Abstract
The skin, the outermost layer of the human body, is exposed to various external stimuli that cause inflammatory skin reactions. These external stimulants trigger external epithelial cell damage and the release of intracellular substances. Following cellular damage or death, intracellular molecules are released that enhance tissue inflammation. As an important substance released from damaged cells, the S100 protein is a low-molecular-weight acidic protein with two calcium-binding sites and EF-hand motif domains. S100 proteins are widely present in systemic organs and interact with other proteins. Recent studies revealed the involvement of S100 in cutaneous inflammatory disorders, psoriasis, and atopic dermatitis. This review provides detailed information on the interactions among various S100 proteins in inflammatory diseases.
1. Introduction
The skin is the human body’s outermost layer organ; thus, it is exposed to a variety of external stimuli associated with inflammatory reactions [1,2,3]. These external stimulants trigger external epithelial cell damage and release intracellular substances [4,5]. Following cellular damage or death, intracellular molecules such as damage-associated molecular patterns (DAMPs) and alarmins are released to enhance tissue inflammatory reactions [6]. These substances serve as “danger signals” that alert neighboring cells to anomalies in the human body. The importance of DAMPs as inducers of inflammatory tissue reactions has been elucidated.
As an important substance released from damaged cells, the S100 protein is a low-molecular-weight acidic protein abundant in nervous system tissues. This protein family was first discovered by Moore [7] and has two calcium-binding sites with EF-hand motif domains. Since S100 proteins are present in systemic organs and can interact with other proteins, they are believed to have a wide variety of functions. S100 proteins alter their morphological form by binding calcium and contacting targeted proteins that influence cellular division [8]. S100 proteins are involved in intra- and extracellular signal transduction [9]. For instance, S100 proteins are released outside the cell and act as ligands for receptors for advanced glycation end product (RAGE), an AGE receptor that subsequently enhances inflammatory reactions as DAMPs.
More than 20 types of S100 proteins have been recognized to date [10] that are widely distributed in various organs. Recent studies have reported their role in human inflammatory diseases. For example, S100A2 is abundantly expressed in epidermal keratinocytes and related to the severity of drug eruption, atopic dermatitis (AD) [11], and the clinical stage of malignant tumors [12]. However, its detailed clinical significance and mechanisms of action remain unclear. To clarify their detailed actions, here, we summarize previous studies of the involvement of S100 proteins in representative inflammatory skin diseases, psoriasis, and AD.
2. Pathogenesis of Psoriasis
Psoriasis is a chronic cutaneous inflammatory disease characterized by deregulation of the cutaneous immune system (Figure 1). Scaly erythematous eruptions are a defining feature of the representative inflammatory skin condition known as psoriasis [13]. External trauma or infection triggers the assembly of host cell-derived nucleotides with keratinocyte-derived antimicrobial peptides to form a complex that triggers the expansion of antigen-specific T lymphocytes in the skin and lymph nodes following the activation of antigen-presenting cells [14]. Type I interferons (IFN) are produced by plasmacyte dendritic cells and induce myeloid dendritic cells to secrete interleukin (IL)-23 and tumor necrosis factor (TNF)-α [15]. These cytokines help Th17 cells produce more IL-17 and IL-22, leading to an enhanced inflammatory response in the epidermis and keratinocyte proliferation [13,16]. Specific cytokine inhibitors exhibit potent anti-inflammatory effects against skin inflammation in psoriasis and have demonstrated significance [17]. While the importance of S100 proteins in psoriasis has been gradually elucidated, numerous aspects of the functions of S100 proteins remain unexplained.
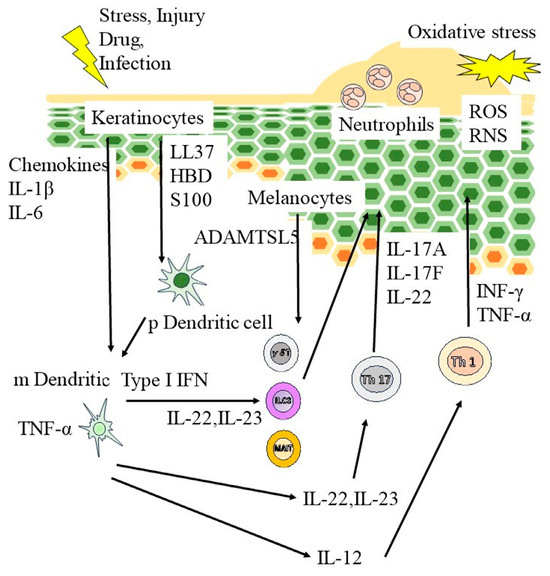
Figure 1.
Pathogenesis of psoriasis. External trauma or infection triggers the assembly of host cell–derived nucleotides with keratinocyte-derived antimicrobial peptides to form a complex that triggers the expansion of antigen-specific T lymphocytes in the skin as well as lymph nodes following the activation of antigen-presenting cells. Type I interferons (IFN) are produced by plasmacyte dendritic cells and induce myeloid dendritic cells to secrete interleukin (IL)-23 and tumor necrosis factor (TNF)-α. These cytokines help Th17 cells produce more IL-17 and IL-22, enhancing the inflammatory response in the epidermis and stimulating keratinocyte proliferation. RNS, reactive nitrogen species; ROS, reactive oxygen species.
3. Pathogenesis of AD
The primary components of the skin barrier are intercellular lipids in the stratum corneum, including ceramides [18,19] (Figure 2). Patients with AD have a reduced stratum corneum ceramide concentration, decreased filaggrin, or loss of function [20]. The epidermis also serves as a crucial barrier to external environmental factors. Tight connections illustrate the adhesive structure between the epidermal cells which prevents foreign substrates from penetrating the skin [21]. Tight connection components are diminished in AD [22], indicating skin barrier disruption in affected patients.
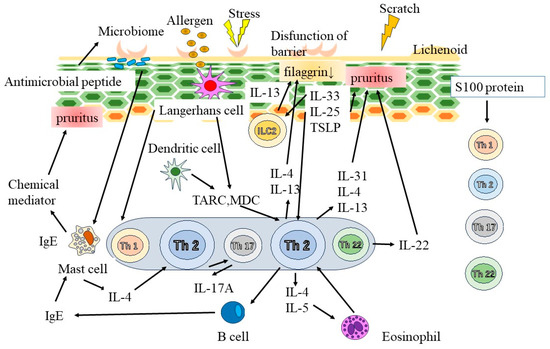
Figure 2.
Pathogenesis of AD. The primary components of the skin barrier are intercellular lipids in the stratum corneum, including ceramides. In addition, tight connections illustrate the adhesive structure between the epidermal cells which prevents foreign substrates from penetrating the skin. Chronic skin inflammation in AD is significantly affected by skin barrier dysfunction. Interleukin (IL)-33 and thymic stromal lymphopoietin (TSLP) are produced by epidermal keratinocytes and are responsible for type 2 immune response-mediated inflammation. Thymus- and activation-related chemokine (TARC) as well as macrophage-derived chemokine (MDC) are produced in atopic skin lesions and contribute to the migration of Th2 cells to skin lesions. IgE, immunoglobulin E.
Chronic skin inflammation in AD is significantly affected by skin barrier dysfunction [21]. IL-33 and thymic stromal lymphopoietin (TSLP) are produced by epidermal keratinocytes and are responsible for type 2 immune response-mediated inflammation [23,24]. Thymus- and activation-related chemokine as well as macrophage-derived chemokine are produced in atopic skin lesions and contribute to the migration of Th2 cells to skin lesions [25]. Additionally, IL-22 promotes the development of epidermal thickness in AD [26]. Neutralizing antibody therapy reduces the symptoms of AD and plays a crucial function in the associated itch development [27,28]. Each race exhibits unique immunological characteristics. Asians have a greater proportion of Th17 cells; additionally, an AD animal model [29] suggested that Th17 cells are crucial to the progression of AD.
4. S100 Proteins and Psoriasis
In the following sections, we describe the actions of S100 proteins in psoriasis and AD in detail. The currently identified S100 proteins include S100A2, S100A4, S100A7, S100A8, S100A9, S100A11, S100A12, S100A15, and S100B.
5. S100A2
Under normal physical conditions, keratinocytes produce dimeric S100A2 [30]. S100A2 is predominantly expressed in human skin; however, its expression is restricted to the basal layer of the epidermis in healthy individuals [11]. In addition, S100A2 is weakly expressed in the cytoplasm, mostly in the nuclei of keratinocytes [31]. In contrast, stress stimuli such as oxidative stress promote cytoplasmic S100A2 translocation from the nucleus, leading to alterations in the distribution pattern of S100A2 in keratinocytes during intracellular Ca2+ level elevations [31]. Furthermore, S100A2 is upregulated by IFN [32] and transforming growth factor [33]. As a result, several inflammatory responses appear associated with the pathological inflammatory responses mediated by S100A2. Consistently, S100A2 expression is markedly elevated in the skin of patients with psoriasis [11]. Moreover, S100A2 expression in the skin increases in AD and is associated with disease severity [11]. However, the detailed mechanism of S100A2 action in inflammatory skin diseases remains unclear.
6. S100A4
S100A4, a polypeptide that can oligomerize according to the Ca2+ concentration, is constructed as an antiparallel homodimer [34]. S100A4 is most frequently found in the cytoplasm of a wide range of cell types. S100A4 is involved in inflammatory reactions [35]. Following certain post-translational changes or stimulation, several investigations revealed its presence in the nucleus [36]. S100A4 can also be released into the extracellular region where it exerts autocrine and paracrine functions [37]. S100A4, when released into the extracellular space attracts immune cells, activates pro-inflammatory pathways and induces cytokine production [38]. Macrophage recruitment to the inflammatory site is consistently impaired in S100A4-deficient mice and these cells exhibit altered chemotaxis and matrix-degrading capacities [39]. Epidermal growth factor receptor, Toll-like receptor 4 (TLR4), annexin-A2, and IL-10 receptor are just a few cell surface receptors with which S100A4 may interact through oligomer formation [40].
S100A4 plays functional roles in psoriasis [41]. It is weakly expressed in the skin, particularly in the upper dermis [41]. S100A4 is predominantly expressed in the upper dermis in psoriasis compared to healthy skin, possibly mediated by p53 [41]. S100A4 inhibitors significantly reduced epidermal hyperplasia and dermal vascularization and impaired keratinocyte proliferation in an animal model of psoriasis [41], suggesting that S100A4 positively regulates skin inflammation in psoriasis.
7. S100A7 in Psoriasis
Although the general structure of S100A7 resembles that of other S100 proteins, there are several significant differences, particularly in the N-terminal EF-hand motif, which has a twisted loop and lacks a critical calcium-binding residue [42].
Psoriatic skin is prone to higher S100A7 levels in the epidermis [43,44]. The severity of psoriatic inflammation indicated by the Psoriasis Area and Severity Index (PASI) was positively correlated with S100A7 expression in the epidermis, which was downregulated during biological treatment with adalimumab, etanercept, ustekinumab [45,46], or a neutralizing antibody against IL-17A [47]. Consistently, S100A7 is highly expressed in psoriatic plaques with joint involvement [48], indicating its role as a booster of psoriatic inflammation in arthritis.
The binding of S100A7 to RAGE is necessary for inflammatory reactions. S100A7 enhances the chemotaxis of inflammatory cell infiltration into the skin [43]. S100A7 produces IL-1α by keratinocytes in a RAGE–p38 MAPK-dependent manner [47]. S100A7 acts on RAGE and subsequently suppresses involucrin, loricrin, keratin 1, and keratin 10 for keratinocyte differentiation through the MyD88-IκB/nuclear factor-kappa B (NF-κB) signaling pathway [49]. S100A7 activates inflammatory cytokine and chemokine production by neutrophils via ERK and p38 MAPK activation [50].
As the regulatory mechanism of S100A7, IL-17 plays a role in its induction. As the signal pathway for the induction of S100A7 mediated by IL-17, IκBζ is an essential regulatory factor in the pathogenesis of psoriasis through IL-17A and IL-17F-mediated actions [51,52]. Furthermore, IL-17A- and IL-17F-mediated IκBκ activation involves the p38 MAPK and NF-κB cascades [51,52]. IL-17C also has the potential to induce S100A7 expression by keratinocytes [53]. Th17 cells are positively associated with NLRP1-mediated caspase-5 activation during cutaneous inflammation mediated by S100A7 [54]. IL-22 enhances S100A7 expression in keratinocytes and the Janus kinase (JAK) inhibitor tofacitinib has been demonstrated as effective in psoriatic skin inflammation and suppresses IL-22-mediated S100A7 in keratinocytes [55]. In contrast, IL-35 inhibits S100A7 expression in keratinocytes [56]. Peripheral blood mononuclear cells in patients with psoriasis produce S100A7 which increases the production of inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-β [57].
Angiogenesis is an essential component of the pathophysiology of inflammatory skin diseases; vascular alterations occur early in the onset of psoriasis [58]. Psoriasis plays a role in angiogenesis hypoxia while reactive oxygen species (ROS) enhance psoriasin expression [59]. The downregulation of S100A7 alters ROS-mediated vascular endothelial growth factor in keratinocytes [59]. S100A7 activates dermal-derived endothelial cell proliferation mediated by the PI3K and NF-kB pathways [59]. Therefore, S100A7 widely affects the pathogenesis of psoriasis and the development of inflammatory reactions within the skin.
S100A7 plays an important role in the pathogenesis of AD. S100A7 is upregulated in AD skin and skin barrier dysfunction upregulates S100A7 in AD skin [60]. S100A7 expression increases in both intrinsic and extrinsic AD skin [61]. Furthermore, the onset of acute AD lesions increases S100A7 production within the skin [62]. Therefore, it is assumed that S100A7 acts in a wide variety of phases and that AD types are involved in its pathogenesis. Regarding the regulatory action of S100A7 in AD, both IL-4 and IL-13 reduced S100A7 [60]. Furthermore, S100A7 in keratinocytes was downregulated by treatment in a JAK2/STAT3-dependent manner, even in the presence of IL-17 [63,64]. STAT3 determines the induction of and influences S100A7 in keratinocytes [63]. JAK inhibitors reduce S100A7 levels in the skin [65]. In contrast, no significant correlation was observed between cutaneous S100A7 expression and the severity of AD skin inflammation or Staphylococcus aureus colonization [66]. Therefore, the inflammatory actions of S100A7 may be limited to certain conditions in patients with AD.
8. S100A8 and S100A9
The S100A8 and S100A9 subunits, which contain 93 and 114 amino acid residues, respectively, are always bound to one another and modify their self-structures to create polymers in the human body, particularly when Ca2+ is present [67,68]. The most common complex was the heterodimer S100A8/A9 [69]. S100A8 is the substance that directly binds to the target molecules whereas S100A9 controls complex activities by guarding S100A8 from degradation, among other actions [70,71].
S100A8 and S100A9 are located in the epidermal suprabasal layers and their expression is negligible in non-lesional psoriatic and healthy skin [72]. Psoriasis severity is associated with S100A8 expression; moreover, S100A9 is highly expressed in psoriatic skin lesions [73]. S100A8 and S100A9 are expressed exclusively in the synovium of patients with psoriatic arthritis (PsA) [74]. In addition, serum S100A8 and S100A9 levels are increased in psoriasis and positively correlated with PASI score [72], indicating that their production is positively correlated with disease development in psoriasis.
Notably, the risk of atherosclerotic events and early cardiovascular disease is associated with skin-derived inflammatory reactions in psoriasis [75]. A lipid-rich necrotic core (LRNC) is a risk factor for coronary plaque development and is linked to future risk of cardiovascular events [76]. Psoriasis severity is associated with LRNC formation and biological treatments can consistently reduce LRNC [77]. S100A8 and S100A9 were reportedly associated with LRNC in patients [75]. A decrease in the LRNCs of S100A8 and S100A9 was observed in patients receiving biological therapy [75]. These findings indicate a potential function of S100A8/A9 in the psoriasis-related formation of coronary plaques, leading to future cardiovascular events.
As a mechanism of S100A8 and S100A9 induction, IL-12/23p40 or anti-IL-23p19 inhibition reduced S100A8 and S100A9 expression in murine skin lesions [78]. Furthermore, IL-17A and IL-17F increase S1008 and S100A9 productions, mainly during the early phases of keratinocyte development [79]. IL-17A consistently enhances S100A9 production by keratinocytes via IκBζ, which was impaired by tacrolimus [80]. These findings indicate that the induction of S100A8 and S100A9 occurs via the IL-23/IL-17 axis.
In addition, S100A8 and S100A9 are induced by TLR4-mediated pathways. Specific peptide sequences within the second calcium-binding EF-hands trigger a TLR4-mediated inflammatory reaction [81]. The S100A8/S100A9 released at the inflammatory lesion was suppressed by calcium-mediated S100A8/S100A9 tetramer formation, leading to the hiding of the TLR4/MD2-binding site in the tetramer shape [81].
The mechanisms of S100A8 and S100A9 in psoriasis were revealed to some degree in previous studies. Recombinant S100A8/A9 proteins induce the expression of several inflammatory cytokine genes and the growth of keratinocytes [82]. S100A9-specific deficient mice show decreased psoriasis-like skin inflammation caused by imiquimod, which is associated with increased psoriasis-associated inflammatory cytokine production in the skin [79].
S100A8 and S100A9 expressions are increased in AD skin [61,62]. They are highly increased in the skin in pediatric-onset AD persisting into adulthood compared with adult-onset AD [83]. S100A8- and S100A9-treated keratinocytes produced inflammatory cytokines and chemokines mediated by p38 MAPK and ERK, respectively, which were impaired by TLR4 inhibitor treatment [84]. S100A8 and S100A9 impair filaggrin and loricrin expression in keratinocytes, indicating their association with skin barrier function in AD [84]. Furthermore, immune cell infiltration depended on S100A8 in the AD skin [85]. A house dust mite stimulates keratinocytes to strongly upregulate S100A8, which is further upregulated in the presence of IL-17A [86]. JAK inhibitors reduce S100A8 in the skin [65]. Emollients also have the potential to reduce S100A8 expression in the skin of AD model mice [87], suggesting that AD treatment may reduce the influence of S100A8 and S100A9 on skin inflammation in AD.
9. S100A11
S100A11 exists as a dimer and each monomer exhibits two EF-hands. The Ca2+ binding state to the C-terminal EF-hand leads to exposure of the hydrophobic cleft of the protein which becomes a protein–protein interaction site in S100A11 [88].
The involvement of S100A11 in AD pathogenesis is as follows: IL-4 and IL-13 downregulate S100A11 expression in keratinocytes, which also influences the upregulation of filaggrin and human beta-defensin 3 expression, indicating the role of the skin barrier function of S100A11 [89]. Although the direct influence of S100A11 on psoriasis and AD remains unclear, S100A11 plays a role in suppressing cellular growth in human keratinocytes [90] and is possibly involved in epidermal hyperplasia in these inflammatory diseases.
10. S100A12
S100A12 is highly expressed in psoriatic skin lesions [91] and substantially associated with PASI and serum S100A12 levels [92]. Etanercept therapy consistently reduced blood levels of S100A12 [92]. S100A12 is associated with PsA. The inflamed synovium expresses S100A12 in the tissue, which is impaired by psoriasis treatment [93]. S100A12 in the serum was positively correlated with PsA disease activity [93]. In contrast, no significant association was observed between LRNC and serum S100A12 in psoriasis [77], possibly indicating a role for S100A12 in the future risk of cardiovascular events.
The epigenetic regulation of DNA or DNA-binding proteins is essential for determining gene expression [94] and is involved in various inflammatory skin diseases [95]. A genome-wide study identified hypermethylation in CpG islands in psoriatic skin and showed a negative association with S100A12 expression, which is highly elevated in psoriasis [96]. Consistently, TNF-α inhibitor treatment accelerates hypomethylation in CpG islands in S100A12 [96], suggesting epigenetic regulation of S100A12 proteins in psoriasis.
S100A12 is increased in AD skin and even in non-lesional skin compared to healthy subjects [97]. However, the detailed mechanisms of action of S100A12 in psoriasis and AD remain unclear.
11. S100A15 in Psoriasis
S100A15 was reportedly overexpressed in psoriasis [43,98]. S100A15 expression is increased in the epidermis of the affected patients [98].
Atherosclerosis is an important issue in psoriasis and S100A15 is associated with atherosclerogenesis. The intima–media thickness of the carotid arteries is associated with S100A15 and PASI scores [99]. Furthermore, S100A15 levels are higher in patients with psoriasis and subclinical atherosclerosis [99], indicating that S100A15 is a particularly helpful marker of subclinical atherosclerosis in patients with psoriasis.
S100A15 is differentially regulated by IL-17A, TNF-α, and IL-22 [100]. S100A15 works with S100A7 to stimulate epidermal keratinocytes to produce TNF-α, IL-6, and IL-8 [100]. S100A15 alone has the potential to increase keratinocyte production of IL-6 [100], indicating the importance of S100A15 in the exacerbation of inflammatory reactions in psoriasis.
12. S100B
Patients with psoriasis show higher S100B levels; however, the S100B level is not associated with psoriasis severity [101]. Notably, S100B is highly expressed in the skin of patients with non-lesional psoriasis versus psoriatic lesions [101]. However, the detailed mechanism of action of S100B in psoriasis remains unknown.
13. Summary of S100 in Psoriasis and AD
Cutaneous Expression of S100 Proteins in Psoriasis and AD
Here, we summarized the distribution of S100 proteins in psoriasis and AD. S100 proteins are widely distributed in the skin. The basal and spinous layers of the healthy epidermis contain S100A7 and S100A11. The basal layer of keratinocytes contains these proteins in the nucleus and cytoplasm. In addition, these proteins are located in the cytoplasm alone in the spinous layer of keratinocytes [102]. S100A10 is found in the cytoplasm of basal and spinous cells and connected to the plasma membrane [102]. In the healthy epidermis, S100A8 and S100A9 are either missing or expressed at low levels [102]. S100A10 and S100A11 levels in the relevant psoriatic tissues were unaffected whereas S100A7, S100A8, and S100A9 were highly expressed [102]. In psoriasis-affected tissues, S100A8 and S100A9 are highly expressed in the basal and spinous layers [102].
14. Regulatory Mechanisms of S100 Proteins in Psoriasis and AD
The regulatory actions of S100 proteins in psoriasis and AD are summarized in Table 1. As a regulatory action of S100 proteins, Th17-associated cytokines activate the expression of S100A proteins whereas Th2-mediated cytokines suppress their expression. IL-4 and IL-13 downregulate S100A7 and S100A15 expression whereas TSLP suppresses S100A8/9 expression. In contrast, IL-17A and IL-22 upregulate the expressions of S100A7, S100A8/9, and S100A15.

Table 1.
Regulatory factors of S100 proteins.
15. Detailed Action of S100 Proteins in Psoriasis and AD
A comprehensive understanding of S100 proteins in psoriasis and AD is summarized in Table 2. Inflammatory cytokine production has been widely observed in AD and psoriasis as a function of S100 proteins. S100A4, S100A7, S100A8, S100A9, and S100A15 are representative inflammatory cytokines. Keratinocyte growth was observed in S100A8, S100A9, and S100A11. Immune cell infiltration involves the S100A4, S100A8, S100A9, and S100A15 proteins. Angiogenesis has a unique function in S100A7.

Table 2.
Regulatory factor of S100 proteins.
16. Conclusions
Although the primary source of inflammatory triggers in psoriasis remains unclear, substances released from keratinocytes are considered essential for the onset of psoriasis and AD. Among multiple causal factors, S100 proteins, particularly those in keratinocytes, are considered the main positive drivers of the pathogenesis of psoriasis and AD. This review also demonstrated the complex relationship between S100 proteins and psoriasis-mediated inflammation in other organs and conditions such as PsA and cardiovascular disease. Therefore, larger cohort studies should ascertain whether S100 protein production is associated with psoriasis and AD.
One of the major challenges for physicians is determining the best options for treating psoriasis and AD skin inflammation. The existing information derived from previous studies found that cytokine-targeted therapy displayed excellent therapeutic effects; however, the entire influence of these options for treatment may not be seen in all of these situations. Therefore, an unknown etiology of the development of psoriasis is necessary to unravel the unidentified influences of psoriatic skin inflammation and S100 proteins might be a clue to answer the unknown etiology of the pathogenesis of these inflammatory diseases.
Author Contributions
N.S.-S. and Y.S. wrote and revised the manuscript. Y.S. has read and agreed with the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Y.S. received a basic research grant from the Japanese Dermatology Association (Shiseido donated) and a research grant from KAKENHI (23K07755) from the Scientific Research Grant Committee of the Japan Society for the Promotion of Science.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Saito-Sasaki, N.; Nakamura, M. Omega 3 fatty acid and skin diseases. Front. Immunol. 2020, 11, 623052. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Vandenabeele, P.; Krysko, D.V. Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 2013, 38, 209–223. [Google Scholar] [CrossRef]
- Honda, T.; Yamamoto, O.; Sawada, Y.; Egawa, G.; Kitoh, A.; Otsuka, A.; Dainichi, T.; Nakajima, S.; Miyachi, Y.; Kabashima, K. Receptor-interacting protein kinase 3 controls keratinocyte activation in a necroptosis-independent manner and promotes psoriatic dermatitis in mice. J. Allergy Clin. Immunol. 2017, 140, 619–622.e6. [Google Scholar] [CrossRef] [PubMed]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Moore, B.W. A soluble protein characteristic of the nervous system. Biochem. Biophys. Res. Commun. 1965, 19, 739–744. [Google Scholar] [CrossRef]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef]
- Sugino, H.; Sawada, Y. Influence of S100A2 in human diseases. Diagnostics 2022, 12, 1756. [Google Scholar] [CrossRef]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 proteins in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118677. [Google Scholar] [CrossRef]
- Yoshioka, M.; Sawada, Y.; Saito-Sasaki, N.; Yoshioka, H.; Hama, K.; Omoto, D.; Ohmori, S.; Okada, E.; Nakamura, M. High S100A2 expression in keratinocytes in patients with drug eruption. Sci. Rep. 2021, 11, 5493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xia, T.; Qi, C.; Du, J.; Ye, C. High expression of S100A2 predicts poor prognosis in patients with endometrial carcinoma. BMC Cancer 2022, 22, 77. [Google Scholar] [CrossRef]
- Tashiro, T.; Sawada, Y. Psoriasis and systemic inflammatory disorders. Int. J. Mol. Sci. 2022, 23, 4457. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef]
- Itamura, M.; Sawada, Y. Involvement of atopic dermatitis in the development of systemic inflammatory diseases. Int. J. Mol. Sci. 2022, 23, 13445. [Google Scholar] [CrossRef]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Nomura, T.; Sandilands, A.; Akiyama, M.; Liao, H.; Evans, A.T.; Sakai, K.; Ota, M.; Sugiura, H.; Yamamoto, K.; Sato, H.; et al. Unique mutations in the filaggrin gene in Japanese patients with ichthyosis vulgaris and atopic dermatitis. J. Allergy Clin. Immunol. 2007, 119, 434–440. [Google Scholar] [CrossRef]
- Egawa, G.; Kabashima, K. Barrier dysfunction in the skin allergy. Allergol. Int. 2018, 67, 3–11. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.e7. [Google Scholar] [CrossRef] [PubMed]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Nakajima, S.; Nonomura, Y.; Otsuka, A.; Egawa, G.; Yoshimoto, T.; Nakamura, M.; Narumiya, S.; et al. Prostaglandin E(2) (PGE(2))-EP2 signaling negatively regulates murine atopic dermatitis-like skin inflammation by suppressing thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2019, 144, 1265–1273.e9. [Google Scholar] [CrossRef]
- Vestergaard, C.; Yoneyama, H.; Murai, M.; Nakamura, K.; Tamaki, K.; Terashima, Y.; Imai, T.; Yoshie, O.; Irimura, T.; Mizutani, H.; et al. Overproduction of Th2-specific chemokines in NC/Nga mice exhibiting atopic dermatitis-like lesions. J. Clin. Investig. 1999, 104, 1097–1105. [Google Scholar] [CrossRef]
- Esaki, H.; Brunner, P.M.; Renert-Yuval, Y.; Czarnowicki, T.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; Johnson, D.B.; et al. Early-onset pediatric atopic dermatitis is T(H)2 but also T(H)17 polarized in skin. J. Allergy Clin. Immunol. 2016, 138, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Furue, M.; Hanifin, J.M.; Pulka, G.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Nakano, M.; Ruzicka, T. Nemolizumab in patients with moderate-to-severe atopic dermatitis: Randomized, phase II, long-term extension study. J. Allergy Clin. Immunol. 2018, 142, 1121–1130.e7. [Google Scholar] [CrossRef]
- Blauvelt, A.; Teixeira, H.D.; Simpson, E.L.; Costanzo, A.; De Bruin-Weller, M.; Barbarot, S.; Prajapati, V.H.; Lio, P.; Hu, X.; Wu, T.; et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2021, 157, 1047–1055. [Google Scholar] [CrossRef]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; de Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef]
- Deshpande, R.; Woods, T.L.; Fu, J.; Zhang, T.; Stoll, S.W.; Elder, J.T. Biochemical characterization of S100A2 in human keratinocytes: Subcellular localization, dimerization, and oxidative cross-linking. J. Investig. Dermatol. 2000, 115, 477–485. [Google Scholar] [CrossRef]
- Zhang, T.; Woods, T.L.; Elder, J.T. Differential responses of S100A2 to oxidative stress and increased intracellular calcium in normal, immortalized, and malignant human keratinocytes. J. Investig. Dermatol. 2002, 119, 1196–1201. [Google Scholar] [CrossRef][Green Version]
- Foser, S.; Redwanz, I.; Ebeling, M.; Heizmann, C.W.; Certa, U. Interferon-alpha and transforming growth factor-beta co-induce growth inhibition of human tumor cells. Cell. Mol. Life Sci. 2006, 63, 2387–2396. [Google Scholar] [CrossRef]
- Naz, S.; Ranganathan, P.; Bodapati, P.; Shastry, A.H.; Mishra, L.N.; Kondaiah, P. Regulation of S100A2 expression by TGF-β-induced MEK/ERK signalling and its role in cell migration/invasion. Biochem. J. 2012, 447, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Boye, K.; Maelandsmo, G.M. S100A4 and metastasis: A small actor playing many roles. Am. J. Pathol. 2010, 176, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Dulyaninova, N.G.; Ruiz, P.D.; Gamble, M.J.; Backer, J.M.; Bresnick, A.R. S100A4 regulates macrophage invasion by distinct myosin-dependent and myosin-independent mechanisms. Mol. Biol. Cell 2018, 29, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.J.; Loeser, R.F.; Yammani, R.R. Sumoylation and nuclear translocation of S100A4 regulate IL-1beta-mediated production of matrix metalloproteinase-13. J. Biol. Chem. 2010, 285, 31517–31524. [Google Scholar] [CrossRef]
- Garrett, S.C.; Varney, K.M.; Weber, D.J.; Bresnick, A.R. S100A4, a mediator of metastasis. J. Biol. Chem. 2006, 281, 677–680. [Google Scholar] [CrossRef]
- Fei, F.; Qu, J.; Li, C.; Wang, X.; Li, Y.; Zhang, S. Role of metastasis-induced protein S100A4 in human non-tumor pathophysiologies. Cell Biosci. 2017, 7, 64. [Google Scholar] [CrossRef]
- Li, Z.H.; Dulyaninova, N.G.; House, R.P.; Almo, S.C.; Bresnick, A.R. S100A4 regulates macrophage chemotaxis. Mol. Biol. Cell 2010, 21, 2598–2610. [Google Scholar] [CrossRef]
- Ambartsumian, N.; Klingelhöfer, J.; Grigorian, M. The multifaceted S100A4 protein in cancer and inflammation. Methods Mol. Biol. 2019, 1929, 339–365. [Google Scholar]
- Zibert, J.R.; Skov, L.; Thyssen, J.P.; Jacobsen, G.K.; Grigorian, M. Significance of the S100A4 protein in psoriasis. J. Investig. Dermatol. 2010, 130, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, D.E.; Etzerodt, M.; Madsen, P.; Celis, J.E.; Thøgersen, H.C.; Nyborg, J.; Kjeldgaard, M. EF-hands at atomic resolution: The structure of human psoriasin (S100A7) solved by MAD phasing. Structure 1998, 6, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Mascia, F.; Dharamsi, A.; Howard, O.M.; Cataisson, C.; Bliskovski, V.; Winston, J.; Feigenbaum, L.; Lichti, U.; Ruzicka, T.; et al. Gene from a psoriasis susceptibility locus primes the skin for inflammation. Sci. Transl. Med. 2010, 2, 61ra90. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.S.; Wong, J.; Polyak, K.; Aronzon, D.; Enerbäck, C. Detection of psoriasin/S100A7 in the sera of patients with psoriasis. Br. J. Dermatol. 2009, 160, 325–332. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Trovato, C.; Skarmoutsou, E.; Rossi, G.A.; Granata, M.; Longo, V.; Gangemi, P.; Pettinato, M.; Mazzarino, M.C. Effects of adalimumab, etanercept and ustekinumab on the expression of psoriasin (S100A7) in psoriatic skin. J. Dermatol. Sci. 2015, 80, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Kobus, S.; Kobus, A.; Tigges, C.; Scola, N.; Altmeyer, P.; Kreuter, A.; Bechara, F.G.; Skrygan, M. Expression of antimicrobial peptides and proteins in etanercept-treated psoriasis patients. Regul. Pept. 2011, 167, 163–166. [Google Scholar] [CrossRef]
- Lei, H.; Li, X.; Jing, B.; Xu, H.; Wu, Y. Human S100A7 induces mature interleukin1α expression by RAGE-p38 MAPK-calpain1 pathway in psoriasis. PLoS ONE 2017, 12, e0169788. [Google Scholar] [CrossRef]
- Cubillos, S.; Norgauer, J. Low vitamin D-modulated calcium-regulating proteins in psoriasis vulgaris plaques: S100A7 overexpression depends on joint involvement. Int. J. Mol. Med. 2016, 38, 1083–1092. [Google Scholar] [CrossRef]
- Son, E.D.; Kim, H.J.; Kim, K.H.; Bin, B.H.; Bae, I.H.; Lim, K.M.; Yu, S.J.; Cho, E.G.; Lee, T.R. S100A7 (psoriasin) inhibits human epidermal differentiation by enhanced IL-6 secretion through IκB/NF-κB signalling. Exp. Dermatol. 2016, 25, 636–641. [Google Scholar] [CrossRef]
- Zheng, Y.; Niyonsaba, F.; Ushio, H.; Ikeda, S.; Nagaoka, I.; Okumura, K.; Ogawa, H. Microbicidal protein psoriasin is a multifunctional modulator of neutrophil activation. Immunology 2008, 124, 357–367. [Google Scholar] [CrossRef]
- Bertelsen, T.; Iversen, L.; Johansen, C. The human IL-17A/F heterodimer regulates psoriasis-associated genes through IκBζ. Exp. Dermatol. 2018, 27, 1048–1052. [Google Scholar] [CrossRef]
- Bertelsen, T.; Ljungberg, C.; Boye Kjellerup, R.; Iversen, L.; Johansen, C. IL-17F regulates psoriasis-associated genes through IκBζ. Exp. Dermatol. 2017, 26, 234–241. [Google Scholar] [CrossRef]
- Sato, E.; Yano, N.; Fujita, Y.; Imafuku, S. Interleukin-17A suppresses granular layer formation in a 3-D human epidermis model through regulation of terminal differentiation genes. J. Dermatol. 2020, 47, 390–396. [Google Scholar] [CrossRef]
- Zwicker, S.; Hattinger, E.; Bureik, D.; Batycka-Baran, A.; Schmidt, A.; Gerber, P.A.; Rothenfusser, S.; Gilliet, M.; Ruzicka, T.; Wolf, R. Th17 micro-milieu regulates NLRP1-dependent caspase-5 activity in skin autoinflammation. PLoS ONE 2017, 12, e0175153. [Google Scholar] [CrossRef]
- Srivastava, A.; Ståhle, M.; Pivarcsi, A.; Sonkoly, E. Tofacitinib represses the janus kinase-signal transducer and activators of transcription signalling pathway in keratinocytes. Acta Derm. Venereol. 2018, 98, 772–775. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.; Li, C.; Zhang, X.; Cheng, L.; Dai, L.; Wang, Y.; Wang, F.; Shi, G.; Li, Y.; et al. IL-35 Decelerates the inflammatory process by regulating inflammatory cytokine secretion and M1/M2 macrophage ratio in psoriasis. J. Immunol. 2016, 197, 2131–2144. [Google Scholar] [CrossRef]
- Batycka-Baran, A.; Hattinger, E.; Zwicker, S.; Summer, B.; Zack Howard, O.M.; Thomas, P.; Szepietowski, J.C.; Ruzicka, T.; Prinz, J.C.; Wolf, R. Leukocyte-derived koebnerisin (S100A15) and psoriasin (S100A7) are systemic mediators of inflammation in psoriasis. J. Dermatol. Sci. 2015, 79, 214–221. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, Y.J.; Kim, M. Angiogenesis in chronic inflammatory skin disorders. Int. J. Mol. Sci. 2021, 22, 12035. [Google Scholar] [CrossRef]
- Vegfors, J.; Ekman, A.K.; Stoll, S.W.; Bivik Eding, C.; Enerbäck, C. Psoriasin (S100A7) promotes stress-induced angiogenesis. Br. J. Dermatol. 2016, 175, 1263–1273. [Google Scholar] [CrossRef]
- Gläser, R.; Meyer-Hoffert, U.; Harder, J.; Cordes, J.; Wittersheim, M.; Kobliakova, J.; Fölster-Holst, R.; Proksch, E.; Schröder, J.M.; Schwarz, T. The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J. Investig. Dermatol. 2009, 129, 641–649. [Google Scholar] [CrossRef]
- Suárez-Fariñas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Gittler, J.K.; Shemer, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ryu, W.I.; Kim, H.J.; Bae, H.C.; Ryu, H.J.; Shin, J.J.; Song, K.H.; Kim, T.W.; Son, S.W. TSLP down-regulates S100A7 and ß-defensin 2 via the JAK2/STAT3-dependent mechanism. J. Investig. Dermatol. 2016, 136, 2427–2435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dai, X.; Muto, J.; Shiraishi, K.; Utsunomiya, R.; Mori, H.; Murakami, M.; Sayama, K. TSLP impairs epidermal barrier integrity by stimulating the formation of nuclear IL-33/phosphorylated STAT3 complex in human keratinocytes. J. Investig. Dermatol. 2022, 142, 2100–2108.e5. [Google Scholar] [CrossRef]
- Pavel, A.B.; Song, T.; Kim, H.J.; Del Duca, E.; Krueger, J.G.; Dubin, C.; Peng, X.; Xu, H.; Zhang, N.; Estrada, Y.D.; et al. Oral janus kinase/SYK inhibition (ASN002) suppresses inflammation and improves epidermal barrier markers in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 144, 1011–1024. [Google Scholar] [CrossRef]
- Harder, J.; Dressel, S.; Wittersheim, M.; Cordes, J.; Meyer-Hoffert, U.; Mrowietz, U.; Fölster-Holst, R.; Proksch, E.; Schröder, J.M.; Schwarz, T.; et al. Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superficial skin injury. J. Investig. Dermatol. 2010, 130, 1355–1364. [Google Scholar] [CrossRef]
- Odink, K.; Cerletti, N.; Brüggen, J.; Clerc, R.G.; Tarcsay, L.; Zwadlo, G.; Gerhards, G.; Schlegel, R.; Sorg, C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature 1987, 330, 80–82. [Google Scholar] [CrossRef]
- Chen, Y.; Ouyang, Y.; Li, Z.; Wang, X.; Ma, J. S100A8 and S100A9 in cancer. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188891. [Google Scholar] [CrossRef]
- Nacken, W.; Roth, J.; Sorg, C.; Kerkhoff, C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc. Res. Tech. 2003, 60, 569–580. [Google Scholar] [CrossRef]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; van Zoelen, M.A.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C.; et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef]
- Vogl, T.; Ludwig, S.; Goebeler, M.; Strey, A.; Thorey, I.S.; Reichelt, R.; Foell, D.; Gerke, V.; Manitz, M.P.; Nacken, W.; et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood 2004, 104, 4260–4268. [Google Scholar] [CrossRef]
- Benoit, S.; Toksoy, A.; Ahlmann, M.; Schmidt, M.; Sunderkötter, C.; Foell, D.; Pasparakis, M.; Roth, J.; Goebeler, M. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br. J. Dermatol. 2006, 155, 62–66. [Google Scholar] [CrossRef]
- Jiménez, C.; Carvajal, D.; Hernández, M.; Valenzuela, F.; Astorga, J.; Fernández, A. Levels of the interleukins 17A, 22, and 23 and the S100 protein family in the gingival crevicular fluid of psoriatic patients with or without periodontitis. An. Bras. Dermatol. 2021, 96, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bierkarre, H.; Harder, J.; Cuthbert, R.; Emery, P.; Leuschner, I.; Mrowietz, U.; Hedderich, J.; McGonagle, D.; Gläser, R. Differential expression of antimicrobial peptides in psoriasis and psoriatic arthritis as a novel contributory mechanism for skin and joint disease heterogeneity. Scand. J. Rheumatol. 2016, 45, 188–196. [Google Scholar] [CrossRef]
- Berg, A.R.; Hong, C.G.; Svirydava, M.; Li, H.; Parel, P.M.; Florida, E.; O’Hagan, R.; Pantoja, C.J.; Lateef, S.S.; Anzenberg, P.; et al. Association of S100A8/A9 with lipid-rich necrotic core and treatment with biologic therapy in patients with psoriasis: Results from an observational cohort study. J. Investig. Dermatol. 2022, 142, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, T.J.; Stolp, J.; van Dam-Nolen, D.; Kassem, M.; Hendrikse, J.; Kooi, M.E.; Bos, D.; Nederkoorn, P.J. Higher leukocyte count is associated with lower presence of carotid lipid-rich necrotic core: A sub-study in the Plaque at RISK (PARISK) study. J. Clin. Med. 2023, 12, 1370. [Google Scholar] [CrossRef]
- Choi, H.; Uceda, D.E.; Dey, A.K.; Abdelrahman, K.M.; Aksentijevich, M.; Rodante, J.A.; Elnabawi, Y.A.; Reddy, A.; Keel, A.; Erb-Alvarez, J.; et al. Treatment of psoriasis with biologic therapy is associated with improvement of coronary artery plaque lipid-rich necrotic core: Results from a prospective, observational study. Circ. Cardiovasc. Imaging 2020, 13, e011199. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kanda, T.; Takaishi, M.; Shiga, T.; Miyoshi, K.; Nakajima, H.; Kamijima, R.; Tarutani, M.; Benson, J.M.; Elloso, M.M.; et al. Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model. J. Immunol. 2011, 186, 4481–4489. [Google Scholar] [CrossRef]
- Christmann, C.; Zenker, S.; Martens, L.; Hübner, J.; Loser, K.; Vogl, T.; Roth, J. Interleukin 17 promotes expression of alarmins S100A8 and S100A9 during the inflammatory response of keratinocytes. Front. Immunol. 2020, 11, 599947. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, J.; Yin, L.; Tu, J.; Yin, Z. Tacrolimus inhibits TNF-α/IL-17A-produced pro-inflammatory effect on human keratinocytes by regulating IκBζ. Inflammation 2020, 43, 692–700. [Google Scholar] [CrossRef]
- Vogl, T.; Stratis, A.; Wixler, V.; Völler, T.; Thurainayagam, S.; Jorch, S.K.; Zenker, S.; Dreiling, A.; Chakraborty, D.; Fröhling, M.; et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J. Clin. Investig. 2018, 128, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Nukui, T.; Ehama, R.; Sakaguchi, M.; Sonegawa, H.; Katagiri, C.; Hibino, T.; Huh, N.H. S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J. Cell. Biochem. 2008, 104, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Facheris, P.; Da Rosa, J.C.; Pagan, A.D.; Angelov, M.; Del Duca, E.; Rabinowitz, G.; Gómez-Arias, P.J.; Rothenberg-Lausell, C.; Estrada, Y.D.; Bose, S.; et al. Age of onset defines two distinct profiles of atopic dermatitis in adults. Allergy 2023, 78, 2202–2214. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Im, M.A.; Lee, J.S.; Mun, J.Y.; Kim, D.H.; Gu, A.; Kim, I.S. Effect of S100A8 and S100A9 on expressions of cytokine and skin barrier protein in human keratinocytes. Mol. Med. Rep. 2019, 20, 2476–2483. [Google Scholar] [CrossRef]
- Peng, S.; Chen, M.; Yin, M.; Feng, H. Identifying the potential therapeutic targets for atopic dermatitis through the immune infiltration analysis and construction of a ceRNA network. Clin. Cosmet. Investig. Dermatol. 2021, 14, 437–453. [Google Scholar] [CrossRef]
- Martel, B.C.; Litman, T.; Hald, A.; Norsgaard, H.; Lovato, P.; Dyring-Andersen, B.; Skov, L.; Thestrup-Pedersen, K.; Skov, S.; Skak, K.; et al. Distinct molecular signatures of mild extrinsic and intrinsic atopic dermatitis. Exp. Dermatol. 2016, 25, 453–459. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Wang, X.; Zhang, L.; Hu, M.; Le, Y.; Chen, L.; Zheng, J. Topical emollient prevents the development of atopic dermatitis and atopic march in mice. Exp. Dermatol. 2023, 32, 1007–1015. [Google Scholar] [CrossRef]
- Rintala-Dempsey, A.C.; Rezvanpour, A.; Shaw, G.S. S100-annexin complexes--structural insights. FEBS. J. 2008, 275, 4956–4966. [Google Scholar] [CrossRef]
- Howell, M.D.; Fairchild, H.R.; Kim, B.E.; Bin, L.; Boguniewicz, M.; Redzic, J.S.; Hansen, K.C.; Leung, D.Y. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J. Investig. Dermatol. 2008, 128, 2248–2258. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Miyazaki, M.; Takaishi, M.; Sakaguchi, Y.; Makino, E.; Kataoka, N.; Yamada, H.; Namba, M.; Huh, N.H. S100C/A11 is a key mediator of Ca(2+)-induced growth inhibition of human epidermal keratinocytes. J. Cell Biol. 2003, 163, 825–835. [Google Scholar] [CrossRef]
- Borsky, P.; Fiala, Z.; Andrys, C.; Beranek, M.; Hamakova, K.; Malkova, A.; Svadlakova, T.; Krejsek, J.; Palicka, V.; Borska, L.; et al. Alarmins HMGB1, IL-33, S100A7, and S100A12 in psoriasis vulgaris. Mediators. Inflamm. 2020, 2020, 8465083. [Google Scholar] [CrossRef]
- Wilsmann-Theis, D.; Wagenpfeil, J.; Holzinger, D.; Roth, J.; Koch, S.; Schnautz, S.; Bieber, T.; Wenzel, J. Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Kane, D.; Bresnihan, B.; Vogl, T.; Nacken, W.; Sorg, C.; Fitzgerald, O.; Roth, J. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology 2003, 42, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Gallo, R.L. Role of epigenetics in the regulation of immune functions of the skin. J. Investig. Dermatol. 2021, 141, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Nakatsuji, T.; Dokoshi, T.; Kulkarni, N.N.; Liggins, M.C.; Sen, G.; Gallo, R.L. Cutaneous innate immune tolerance is mediated by epigenetic control of MAP2K3 by HDAC8/9. Sci. Immunol. 2021, 6, eabe1935. [Google Scholar] [CrossRef]
- Roberson, E.D.; Liu, Y.; Ryan, C.; Joyce, C.E.; Duan, S.; Cao, L.; Martin, A.; Liao, W.; Menter, A.; Bowcock, A.M. A subset of methylated CpG sites differentiate psoriatic from normal skin. J. Investig. Dermatol. 2012, 132, 583–592. [Google Scholar] [CrossRef]
- Pavel, A.B.; Zhou, L.; Diaz, A.; Ungar, B.; Dan, J.; He, H.; Estrada, Y.D.; Xu, H.; Fernandes, M.; Renert-Yuval, Y.; et al. The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature. J. Am. Acad. Dermatol. 2020, 82, 690–699. [Google Scholar] [CrossRef]
- Wolf, R.; Mirmohammadsadegh, A.; Walz, M.; Lysa, B.; Tartler, U.; Remus, R.; Hengge, U.; Michel, G.; Ruzicka, T. Molecular cloning and characterization of alternatively spliced mRNA isoforms from psoriatic skin encoding a novel member of the S100 family. FASEB J. 2003, 17, 1969–1971. [Google Scholar] [CrossRef]
- Awad, S.M.; Attallah, D.A.; Salama, R.H.; Mahran, A.M.; Abu El-Hamed, E. Serum levels of psoriasin (S100A7) and koebnerisin (S100A15) as potential markers of atherosclerosis in patients with psoriasis. Clin. Exp. Dermatol. 2018, 43, 262–267. [Google Scholar] [CrossRef]
- Hegyi, Z.; Zwicker, S.; Bureik, D.; Peric, M.; Koglin, S.; Batycka-Baran, A.; Prinz, J.C.; Ruzicka, T.; Schauber, J.; Wolf, R. Vitamin D analog calcipotriol suppresses the Th17 cytokine-induced proinflammatory S100 “alarmins” psoriasin (S100A7) and koebnerisin (S100A15) in psoriasis. J. Investig. Dermatol. 2012, 132, 1416–1424. [Google Scholar] [CrossRef]
- Salem, S.A.M.; El-Khateeb, E.A.; Harvy, M.; Emam, H.M.E.; Abdelaal, W.; Nemr, R.E.; El-Hagry, O.O. Study of serum levels and skin expression of S100B protein in psoriasis. An. Bras. Dermatol. 2017, 92, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Broome, A.M.; Ryan, D.; Eckert, R.L. S100 protein subcellular localization during epidermal differentiation and psoriasis. J. Histochem. Cytochem. 2003, 51, 675–685. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).