The Potential Use of Artificial Intelligence in Irritable Bowel Syndrome Management
Abstract
:1. Introduction
2. The Development of Artificial Intelligence in the Medical Field
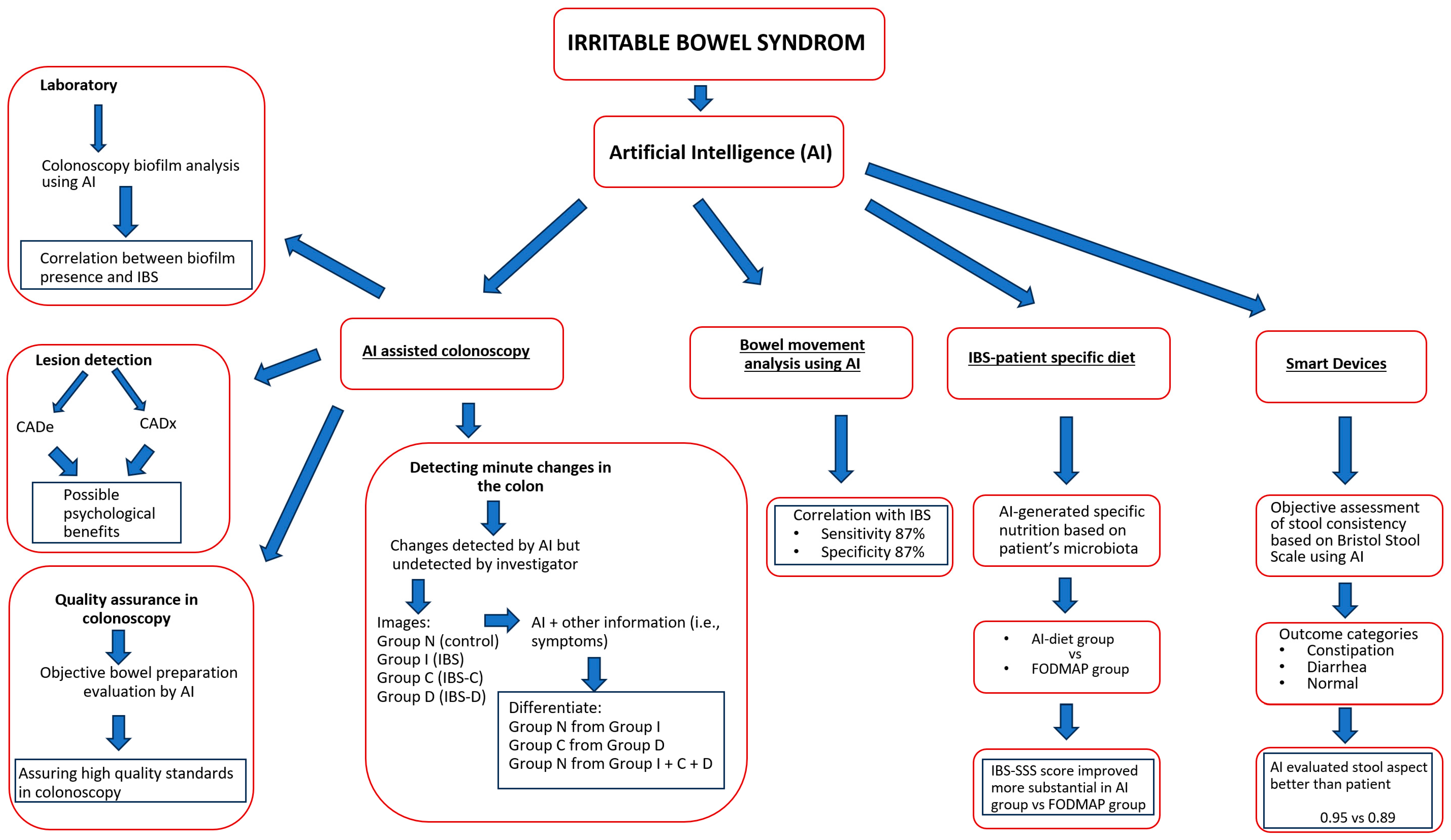
3. IBS and Artificial Intelligence
3.1. Artificial Intelligence-Assisted Colonoscopy in IBS
3.2. The Analysis of Acoustic Bowel Movements Using Artificial Intelligence
3.3. Artificial Intelligence-Generated Personalized Diet in IBS
3.4. Smartphone Application Using Artificial Intelligence to Monitor IBS Symptoms
4. Future Trends
- ➢
- Diagnose IBS early by analyzing patient data, symptoms and patterns, allowing for a more accurate and timely diagnosis.
- ➢
- A personalized treatment plan can be developed by AI, based on a patient’s personal data, lifestyle and preferences, thereby optimizing symptom management.
- ➢
- An AI-powered application can continuously monitor symptoms, providing real-time feedback and suggesting changes to diet or lifestyle.
- ➢
- AI chatbots and virtual assistants can provide instant answers to IBS patients’ questions and assist them in managing their symptoms.
- ➢
- By using Natural Language Processing (NLP) algorithms, it is possible to extract valuable insights from patients’ descriptions of their symptoms and experiences, which in turn can be used to assist in diagnosis and treatment.
- ➢
- AI can provide personalized dietary advice, helping patients identify trigger foods and create IBS-friendly meal plans.
- ➢
- Incorporating AI into telemedicine consulting can enhance the quality of telemedicine consultations by providing physicians with decision support and assisting them in making better treatment recommendations.
- ➢
- By analyzing vast datasets and identifying potential therapeutic targets, AI can accelerate IBS treatment discovery.
- ➢
- An AI-driven platform can facilitate the connection between patients with IBS and support communities and resources, fostering a sense of camaraderie and sharing coping strategies for IBS.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karakan, T.; Gundogdu, A.; Alagözlü, H.; Ekmen, N.; Ozgul, S.; Tunali, V.; Hora, M.; Beyazgul, D.; Nalbantoglu, O.U. Artificial intelligence-based personalized diet: A pilot clinical study for irritable bowel syndrome. Gut Microbes 2022, 14, 2138672. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A. Irritable bowel syndrome. Clin. Drug Investig. 2007, 27, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Prim. 2016, 2, 1–24. [Google Scholar] [CrossRef]
- Baumgartner, M.; Lang, M.; Holley, H.; Crepaz, D.; Hausmann, B.; Pjevac, P.; Moser, D.; Haller, F.; Hof, F.; Beer, A.; et al. Mucosal Biofilms Are an Endoscopic Feature of Irritable Bowel Syndrome and Ulcerative Colitis. Gastroenterology 2021, 161, 1245–1256.e20. [Google Scholar] [CrossRef]
- Hellström, P.M.; Benno, P. The Rome IV: Irritable bowel syndrome—A functional disorder. Best Pract. Res. Clin. Gastroenterol. 2019, 40, 101634. [Google Scholar] [CrossRef]
- Flacco, M.E.; Manzoli, L.; de Giorgio, R.; Gasbarrini, A.; Cicchetti, A.; Bravi, F.; Altini, M.; Caio, G.; Ursini, F. Costs of irritable bowel syndrome in European countries with universal healthcare coverage: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2986–3000. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2020, 116, 17–44. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106. [Google Scholar] [CrossRef]
- Lieberman, D.A.; Holub, J.; Eisen, G.; Kraemer, D.; Morris, C.D. Utilization of colonoscopy in the United States: Results from a national consortium. Gastrointest. Endosc. 2005, 62, 875–883. [Google Scholar] [CrossRef]
- Turing, A. Computing Machinery and Intelligence (1950). In The Essential Turing; Oxford Academic: Oxford, UK; Oxford University Press: Oxford, UK, 2004; pp. 433–464. [Google Scholar]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Buchanan, B.G.; Shortliffe, E.H. Rule-Based Expert Systems: The MYCIN Experiments of the Stanford Heuristic Programming Project; Addison-Wesley: Reading, MA, USA, 1984. [Google Scholar]
- Vulpoi, R.-A.; Luca, M.; Ciobanu, A.; Olteanu, A.; Barboi, O.-B.; Drug, V.L. Artificial Intelligence in Digestive Endoscopy—Where Are We and Where Are We Going? Diagnostics 2022, 12, 927. [Google Scholar] [CrossRef]
- Madiajagan, M.; Raj, S.S. Parallel Machine Learning and Deep Learning Approaches for Bioinformatics. In Deep Learning and Parallel Computing Environment for Bioengineering Systems; Academic Press: Cambridge, MA, USA, 2019; pp. 245–255. [Google Scholar] [CrossRef]
- He, J.; Baxter, S.L.; Xu, J.; Xu, J.; Zhou, X.; Zhang, K. The practical implementation of artificial intelligence technologies in medicine. Nat. Med. 2019, 25, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Jiang, Y.; Zhi, H.; Dong, Y.; Li, H.; Ma, S.; Wang, Y.; Dong, Q.; Shen, H.; Wang, Y. Artificial intelligence in healthcare: Past, present and future. Stroke Vasc. Neurol. 2017, 2, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Nakashima, H.; Nakamura, K.; Nagahama, R.; Saito, Y. Performance of Computer-Aided Detection and Diagnosis of Colorectal Polyps Compares to That of Experienced Endoscopists. Dig. Dis. Sci. 2021, 67, 3976–3983. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Allwood, G.; Webberley, K.M.; Inderjeeth, A.J.; Osseiran, A.; Marshall, B.J. Noninvasive Diagnosis of Irritable Bowel Syndrome via Bowel Sound Features: Proof of Concept. Clin. Transl. Gastroenterol. 2019, 10, e00017. [Google Scholar] [CrossRef]
- Canavan, C.; West, J.; Card, T. Review article: The economic impact of the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2014, 40, 1023–1034. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Lv, L. Diagnostic yield of colonoscopy for organic disease in irritable bowel syndrome and its risk factors: A meta-analysis. Neurogastroenterol. Motil. 2022, 35, e14481. [Google Scholar] [CrossRef]
- Tabata, K.; Mihara, H.; Nanjo, S.; Motoo, I.; Ando, T.; Teramoto, A.; Fujinami, H.; Yasuda, I. Artificial intelligence model for analyzing colonic endoscopy images to detect changes associated with irritable bowel syndrome. PLoS Digit. Health 2023, 2, e0000058. [Google Scholar] [CrossRef]
- Taghiakbari, M.; Mori, Y.; von Renteln, D. Artificial intelligence-assisted colonoscopy: A review of current state of practice and research. World J. Gastroenterol. 2021, 27, 8103–8122. [Google Scholar] [CrossRef]
- Repici, A.; Badalamenti, M.; Maselli, R.; Correale, L.; Radaelli, F.; Rondonotti, E.; Ferrara, E.; Spadaccini, M.; Alkandari, A.; Fugazza, A.; et al. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 2020, 159, 512–520.e7. [Google Scholar] [CrossRef] [PubMed]
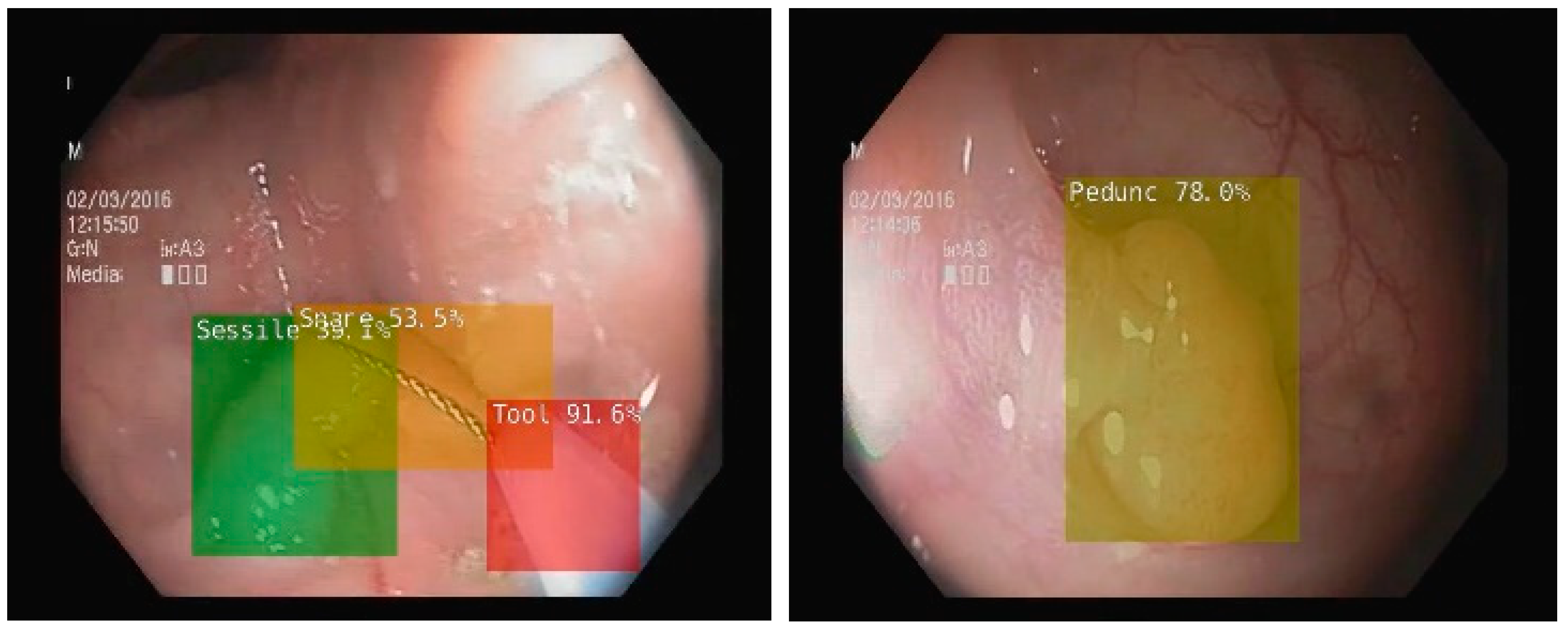
- Ciobanu, A.; Luca, M.; Barbu, T.; Drug, V.; Olteanu, A.; Vulpoi, R. Experimental Deep Learning Object Detection in Real-time Colonoscopies. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021; Institute of Electrical and Electronics Engineers (IEEE): Iasi, Romania, 2021. [Google Scholar] [CrossRef]
- Kulkarni, U.; Meena, S.M.; Gurlahosur, S.V.; Bhogar, G. Quantization Friendly MobileNet (QF-MobileNet) Architecture for Vision Based Applications on Embedded Platforms. Neural Netw. 2020, 136, 28–39. [Google Scholar] [CrossRef]
- van der Zander, Q.E.W.; Schreuder, R.M.; Fonollà, R.; Scheeve, T.; van der Sommen, F.; Winkens, B.; Aepli, P.; Hayee, B.; Pischel, A.B.; Stefanovic, M.; et al. Optical diagnosis of colorectal polyp images using a newly developed computer-aided diagnosis system (CADx) compared with intuitive optical diagnosis. Endoscopy 2020, 53, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Le, Q.V. EfficientNet: Rethinking Model Scaling for Convolutional Neural Networks. PMLR, 24 May 2019; pp. 6105–6114. Available online: https://proceedings.mlr.press/v97/tan19a.html (accessed on 11 March 2023).
- Lee, J.Y.; Calderwood, A.H.; Karnes, W.; Requa, J.; Jacobson, B.C.; Wallace, M.B. Artificial intelligence for the assessment of bowel preparation. Gastrointest. Endosc. 2021, 95, 512–518.e1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, L.; Wan, X.; Shen, L.; Liu, J.; Zhang, J.; Jiang, X.; Wang, Z.; Yu, S.; Kang, J.; et al. A novel artificial intelligence system for the assessment of bowel preparation (with video). Gastrointest. Endosc. 2019, 91, 428–435.e2. [Google Scholar] [CrossRef] [PubMed]
- Craine, B.L.; Silpa, M.; O’Toole, C.J. Computerized auscultation applied to irritable bowel syndrome. Dig. Dis. Sci. 1999, 44, 1887–1892. [Google Scholar] [CrossRef]
- Nowak, J.K.; Nowak, R.; Radzikowski, K.; Grulkowski, I.; Walkowiak, J. Automated Bowel Sound Analysis: An Overview. Sensors 2021, 21, 5294. [Google Scholar] [CrossRef]
- Inderjeeth, A.J.; Webberley, K.M.; Muir, J.; Marshall, B.J. The potential of computerised analysis of bowel sounds for diagnosis of gastrointestinal conditions: A systematic review. Syst. Rev. 2018, 7, 1–18. [Google Scholar] [CrossRef]
- Du, X.; Allwood, G.; Webberley, K.M.; Osseiran, A.; Marshall, B.J. Bowel Sounds Identification and Migrating Motor Complex Detection with Low-Cost Piezoelectric Acoustic Sensing Device. Sensors 2018, 18, 4240. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Scholz, M.; Lomer, M.C.; Ralph, F.S.; Irving, P.M.; Lindsay, J.O.; Fava, F.; Tuohy, K.; Whelan, K. Gut microbiota associations with diet in irritable bowel syndrome and the effect of low FODMAP diet and probiotics. Clin. Nutr. 2020, 40, 1861–1870. [Google Scholar] [CrossRef]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Hyde, E.; Debelius, J.W.; Morton, J.T.; Gonzalez, A.; Ackermann, G.; Aksenov, A.A.; Behsaz, B.; Brennan, C.; Chen, Y.; et al. American Gut: An Open Platform for Citizen Science Microbiome Research. mSystems 2018, 3, e00031-18. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef]
- Vandeputte, D.; Vanleeuwen, R.; Falony, G.; Joossens, M.; Raes, J. Perspectives and pitfalls of microbiome research through home based fecal sampling: The Flemish Gut Flora Project experience. Arch. Public Health 2015, 73 (Suppl. S1), P33. [Google Scholar] [CrossRef]
- Pimentel, M.; Mathur, R.; Wang, J.; Chang, C.; Hosseini, A.; Fiorentino, A.; Rashid, M.; Pichetshote, N.; Basseri, B.; Treyzon, L.; et al. A Smartphone Application Using Artificial Intelligence Is Superior To Subject Self-Reporting When Assessing Stool Form. Am. J. Gastroenterol. 2022, 117, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Kordi, M.; Dehghan, M.J.; Shayesteh, A.A.; Azizi, A. The impact of artificial intelligence algorithms on management of patients with irritable bowel syndrome: A systematic review. Inform. Med. Unlocked 2022, 29, 100891. [Google Scholar] [CrossRef]
- Fukui, H.; Nishida, A.; Matsuda, S.; Kira, F.; Watanabe, S.; Kuriyama, M.; Kawakami, K.; Aikawa, Y.; Oda, N.; Arai, K.; et al. Usefulness of Machine Learning-Based Gut Microbiome Analysis for Identifying Patients with Irritable Bowels Syndrome. J. Clin. Med. 2020, 9, 2403. [Google Scholar] [CrossRef]
| Product | Manufacturer | Place of Approval and Year | Computer System Used |
|---|---|---|---|
| EndoBRAIN | Cybernet System Corp./ Olympus Corp. | Japan 2018 | CADx |
| EndoBRAIN-EYE | Cybernet System Corp./ Olympus Corp. | Japan 2020 | CADe |
| EndoBrain-PLUS | Cybernet System Corp./ Olympus Corp. | Japan 2020 | CADx |
| EndoBrain-UC | Cybernet System Corp./ Olympus Corp. | Japan 2020 | CADx |
| GI Genius | Medtronic Corp. | Europe 2019 United States 2021 | CADe |
| ENDO-AID | Olympus Corp. | Europe 2020 | CADe |
| CAD EYE | Fujifilm Corp. | Europe 2020 Japan 2020 | CADe/ CADx |
| DISCOVERY | Pentax Corp. | Europe 2020 | CADe |
| WISE VISION | NEC Corp. | Europe 2021 Japan 2021 | CADe |
| CADDIE | Odin Vision | Europe 2021 | CADe |
| ME-APDS | Magentiq Eye | Europe 2021 | CADe |
| EndoAngel | Wuhan EndoAngel Medical Technology Company | China 2020 | CADe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vulpoi, R.A.; Luca, M.; Ciobanu, A.; Olteanu, A.; Bărboi, O.; Iov, D.-E.; Nichita, L.; Ciortescu, I.; Cijevschi Prelipcean, C.; Ștefănescu, G.; et al. The Potential Use of Artificial Intelligence in Irritable Bowel Syndrome Management. Diagnostics 2023, 13, 3336. https://doi.org/10.3390/diagnostics13213336
Vulpoi RA, Luca M, Ciobanu A, Olteanu A, Bărboi O, Iov D-E, Nichita L, Ciortescu I, Cijevschi Prelipcean C, Ștefănescu G, et al. The Potential Use of Artificial Intelligence in Irritable Bowel Syndrome Management. Diagnostics. 2023; 13(21):3336. https://doi.org/10.3390/diagnostics13213336
Chicago/Turabian StyleVulpoi, Radu Alexandru, Mihaela Luca, Adrian Ciobanu, Andrei Olteanu, Oana Bărboi, Diana-Elena Iov, Loredana Nichita, Irina Ciortescu, Cristina Cijevschi Prelipcean, Gabriela Ștefănescu, and et al. 2023. "The Potential Use of Artificial Intelligence in Irritable Bowel Syndrome Management" Diagnostics 13, no. 21: 3336. https://doi.org/10.3390/diagnostics13213336
APA StyleVulpoi, R. A., Luca, M., Ciobanu, A., Olteanu, A., Bărboi, O., Iov, D.-E., Nichita, L., Ciortescu, I., Cijevschi Prelipcean, C., Ștefănescu, G., Mihai, C., & Drug, V. L. (2023). The Potential Use of Artificial Intelligence in Irritable Bowel Syndrome Management. Diagnostics, 13(21), 3336. https://doi.org/10.3390/diagnostics13213336







