Effect of Moderate Exercise on the Superficial Zone of Articular Cartilage in Age-Related Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Subchondral Bone Change after Exercise
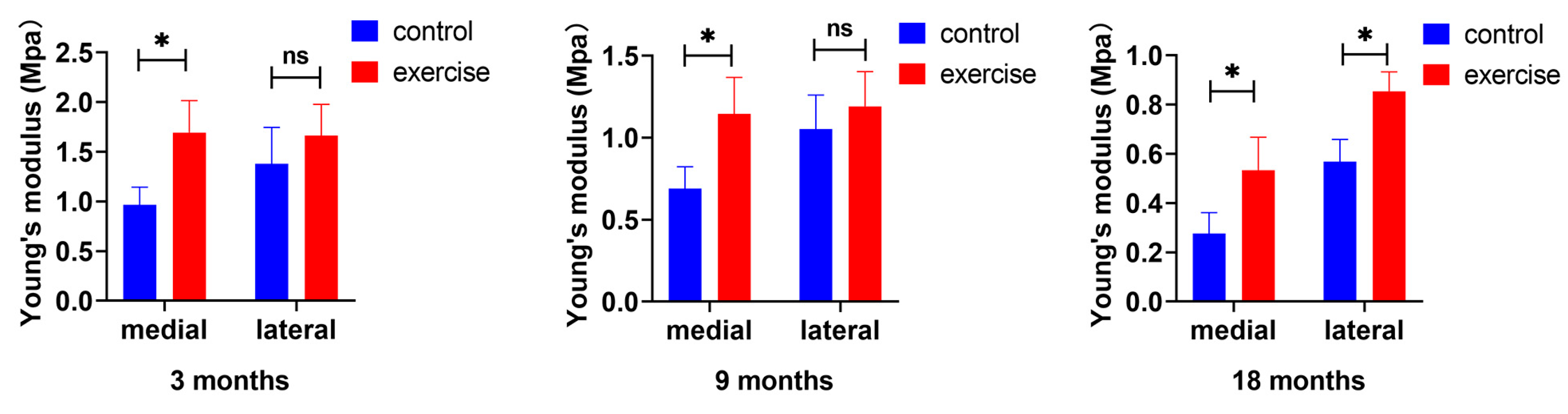
3.2. Change in Cartilage Nanoindentation Modulus after Exercise
3.3. Change in the Number and Distribution of SFZ Chondrocytes after Exercise
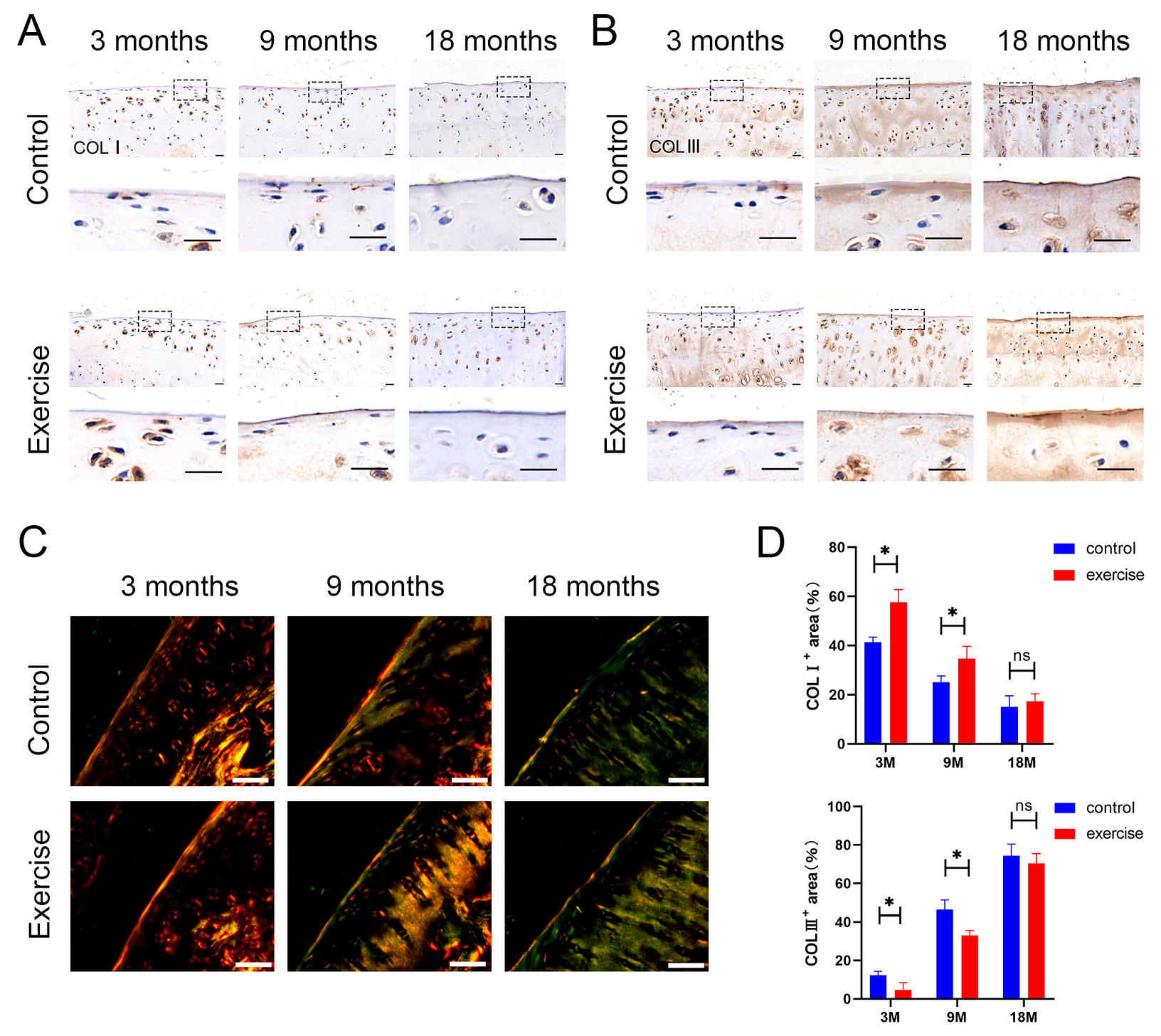
3.4. Change in the Distribution and Content of Type Ⅰ and Type Ⅲ Collagens in SFZ after Exercise
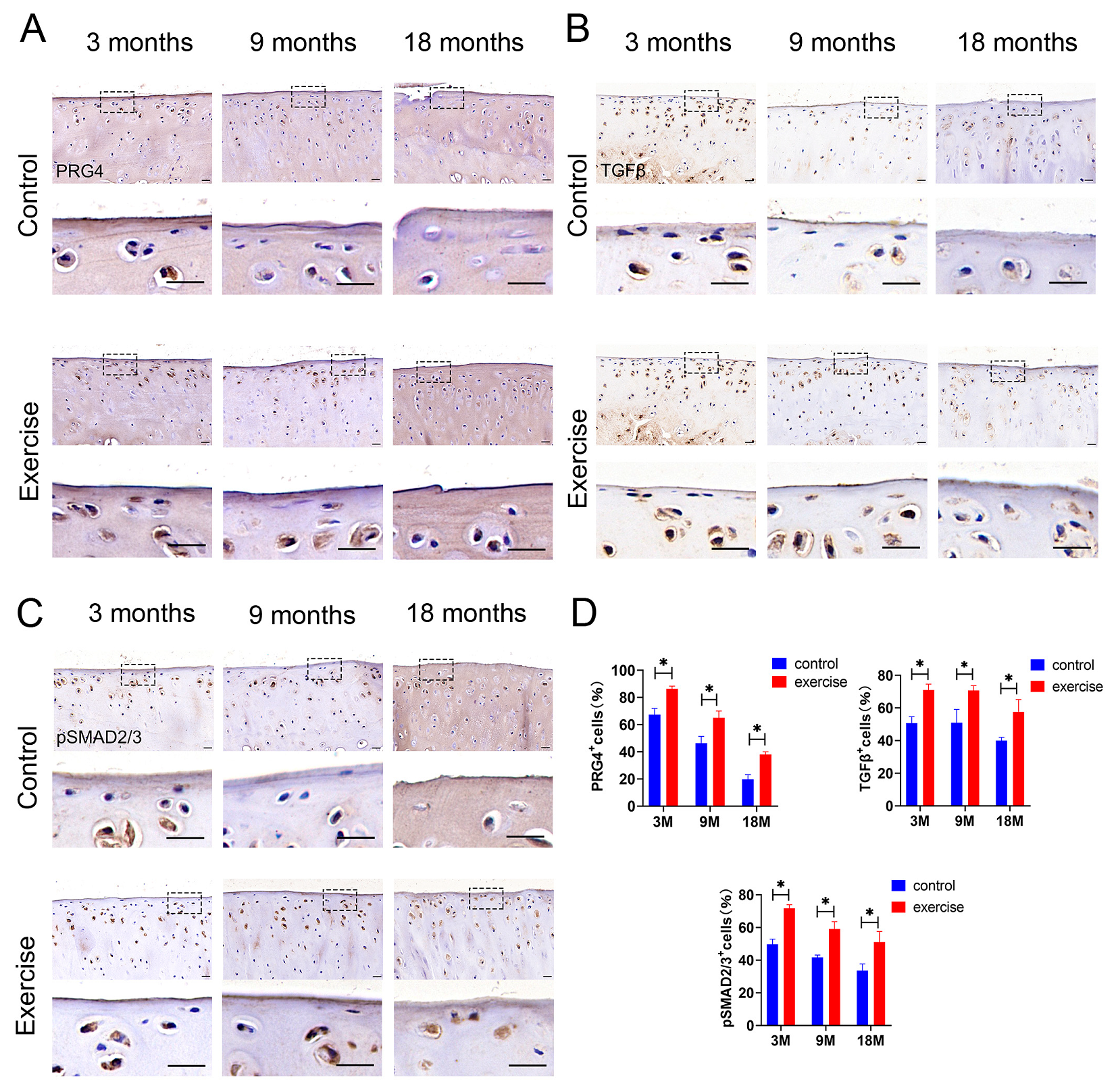
3.5. Change in Expression of Lubricin/Prg4 and TGFβ-pSmad2/3 in SFZ after Exercise In Vivo
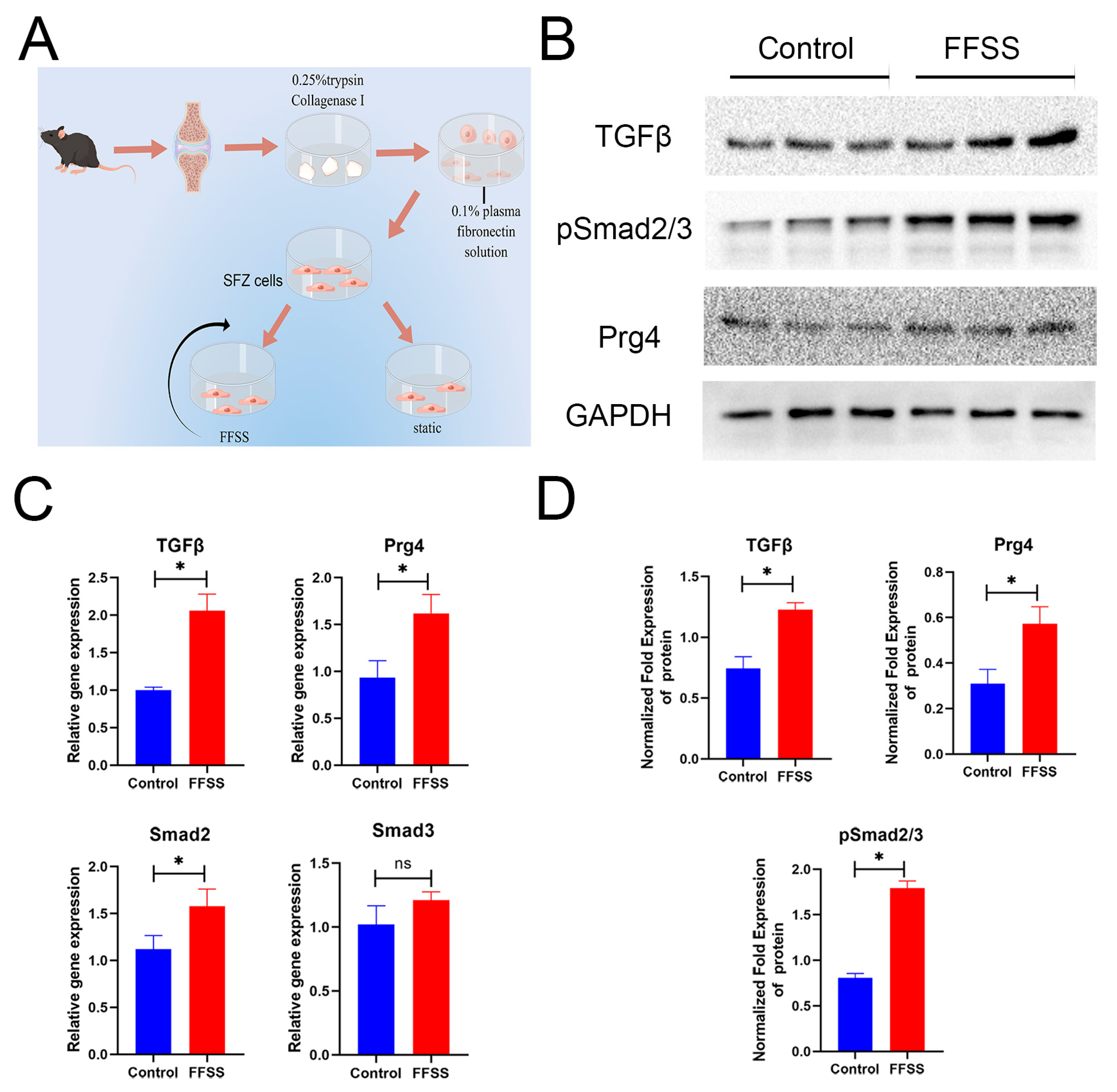
3.6. SFZ Cells Change after FFSS In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. Jama 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Nelligan, R.K.; Hinman, R.S.; Kasza, J.; Crofts, S.J.C.; Bennell, K.L. Effects of a Self-directed Web-Based Strengthening Exercise and Physical Activity Program Supported by Automated Text Messages for People with Knee Osteoarthritis: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Semanik, P.A.; Chang, R.W.; Dunlop, D.D. Aerobic activity in prevention and symptom control of osteoarthritis. PM R J. Inj. Funct. Rehabil. 2012, 4, S37–S44. [Google Scholar] [CrossRef]
- Cormier, J.; Cone, K.; Lanpher, J.; Kinens, A.; Henderson, T.; Liaw, L.; Bilsky, E.J.; King, T.; Rosen, C.J.; Stevenson, G.W. Exercise reverses pain-related weight asymmetry and differentially modulates trabecular bone microarchitecture in a rat model of osteoarthritis. Life Sci. 2017, 180, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Di Rosa, M.; Ravalli, S.; Castorina, A.; Guglielmino, C.; Imbesi, R.; Vecchio, M.; Drago, F.; Szychlinska, M.A.; Musumeci, G. Moderate Physical Activity as a Prevention Method for Knee Osteoarthritis and the Role of Synoviocytes as Biological Key. Int. J. Mol. Sci. 2019, 20, 511. [Google Scholar] [CrossRef] [PubMed]
- Stagg, N.J.; Mata, H.P.; Ibrahim, M.M.; Henriksen, E.J.; Porreca, F.; Vanderah, T.W.; Philip Malan, T., Jr. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: Role of endogenous opioids. Anesthesiology 2011, 114, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, M.H.; Tarum, F.; Rauf, I.Z.; Low, L.A.; Bushnell, C. Modest Amounts of Voluntary Exercise Reduce Pain- and Stress-Related Outcomes in a Rat Model of Persistent Hind Limb Inflammation. J. Pain 2017, 18, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Kuphal, K.E.; Fibuch, E.E.; Taylor, B.K. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J. Pain 2007, 8, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Imbert, I.; Havelin, J.; Henderson, T.; Stevenson, G.; Liaw, L.; King, T. Effects of Treadmill Exercise on Advanced Osteoarthritis Pain in Rats. Arthritis Rheumatol. 2017, 69, 1407–1417. [Google Scholar] [CrossRef]
- Galois, L.; Etienne, S.; Grossin, L.; Watrin-Pinzano, A.; Cournil-Henrionnet, C.; Loeuille, D.; Netter, P.; Mainard, D.; Gillet, P. Dose-response relationship for exercise on severity of experimental osteoarthritis in rats: A pilot study. Osteoarthr. Cartil. 2004, 12, 779–786. [Google Scholar] [CrossRef]
- Cifuentes, D.J.; Rocha, L.G.; Silva, L.A.; Brito, A.C.; Rueff-Barroso, C.R.; Porto, L.C.; Pinho, R.A. Decrease in oxidative stress and histological changes induced by physical exercise calibrated in rats with osteoarthritis induced by monosodium iodoacetate. Osteoarthr. Cartil. 2010, 18, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.B.; Mendonça, V.A.; Aguiar, G.C.; da Fonseca, S.F.; Dos Santos, J.M.; Tossige-Gomes, R.; Melo, D.S.; Oliveira, M.X.; Leite, H.R.; Camargos, A.C.R.; et al. Effect of a Moderate-Intensity Aerobic Training on Joint Biomarkers and Functional Adaptations in Rats Subjected to Induced Knee Osteoarthritis. Front. Physiol. 2019, 10, 1168. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wu, J.P.; Chen, H.H.; Kirk, T.B.; Xu, J. Elastin fibers display a versatile microfibril network in articular cartilage depending on the mechanical microenvironments. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2013, 31, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R. An introduction to the pathophysiology of osteoarthritis. Front. Biosci. J. Virtual Libr. 1999, 4, D662–D670. [Google Scholar] [CrossRef] [PubMed]
- Fukui, N.; Ikeda, Y.; Ohnuki, T.; Tanaka, N.; Hikita, A.; Mitomi, H.; Mori, T.; Juji, T.; Katsuragawa, Y.; Yamamoto, S.; et al. Regional differences in chondrocyte metabolism in osteoarthritis: A detailed analysis by laser capture microdissection. Arthritis Rheum. 2008, 58, 154–163. [Google Scholar] [CrossRef]
- Aigner, T.; Bertling, W.; Stöss, H.; Weseloh, G.; von der Mark, K. Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J. Clin. Investig. 1993, 91, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Hosseininia, S.; Weis, M.A.; Rai, J.; Kim, L.; Funk, S.; Dahlberg, L.E.; Eyre, D.R. Evidence for enhanced collagen type III deposition focally in the territorial matrix of osteoarthritic hip articular cartilage. Osteoarthr. Cartil. 2016, 24, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, J.; Carpten, J.D.; Suwairi, W.M.; Gutierrez, O.M.; Schwartz, S.; Robbins, C.; Sood, R.; Makalowska, I.; Baxevanis, A.; Johnstone, B.; et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat. Genet. 1999, 23, 319–322. [Google Scholar] [CrossRef]
- Jay, G.D.; Cha, C.J. The effect of phospholipase digestion upon the boundary lubricating ability of synovial fluid. J. Rheumatol. 1999, 26, 2454–2457. [Google Scholar] [PubMed]
- Saito, T. The superficial zone of articular cartilage. Inflamm. Regen. 2022, 42, 14. [Google Scholar] [CrossRef] [PubMed]
- Furumatsu, T.; Matsumoto, E.; Kanazawa, T.; Fujii, M.; Lu, Z.; Kajiki, R.; Ozaki, T. Tensile strain increases expression of CCN2 and COL2A1 by activating TGF-β-Smad2/3 pathway in chondrocytic cells. J. Biomech. 2013, 46, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Bougault, C.; Aubert-Foucher, E.; Paumier, A.; Perrier-Groult, E.; Huot, L.; Hot, D.; Duterque-Coquillaud, M.; Mallein-Gerin, F. Dynamic compression of chondrocyte-agarose constructs reveals new candidate mechanosensitive genes. PLoS ONE 2012, 7, e36964. [Google Scholar] [CrossRef]
- DuRaine, G.; Neu, C.P.; Chan, S.M.; Komvopoulos, K.; June, R.K.; Reddi, A.H. Regulation of the friction coefficient of articular cartilage by TGF-beta1 and IL-1beta. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2009, 27, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Niikura, T.; Reddi, A.H. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007, 56, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Neu, C.P.; Khalafi, A.; Komvopoulos, K.; Schmid, T.M.; Reddi, A.H. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum. 2007, 56, 3706–3714. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.X.; Liu, S.Y.; Lei, L.; Li, Z.; Zhou, Y.Z.; Zhan, L.Q. Intensity-dependent effect of treadmill running on knee articular cartilage in a rat model. BioMed Res. Int. 2013, 2013, 172392. [Google Scholar] [CrossRef] [PubMed]
- Bos, E.J.; van der Laan, K.; Helder, M.N.; Mullender, M.G.; Iannuzzi, D.; van Zuijlen, P.P. Noninvasive Measurement of Ear Cartilage Elasticity on the Cellular Level: A New Method to Provide Biomechanical Information for Tissue Engineering. Plastic and reconstructive surgery. Glob. Open 2017, 5, e1147. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S24–S34. [Google Scholar] [CrossRef]
- Yasuhara, R.; Ohta, Y.; Yuasa, T.; Kondo, N.; Hoang, T.; Addya, S.; Fortina, P.; Pacifici, M.; Iwamoto, M.; Enomoto-Iwamoto, M. Roles of β-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab. Investig. J. Tech. Methods Pathol. 2011, 91, 1739–1752. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.J.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A.; Whatley, R.E. Shear stress activates cytosolic phospholipase A2 (cPLA2) and MAP kinase in human endothelial cells. Biochem. Biophys. Res. Commun. 1996, 218, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Kozhemyakina, E.; Hung, H.H.; Grodzinsky, A.J.; Lassar, A.B. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev. 2014, 28, 127–139. [Google Scholar] [CrossRef]
- Rios, J.L.; Boldt, K.R.; Mather, J.W.; Seerattan, R.A.; Hart, D.A.; Herzog, W. Quantifying the Effects of Different Treadmill Training Speeds and Durations on the Health of Rat Knee Joints. Sports Med. Open 2018, 4, 15. [Google Scholar] [CrossRef]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Imbesi, R.; Giunta, S.; Szychlinska, M.A.; Loreto, C.; Castorina, S.; Mobasheri, A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand. J. Med. Sci. Sports 2015, 25, e222–e230. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Flowers, S.A.; Jin, C.; Bennet, E.P.; Ekwall, A.K.; Karlsson, N.G. The O-glycomap of lubricin, a novel mucin responsible for joint lubrication, identified by site-specific glycopeptide analysis. Mol. Cell. Proteom. MCP 2014, 13, 3396–3409. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.M.; Leonard, C.; Regmi, S.C.; De Rantere, D.; Tailor, P.; Ren, G.; Ishida, H.; Hsu, C.; Abubacker, S.; Pang, D.S.; et al. Lubricin/Proteoglycan 4 binds to and regulates the activity of Toll-Like Receptors In Vitro. Sci. Rep. 2016, 6, 18910. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharif, A.; Jamal, M.; Zhang, L.X.; Larson, K.; Schmidt, T.A.; Jay, G.D.; Elsaid, K.A. Lubricin/Proteoglycan 4 Binding to CD44 Receptor: A Mechanism of the Suppression of Proinflammatory Cytokine-Induced Synoviocyte Proliferation by Lubricin. Arthritis Rheumatol. 2015, 67, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Alquraini, A.; Jamal, M.; Zhang, L.; Schmidt, T.; Jay, G.D.; Elsaid, K.A. The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Res. Ther. 2017, 19, 89. [Google Scholar] [CrossRef]
- Maenohara, Y.; Chijimatsu, R.; Tachibana, N.; Uehara, K.; Xuan, F.; Mori, D.; Murahashi, Y.; Nakamoto, H.; Oichi, T.; Chang, S.H.; et al. Lubricin Contributes to Homeostasis of Articular Cartilage by Modulating Differentiation of Superficial Zone Cells. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2021, 36, 792–802. [Google Scholar] [CrossRef]
- Madej, W.; van Caam, A.; Blaney Davidson, E.; Buma, P.; van der Kraan, P.M. Unloading results in rapid loss of TGFβ signaling in articular cartilage: Role of loading-induced TGFβ signaling in maintenance of articular chondrocyte phenotype? Osteoarthr. Cartil. 2016, 24, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Madej, W.; van Caam, A.; Blaney Davidson, E.N.; van der Kraan, P.M.; Buma, P. Physiological and excessive mechanical compression of articular cartilage activates Smad2/3P signaling. Osteoarthr. Cartil. 2014, 22, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Andrades, J.A.; Motaung, S.C.; Jiménez-Palomo, P.; Claros, S.; López-Puerta, J.M.; Becerra, J.; Schmid, T.M.; Reddi, A.H. Induction of superficial zone protein (SZP)/lubricin/PRG 4 in muscle-derived mesenchymal stem/progenitor cells by transforming growth factor-β1 and bone morphogenetic protein-7. Arthritis Res. Ther. 2012, 14, R72. [Google Scholar] [CrossRef]
- Iwakura, T.; Sakata, R.; Reddi, A.H. Induction of chondrogenesis and expression of superficial zone protein in synovial explants with TGF-β1 and BMP-7. Tissue Eng. Part A 2013, 19, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R. Collagens and cartilage matrix homeostasis. Clin. Orthop. Relat. Res. 2004, 427, S118–S122. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef]
- Wu, J.J.; Weis, M.A.; Kim, L.S.; Eyre, D.R. Type III collagen, a fibril network modifier in articular cartilage. J. Biol. Chem. 2010, 285, 18537–18544. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Brisson, B.K.; Terajima, M.; Li, Q.; Hoxha, K.; Han, B.; Goldberg, A.M.; Sherry Liu, X.; Marcolongo, M.S.; Enomoto-Iwamoto, M.; et al. Type III collagen is a key regulator of the collagen fibrillar structure and biomechanics of articular cartilage and meniscus. Matrix Biol. J. Int. Soc. Matrix Biol. 2020, 85–86, 47–67. [Google Scholar] [CrossRef]
- Castañeda, S.; Roman-Blas, J.A.; Largo, R.; Herrero-Beaumont, G. Subchondral bone as a key target for osteoarthritis treatment. Biochem. Pharmacol. 2012, 83, 315–323. [Google Scholar] [CrossRef] [PubMed]


| Primers | Sequences (5’ to 3’) |
|---|---|
| Prg4 | CACCATCTCCACCACGCAGAAT |
| TGCTGAATGTTGCCACCTCTCTTG | |
| Erg | GGTCTTGAAGGTCCCGATGC |
| CACTCTGCGCTCATTTGTGG | |
| TenascinC | CAGTACCACGGCTACCACAG |
| CATTCTCCGATGCCGTCCAG | |
| TGFβ | ATGGTGGACCGCAACAACGC |
| GGCACTGCTTCCCGAATGTCTG | |
| Smad2 | CCGTGCTCCCTCCGTCTTCC |
| CTGCCGCCCGCTGATTGG | |
| Smad3 | TTGACAGAGAGCAACACAGTAT |
| CTTCATCCAGATCGATTGCTTG | |
| GAPDH | GGTCCCAGCTTAGGTTCATCA |
| ATCCGTTCACACCGACCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Zhang, Y.; Guo, L.; Li, P.; Wang, D.; Huang, L.; Zhao, X.; Wu, G.; Li, L.; Wei, X. Effect of Moderate Exercise on the Superficial Zone of Articular Cartilage in Age-Related Osteoarthritis. Diagnostics 2023, 13, 3193. https://doi.org/10.3390/diagnostics13203193
Yin Y, Zhang Y, Guo L, Li P, Wang D, Huang L, Zhao X, Wu G, Li L, Wei X. Effect of Moderate Exercise on the Superficial Zone of Articular Cartilage in Age-Related Osteoarthritis. Diagnostics. 2023; 13(20):3193. https://doi.org/10.3390/diagnostics13203193
Chicago/Turabian StyleYin, Yukun, Yuanyu Zhang, Li Guo, Pengcui Li, Dongming Wang, Lingan Huang, Xiaoqin Zhao, Gaige Wu, Lu Li, and Xiaochun Wei. 2023. "Effect of Moderate Exercise on the Superficial Zone of Articular Cartilage in Age-Related Osteoarthritis" Diagnostics 13, no. 20: 3193. https://doi.org/10.3390/diagnostics13203193
APA StyleYin, Y., Zhang, Y., Guo, L., Li, P., Wang, D., Huang, L., Zhao, X., Wu, G., Li, L., & Wei, X. (2023). Effect of Moderate Exercise on the Superficial Zone of Articular Cartilage in Age-Related Osteoarthritis. Diagnostics, 13(20), 3193. https://doi.org/10.3390/diagnostics13203193






