In Vivo Preclinical Assessment of the VEGF Targeting Potential of the Newly Synthesized [52Mn]Mn-DOTAGA-Bevacizumab Using Experimental Cervix Carcinoma Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Chemicals
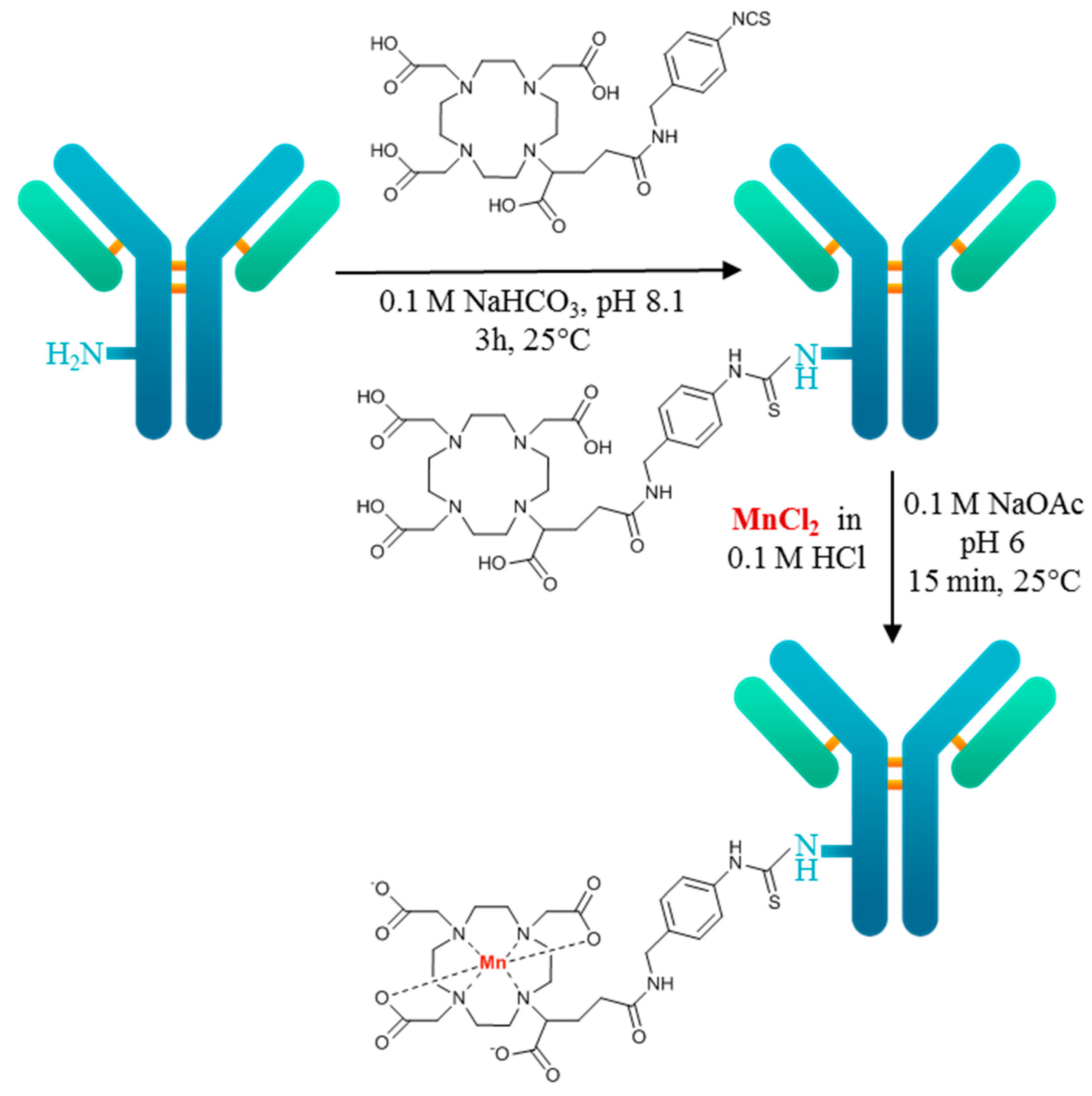
2.3. Radiolabeling
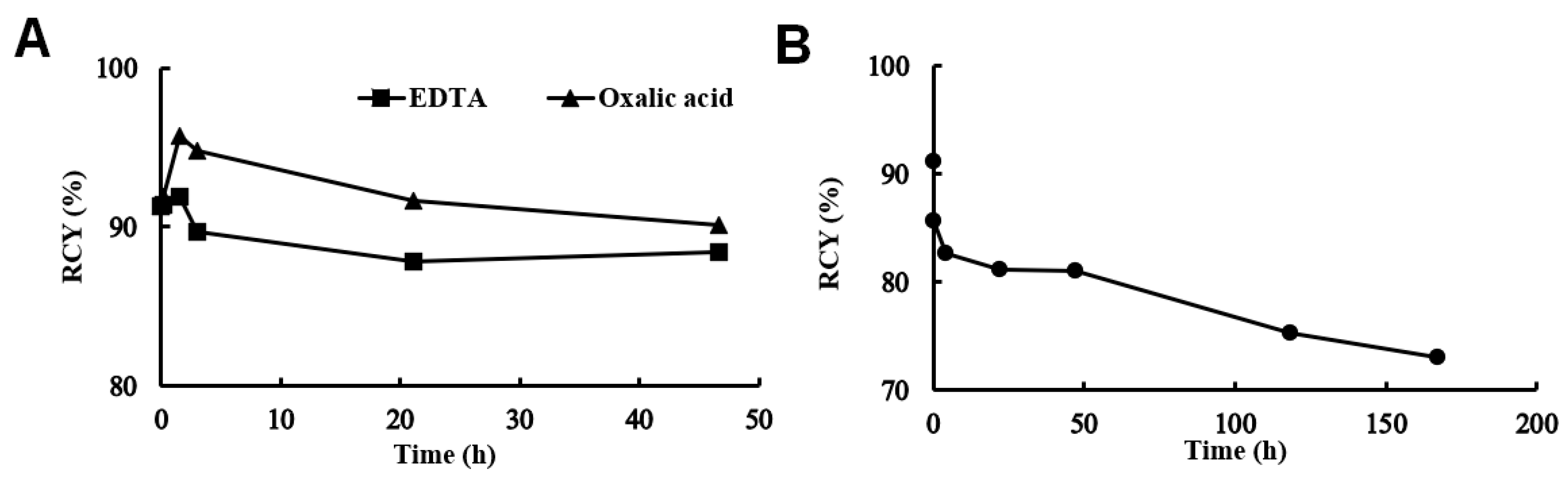
2.4. Determination of In Vitro Stability of [52Mn]Mn-DOTAGA-Bevacizumab
2.5. Cell Lines
2.6. In Vivo Cervix Carcinoma Tumor Model
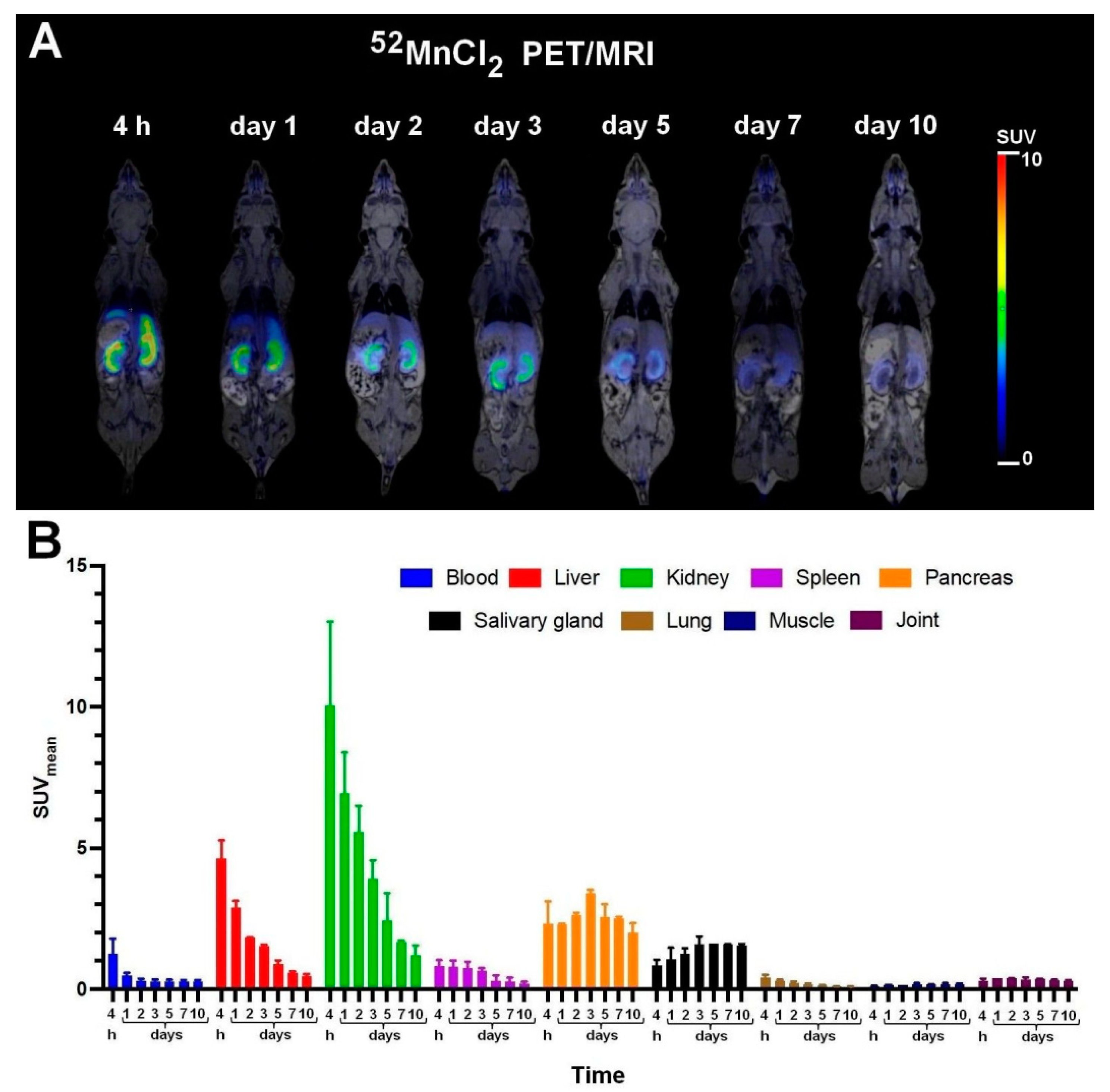
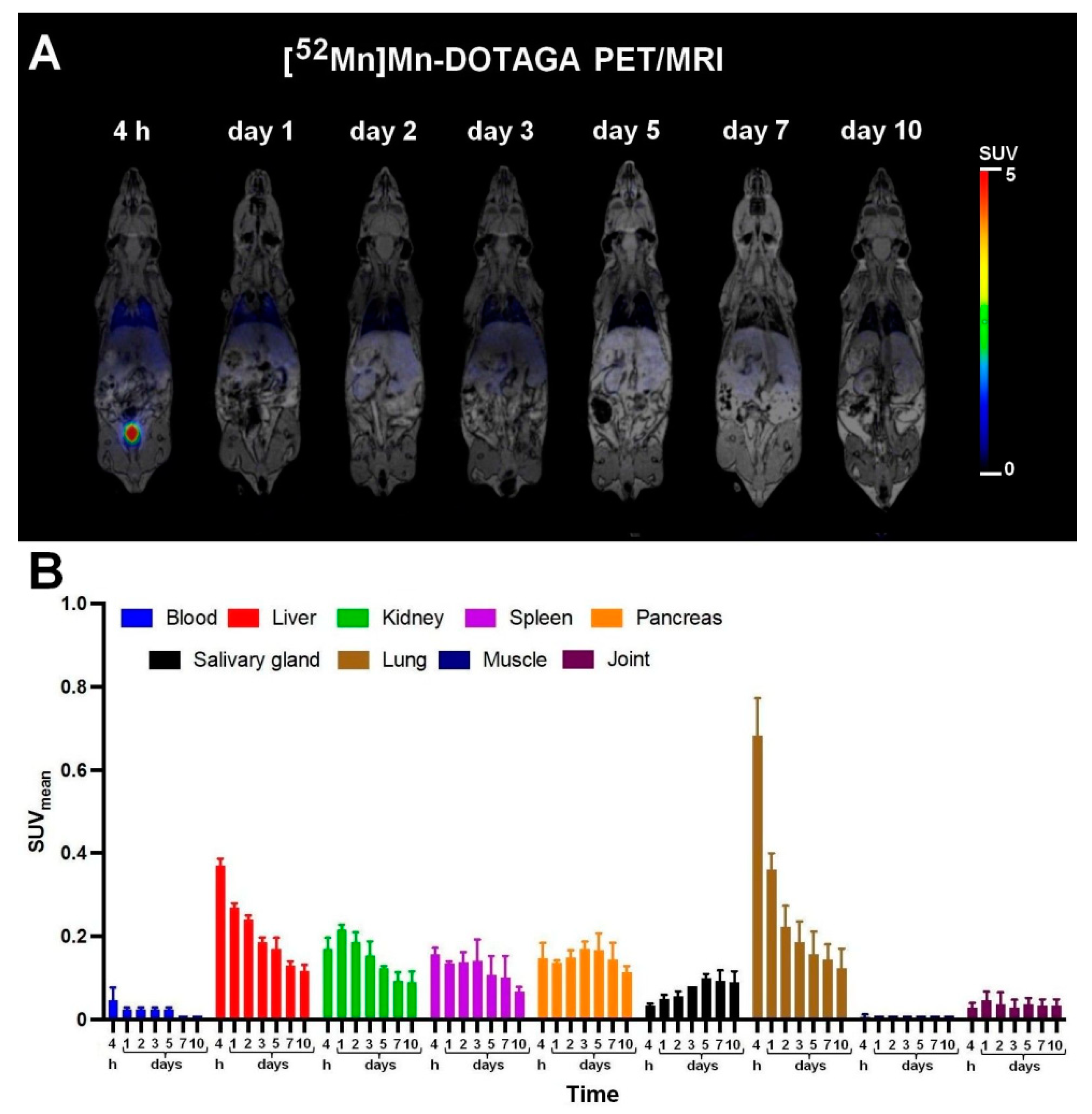
2.7. In Vivo PET/MRI Imaging
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
3.1. Radiolabeling and Characterization of [52Mn]Mn-DOTAGA-Bevacizumab
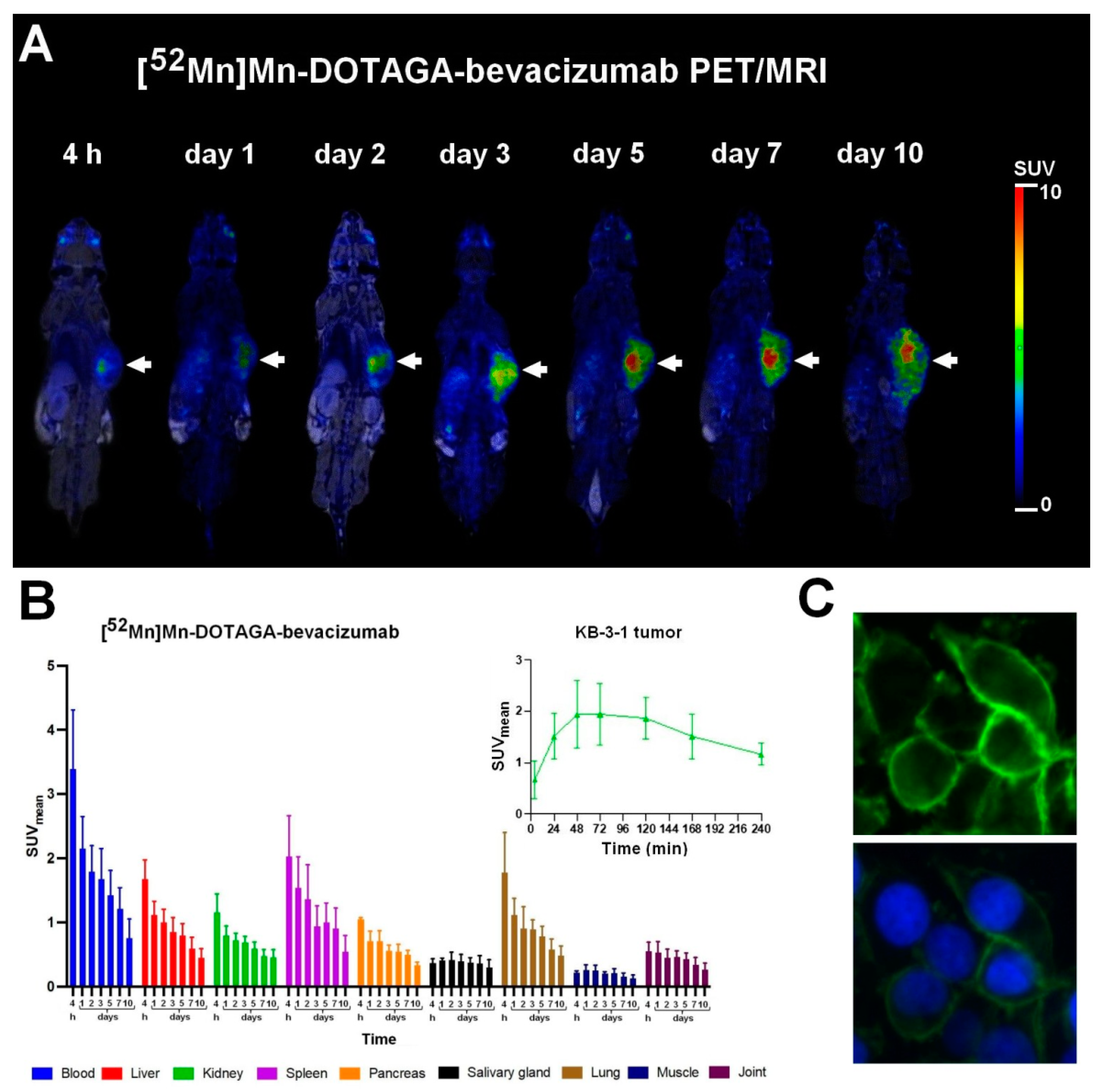
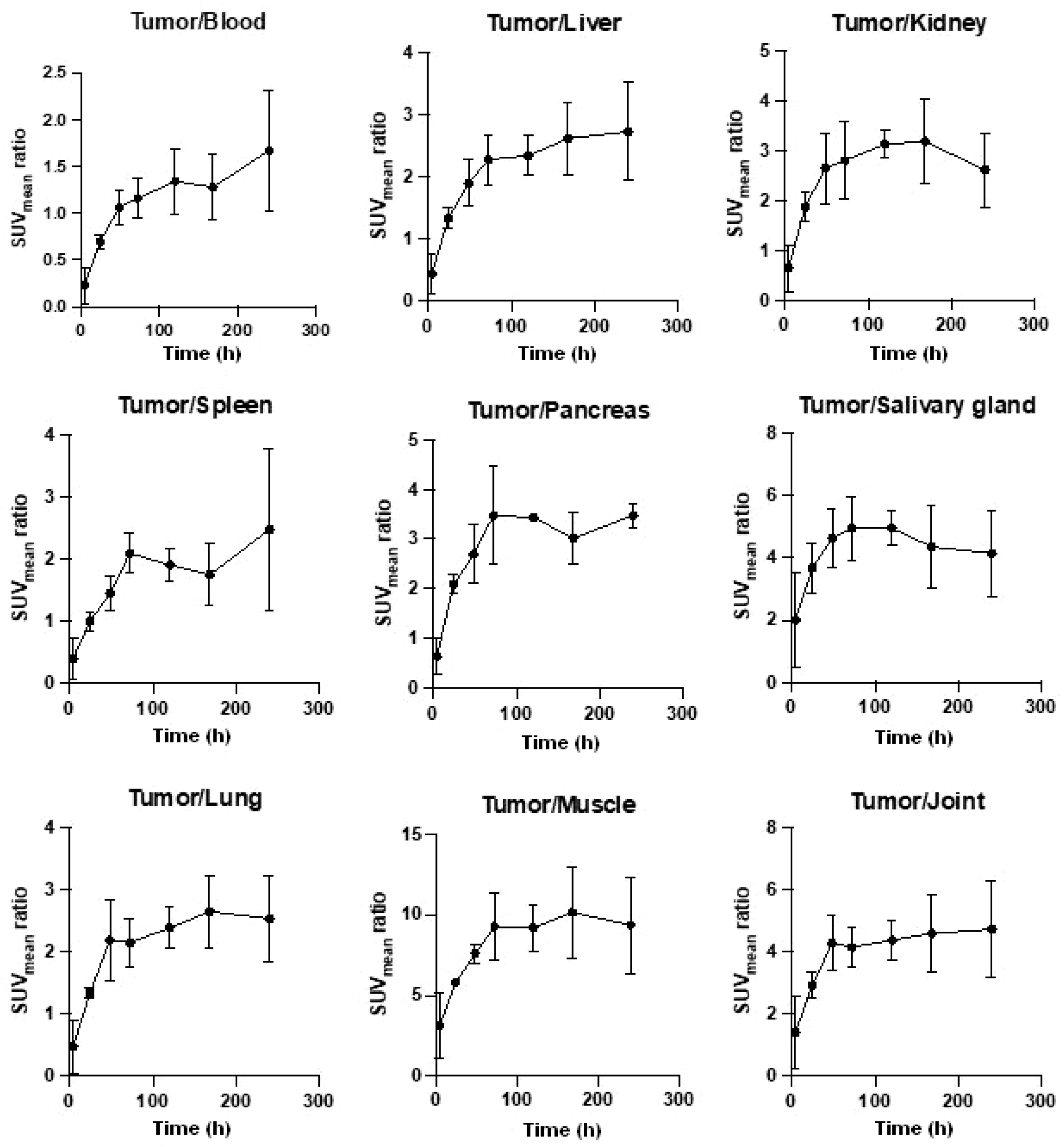
3.2. Biodistribution and PET/MRI Imaging Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 52Mn | manganese-52 isotope |
| DOTAGA | 2,2′,2″-(10-(1-carboxy-4-((4-isothiocyanatobenzyl)amino)-4-oxobutyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid |
| PET | positron emission tomography |
| RCY | radiochemical yield |
| VEGF | vascular endothelial growth factor |
References
- Buskwofie, A.; David-West, G.; Clare, C.A. A Review of Cervical Cancer: Incidence and Disparities. J. Natl. Med. Assoc. 2020, 112, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Cervical Cancer Survival Rates. Cancer 5 Year Survival Rates. Available online: https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/survival.html (accessed on 21 November 2022).
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of Incidence and Mortality of Cervical Cancer in 2018: A Worldwide Analysis. Lancet Glob. Health 2019, 8, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2019, 77, 1745–1770. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical Cancer Therapies: Current Challenges and Future Perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in Cancer Treatment: A Review of 15 Years of Clinical Experience and Future Outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Herrera, F.G.; Prior, J.O. The Role of PET/CT in Cervical Cancer. Front. Oncol. 2013, 3, 34. [Google Scholar] [PubMed]
- Chomet, M.; van Dongen, G.A.M.S.; Vugts, D.J. State of the Art in Radiolabeling of Antibodies with Common and Uncommon Radiometals for Preclinical and Clinical Immuno-PET. Bioconjug. Chem. 2021, 32, 1315–1330. [Google Scholar] [CrossRef]
- Hofmann, M.; Pichler, B.; Schölkopf, B.; Beyer, T. Towards Quantitative PET/MRI: A Review of MR-Based Attenuation Correction Techniques. Eur. J. Nucl. Med. Mol. Imaging 2008, 36, 93–104. [Google Scholar] [CrossRef]
- Taurone, S.; Galli, F.; Signore, A.; Agostinelli, E.; Dierckx, R.A.J.O.; Minni, A.; Pucci, M.; Artico, M. VEGF in Nuclear Medicine: Clinical Application in Cancer and Future Perspectives (Review). Int. J. Oncol. 2016, 49, 437–447. [Google Scholar] [CrossRef]
- Raavé, R.; Sandker, G.; Adumeau, P.; Jacobsen, C.B.; Mangin, F.; Meyer, M.; Moreau, M.; Bernhard, C.; Da Costa, L.; Dubois, A.; et al. Direct Comparison of the in Vitro and in Vivo Stability of DFO, DFO* and DFOcyclo* for 89Zr-ImmunoPET. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- Csupász, T.; Szücs, D.; Kálmán, F.K.; Hollóczki, O.; Fekete, A.; Szikra, D.; Tóth, É.; Tóth, I.; Tircsó, G. A New Oxygen Containing Pyclen-Type Ligand as a Manganese(II) Binder for MRI and 52Mn PET Applications: Equilibrium, Kinetic, Relaxometric, Structural and Radiochemical Studies. Molecules 2022, 27, 371. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Schmieder, A.H.; Wickline, S.A.; Lanza, G.M. Manganese-Based MRI Contrast Agents: Past, Present, and Future. Tetrahedron 2011, 67, 8431–8444. [Google Scholar] [CrossRef] [PubMed]
- Devreux, M.; Henoumont, C.; Dioury, F.; Boutry, S.; Vacher, O.; Elst, L.V.; Port, M.; Muller, R.N.; Sandre, O.; Laurent, S. Mn2+ Complexes with Pyclen-Based Derivatives as Contrast Agents for Magnetic Resonance Imaging: Synthesis and Relaxometry Characterization. Inorg. Chem. 2021, 60, 3604–3619. [Google Scholar] [CrossRef]
- Gale, E.M.; Atanasova, I.P.; Blasi, F.; Ay, I.; Caravan, P. A Manganese Alternative to Gadolinium for MRI Contrast. J. Am. Chem. Soc. 2015, 137, 15548–15557. [Google Scholar] [CrossRef]
- Krasznai, Z.T.; Trencsényi, G.; Krasznai, Z.; Mikecz, P.; Nizsalóczki, E.; Szalóki, G.; Szabó, J.P.; Balkay, L.; Márián, T.; Goda, K. 18FDG a PET Tumor Diagnostic Tracer Is Not a Substrate of the ABC Transporter P-Glycoprotein. Eur. J. Pharm. Sci. 2014, 64, 1–8. [Google Scholar] [CrossRef]
- Yun, J.W.; Lee, S.; Ryu, D.; Park, S.; Park, W.Y.; Joung, J.G.; Jeong, J. Biomarkers Associated with Tumor Heterogeneity in Prostate Cancer. Transl. Oncol. 2019, 12, 43–48. [Google Scholar] [CrossRef]
- Volkova, L.V.; Pashov, A.I.; Omelchuk, N.N. Cervical Carcinoma: Oncobiology and Biomarkers. Int. J. Mol. Sci. 2021, 22, 12571. [Google Scholar] [CrossRef]
- Lugat, A.; Bailly, C.; Chérel, M.; Rousseau, C.; Kraeber-Bodéré, F.; Bodet-Milin, C.; Bourgeois, M. Immuno-PET: Design Options and Clinical Proof-of-Concept. Front. Med. 2022, 9, 1026083. [Google Scholar] [CrossRef]
- Dewulf, J.; Adhikari, K.; Vangestel, C.; Wyngaert, T.V.D.; Elvas, F. Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders—An Update. Cancers 2020, 12, 1868. [Google Scholar] [CrossRef]
- Demir, I.; Muftuler, F.Z.B.; Unak, P.; Acar, C. In Vivo Investigation of Radiolabeled Bevacizumab in Healthy Rat Tissues. Braz. Arch. Biol. Technol. 2011, 54, 73–79. [Google Scholar] [CrossRef]
- Ashrafi, S.A.; Hosseinimehr, S.J.; Varmira, K.; Abedi, S.M. Radioimmunotherapy with 131I-Bevacizumab as a Specific Molecule for Cells with Overexpression of the Vascular Endothelial Growth Factor. Cancer Biother. Radiopharm. 2012, 27, 420–425. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chatterjee, G.; Ghosh, M.; Das, B.; Majumder, D. Efficacy and Toxicity Assessment of Different Antibody Based Antiangiogenic Drugs by Computational Docking Method. Adv. Bioinform. 2016, 2016, 7053712. [Google Scholar] [CrossRef]
- Yoshida, K.; Suzuki, S.; Sakata, J.; Utsumi, F.; Niimi, K.; Yoshikawa, N.; Nishino, K.; Shibata, K.; Kikkawa, F.; Kajiyama, H. The Upregulated Expression of Vascular Endothelial Growth Factor in Surgically Treated Patients with Recurrent/Radioresistant Cervical Cancer of the Uterus. Oncol. Lett. 2018, 16, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Zhu, C.; He, J.; Chen, J.; Liang, Y.; Yang, F.; Wu, X.; Ma, X. Prognostic Role of Vascular Endothelial Growth Factor in Cervical Cancer: A Meta-Analysis. Oncotarget 2017, 8, 24797–24803. [Google Scholar] [CrossRef] [PubMed]
- Khorami-Moghadam, A.; Bolouri, B.; Jalilian, A.R.; Bahrami-Samani, N.M.A.; Mazidi, S.M.; Alirezapour, B. Preclinical Evaluation of Holmium-166 Labeled Anti-VEGF-A(Bevacizumab). J. Label. Comp. Radiopharm. 2013, 56, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Nagengast, W.B.; de Vries, E.G.; Hospers, G.A.; Mulder, N.H.; de Jong, J.R.; Hollema, H.; Brouwers, A.H.; van Dongen, G.A.; Perk, L.R.; Lub-de Hooge, M.N. In Vivo VEGF Imaging with Radiolabeled Bevacizumab in a Human Ovarian Tumor Xenograft. J. Nucl. Med. 2007, 48, 1313–1319. [Google Scholar] [CrossRef]
- Stollman, T.H.; Scheer, M.G.W.; Leenders, W.P.J.; Verrijp, K.C.N.; Soede, A.C.; Oyen, W.J.G.; Ruers, T.J.M.; Boerman, O.C. Specific Imaging of VEGF-A Expression with Radiolabeled Anti-VEGF Monoclonal Antibody. Int. J. Cancer 2008, 122, 2310–2314. [Google Scholar] [CrossRef]
- Nayak, T.K.; Garmestani, K.; Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. PET Imaging of Tumor Angiogenesis in Mice with VEGF-A-Targeted 86Y-CHX-A″-DTPA-Bevacizumab. Int. J. Cancer 2010, 128, 920–926. [Google Scholar] [CrossRef]
- Camacho, X.; García, M.F.; Calzada, V.; Fernández, M.; Chabalgoity, J.A.; Moreno, M.; Barbosa de Aguiar, R.; Alonso, O.; Gambini, J.P.; Chammas, R.; et al. [99mTc(CO)3]-Radiolabeled Bevacizumab: In Vitro and in Vivo Evaluation in a Melanoma Model. Oncology 2013, 84, 200–209. [Google Scholar] [CrossRef]
- Gaykema, S.B.M.; Brouwers, A.H.; Lub-de Hooge, M.N.; Pleijhuis, R.G.; Timmer-Bosscha, H.; Pot, L.; van Dam, G.M.; van der Meulen, S.B.; de Jong, J.R.; Bart, J.; et al. 89Zr-Bevacizumab PET Imaging in Primary Breast Cancer. J. Nucl. Med. 2013, 54, 1014–1018. [Google Scholar] [CrossRef]
- Menke-van der Houven van Oordt, C.W.; McGeoch, A.; Bergstrom, M.; McSherry, I.; Smith, D.A.; Cleveland, M.; Al-Azzam, W.; Chen, L.; Verheul, H.; Hoekstra, O.S.; et al. Immuno-PET Imaging to Assess Target Engagement: Experience from 89Zr-Anti-HER3 MAb (GSK2849330) in Patients with Solid Tumors. J. Nucl. Med. 2019, 60, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.H.; Veldhuijzen van Zanten, S.E.M.; van Vuurden, D.G.; Huisman, M.C.; Vugts, D.J.; Hoekstra, O.S.; van Dongen, G.A.; Kaspers, G.J.L. Molecular Drug Imaging: 89Zr-Bevacizumab PET in Children with Diffuse Intrinsic Pontine Glioma. J. Nucl. Med. 2016, 58, 711–716. [Google Scholar] [CrossRef]
- Gaykema, S.B.M.; Schröder, C.P.; Vitfell-Rasmussen, J.; Chua, S.; Oude Munnink, T.H.; Brouwers, A.H.; Bongaerts, A.H.H.; Akimov, M.; Fernandez-Ibarra, C.; Lub-de Hooge, M.N.; et al. 89Zr-Trastuzumab and 89Zr-Bevacizumab PET to Evaluate the Effect of the HSP90 Inhibitor NVP-AUY922 in Metastatic Breast Cancer Patients. Clin. Cancer Res. 2014, 20, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Hegi-Johnson, F.; Rudd, S.E.; Wichmann, C.; Akhurst, T.; Roselt, P.; Trinh, J.; John, T.; Devereux, L.; Donnelly, P.S.; Hicks, R.; et al. ImmunoPET: IMaging of Cancer ImMUNOtherapy Targets with Positron Emission Tomography: A Phase 0/1 Study Characterising PD-L1 with89Zr-Durvalumab (MEDI4736) PET/CT in Stage III NSCLC Patients Receiving Chemoradiation Study Protocol. BMJ Open 2022, 12, e056708. [Google Scholar] [CrossRef] [PubMed]
- Jauw, Y.W.S.; Huisman, M.C.; Nayak, T.K.; Vugts, D.J.; Christen, R.; Naegelen, V.M.; Ruettinger, D.; Heil, F.; Lammertsma, A.A.; Verheul, H.M.W.; et al. Assessment of Target-Mediated Uptake with Immuno-PET: Analysis of a Phase I Clinical Trial with an Anti-CD44 Antibody. EJNMMI Res. 2018, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Wojdowska, W.; Karczmarczyk, U.; Balog, L.; Sawicka, A.; Pöstényi, Z.; Kovács-Haász, V.; Polyák, A.; Laszuk, E.; Mikołajczak, R.; Garnuszek, P. Impact of DOTA-Chelators on the Antitumor Activity of 177Lu-DOTA-Rituximab Preparations in Lymphoma Tumor-Bearing Mice. Cancer Biother. Radiopharm. 2020, 35, 558–562. [Google Scholar] [CrossRef]
- Thiele, N.A.; Wilson, J.J. Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother. Radiopharm. 2018, 33, 336–348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csikos, C.; Vágner, A.; Nagy, G.; Kálmán-Szabó, I.; Szabó, J.P.; Ngo, M.T.; Szoboszlai, Z.; Szikra, D.; Krasznai, Z.T.; Trencsényi, G.; et al. In Vivo Preclinical Assessment of the VEGF Targeting Potential of the Newly Synthesized [52Mn]Mn-DOTAGA-Bevacizumab Using Experimental Cervix Carcinoma Mouse Model. Diagnostics 2023, 13, 236. https://doi.org/10.3390/diagnostics13020236
Csikos C, Vágner A, Nagy G, Kálmán-Szabó I, Szabó JP, Ngo MT, Szoboszlai Z, Szikra D, Krasznai ZT, Trencsényi G, et al. In Vivo Preclinical Assessment of the VEGF Targeting Potential of the Newly Synthesized [52Mn]Mn-DOTAGA-Bevacizumab Using Experimental Cervix Carcinoma Mouse Model. Diagnostics. 2023; 13(2):236. https://doi.org/10.3390/diagnostics13020236
Chicago/Turabian StyleCsikos, Csaba, Adrienn Vágner, Gábor Nagy, Ibolya Kálmán-Szabó, Judit P. Szabó, Minh Toan Ngo, Zoltán Szoboszlai, Dezső Szikra, Zoárd Tibor Krasznai, György Trencsényi, and et al. 2023. "In Vivo Preclinical Assessment of the VEGF Targeting Potential of the Newly Synthesized [52Mn]Mn-DOTAGA-Bevacizumab Using Experimental Cervix Carcinoma Mouse Model" Diagnostics 13, no. 2: 236. https://doi.org/10.3390/diagnostics13020236
APA StyleCsikos, C., Vágner, A., Nagy, G., Kálmán-Szabó, I., Szabó, J. P., Ngo, M. T., Szoboszlai, Z., Szikra, D., Krasznai, Z. T., Trencsényi, G., & Garai, I. (2023). In Vivo Preclinical Assessment of the VEGF Targeting Potential of the Newly Synthesized [52Mn]Mn-DOTAGA-Bevacizumab Using Experimental Cervix Carcinoma Mouse Model. Diagnostics, 13(2), 236. https://doi.org/10.3390/diagnostics13020236







