.923 | 0.932 | 0.945 | 0.962 | 0.968 | 0.932 | 0.945 |
| Observer 3 | 0.735 | 0.911 | 0.865 | 0.939 | 0.849 | 0.937 | 0.865 | 0.939 |
| Score | Kappa | Observer 1 | Observer 2 | Observer 3 |
|---|---|---|---|---|
| Multiple | Weighted | 0.749 | 0.713 | 0.607 |
| Cohen’s | 0.438 | 0.349 | 0.308 | |
| Binary | Cohen’s | 0.816 | 0.683 | 0.410 |
| n | Absence | Presence | |
|---|---|---|---|
| Observer 1 | Unaided | 15,302 | 4634 |
| Aided | 14,225 | 5711 | |
| Observer 2 | Unaided | 14,756 | 5180 |
| Aided | 14,253 | 5683 | |
| Observer 3 | Unaided | 11,604 | 8332 |
| Aided | 13,584 | 6352 | |
| Ground Truth | 13,928 | 6008 | |
| Fleiss Kappa Coefficient | Binary | |
|---|---|---|
| Tooth Condition | Unaided | Aided |
| 0.831 | 0.928 |
| 0.612 | 0.829 |
| Overall Consensus | 0.443 | 0.757 |
| Fleiss Kappa Coefficient | Multiple | |
|---|---|---|
| Tooth Condition | Unaided | Aided |
| 0.534 | 0.581 |
| 0.494 | 0.628 |
| 0.420 | 0.330 |
| 0.305 | 0.709 |
| 0.561 | 0.668 |
| Overall Consensus | 0.325 | 0.468 |
| TP | TN | FP | FN | Sensitivity | Specificity | Accuracy | Kappa | ||
|---|---|---|---|---|---|---|---|---|---|
| Observer 1 | Unaided | 4377 | 13,671 | 257 | 1631 | 0.729 | 0.982 | 0.905 | 0.759 |
| Aided | 5248 | 13,465 | 463 | 760 | 0.874 | 0.967 | 0.939 | 0.852 | |
| Observer 2 | Unaided | 4609 | 13,357 | 571 | 1399 | 0.767 | 0.959 | 0.901 | 0.756 |
| Aided | 5210 | 13,455 | 473 | 798 | 0.867 | 0.966 | 0.936 | 0.846 | |
| Observer 3 | Unaided | 4588 | 10,184 | 3744 | 1420 | 0.764 | 0.731 | 0.741 | 0.446 |
| Aided | 5299 | 12,875 | 1053 | 709 | 0.882 | 0.924 | 0.912 | 0.793 |
| Observer | Five-Point Confidence Scale | Binary Score | ||||||
|---|---|---|---|---|---|---|---|---|
| Weighted Kappa | Cohen’s Kappa | |||||||
| 1 | 2 | 3 | Unaided | Aided | Unaided | Aided | Unaided | Aided |
| X | X | 0.557 | 0.859 | 0.332 | 0.664 | 0.583 | 0.810 | |
| X | X | 0.406 | 0.578 | 0.355 | 0.420 | 0.388 | 0.740 | |
| X | X | 0.435 | 0.574 | 0.317 | 0.373 | 0.408 | 0.724 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amasya, H.; Alkhader, M.; Serindere, G.; Futyma-Gąbka, K.; Aktuna Belgin, C.; Gusarev, M.; Ezhov, M.; Różyło-Kalinowska, I.; Önder, M.; Sanders, A.; et al. Evaluation of a Decision Support System Developed with Deep Learning Approach for Detecting Dental Caries with Cone-Beam Computed Tomography Imaging. Diagnostics 2023, 13, 3471. https://doi.org/10.3390/diagnostics13223471
Amasya H, Alkhader M, Serindere G, Futyma-Gąbka K, Aktuna Belgin C, Gusarev M, Ezhov M, Różyło-Kalinowska I, Önder M, Sanders A, et al. Evaluation of a Decision Support System Developed with Deep Learning Approach for Detecting Dental Caries with Cone-Beam Computed Tomography Imaging. Diagnostics. 2023; 13(22):3471. https://doi.org/10.3390/diagnostics13223471
Chicago/Turabian StyleAmasya, Hakan, Mustafa Alkhader, Gözde Serindere, Karolina Futyma-Gąbka, Ceren Aktuna Belgin, Maxim Gusarev, Matvey Ezhov, Ingrid Różyło-Kalinowska, Merve Önder, Alex Sanders, and et al. 2023. "Evaluation of a Decision Support System Developed with Deep Learning Approach for Detecting Dental Caries with Cone-Beam Computed Tomography Imaging" Diagnostics 13, no. 22: 3471. https://doi.org/10.3390/diagnostics13223471
APA StyleAmasya, H., Alkhader, M., Serindere, G., Futyma-Gąbka, K., Aktuna Belgin, C., Gusarev, M., Ezhov, M., Różyło-Kalinowska, I., Önder, M., Sanders, A., Costa, A. L. F., Castro Lopes, S. L. P. d., & Orhan, K. (2023). Evaluation of a Decision Support System Developed with Deep Learning Approach for Detecting Dental Caries with Cone-Beam Computed Tomography Imaging. Diagnostics, 13(22), 3471. https://doi.org/10.3390/diagnostics13223471









