Artificial Intelligence and Radiomics: Clinical Applications for Patients with Advanced Melanoma Treated with Immunotherapy
Abstract
:1. Introduction
2. Development of Immunotherapy
3. New Patterns of Response and Progression with Immunotherapy
4. Melanoma Response to Immunotherapy
5. Immune-Related Adverse Events
6. Intratumoral Immunotherapy
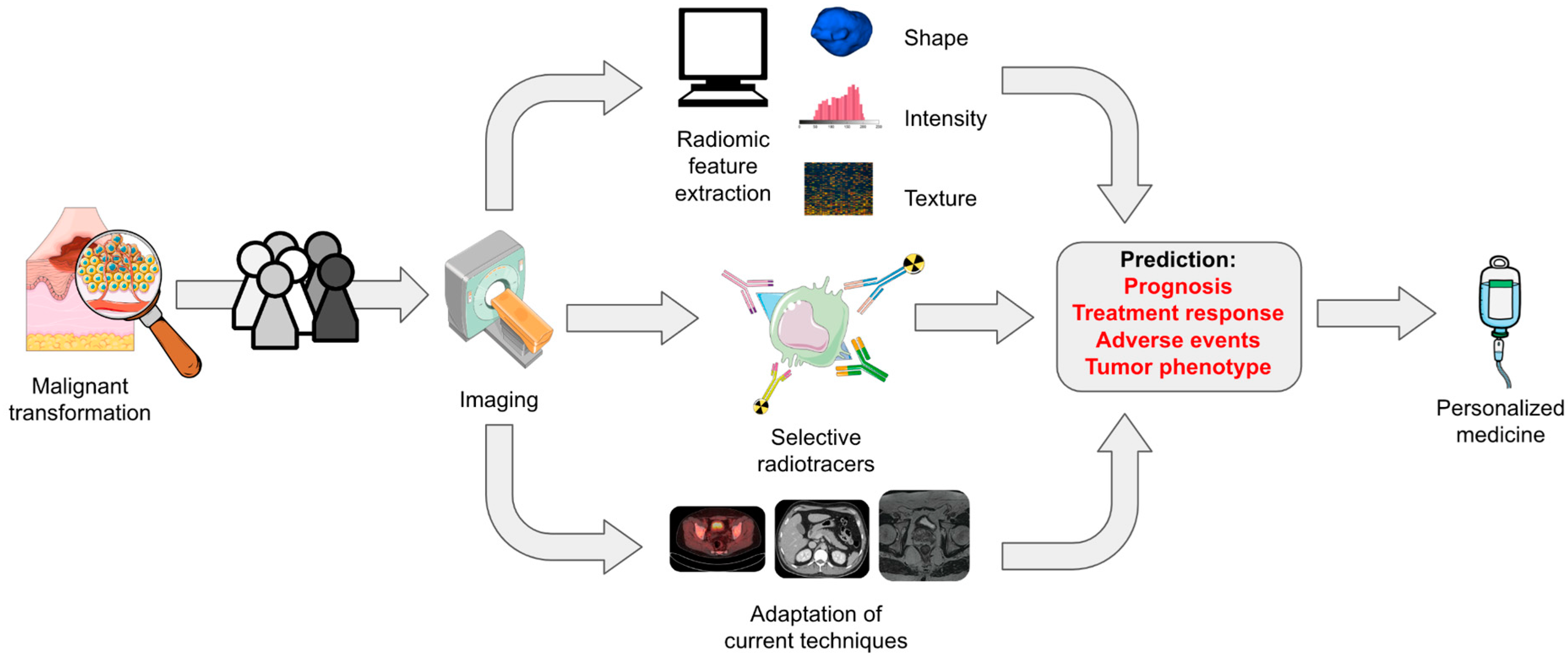
7. AI and Radiomics: Concept
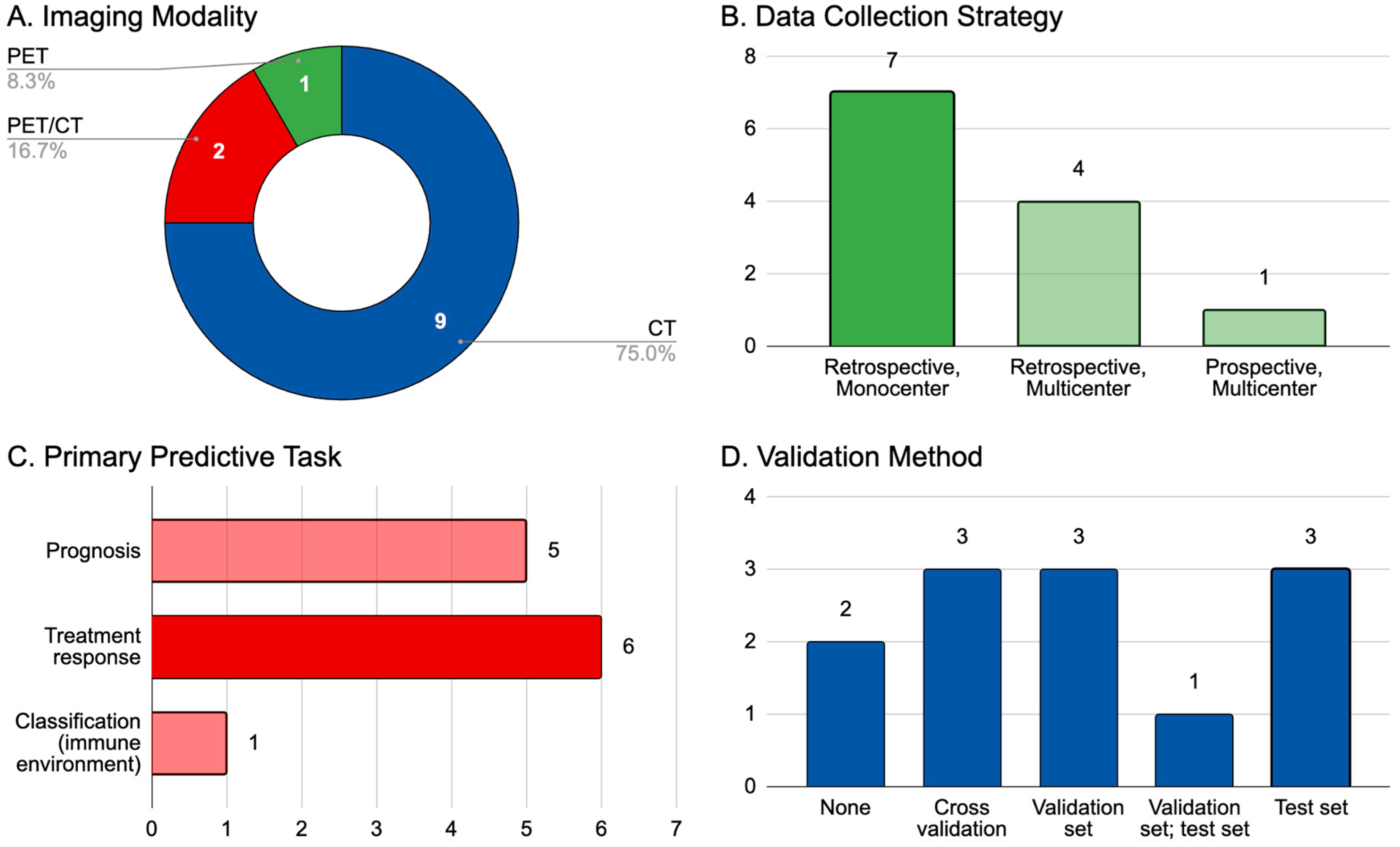
8. AI and Radiomics: Current Landscape in Relation to Melanoma Imaging
9. AI in Radiomics: Predictive Aims
10. AI and Radiomics: Technical Limitations in the Current Literature
11. New Advances and Future Directions
11.1. CT-Based Sarcopenia Measurement
11.2. Adaptation of Existing Imaging Techniques
11.3. Quantification of Inter-Lesion Heterogeneity
11.4. Optical Coherence Tomography
11.5. ImmunoPET Imaging
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shen, W.; Sakamoto, N.; Yang, L. Melanoma-Specific Mortality and Competing Mortality in Patients with Non-Metastatic Malignant Melanoma: A Population-Based Analysis. BMC Cancer 2016, 16, 413. [Google Scholar] [CrossRef] [PubMed]
- Melanoma Research Alliance Melanoma Survival Rates. Available online: https://www.curemelanoma.org/about-melanoma/melanoma-staging/melanoma-survival-rates (accessed on 27 March 2023).
- Gurzu, S.; Beleaua, M.A.; Jung, I. The Role of Tumor Microenvironment in Development and Progression of Malignant Melanomas—A Systematic Review. Rom. J. Morphol. Embryol. 2018, 59, 23–28. [Google Scholar] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Chiou, V.L.; Burotto, M. Pseudoprogression and Immune-Related Response in Solid Tumors. J. Clin. Oncol. 2015, 33, 3541–3543. [Google Scholar] [CrossRef]
- Egen, J.G.; Kuhns, M.S.; Allison, J.P. CTLA-4: New Insights into Its Biological Function and Use in Tumor Immunotherapy. Nat. Immunol. 2002, 3, 611–618. [Google Scholar] [CrossRef]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A Rheostat for Immune Responses: The Unique Properties of PD-1 and Their Advantages for Clinical Application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non–Small Cell Lung Cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Batlevi, C.L.; Matsuki, E.; Brentjens, R.J.; Younes, A. Novel Immunotherapies in Lymphoid Malignancies. Nat. Rev. Clin. Oncol. 2016, 13, 25–40. [Google Scholar]
- Hemminki, O.; Dos Santos, J.M.; Hemminki, A. Oncolytic Viruses for Cancer Immunotherapy. J. Hematol. Oncol. 2020, 13, 84. [Google Scholar] [CrossRef]
- Lathwal, A.; Kumar, R.; Raghava, G.P.S. OvirusTdb: A Database of Oncolytic Viruses for the Advancement of Therapeutics in Cancer. Virology 2020, 548, 109–116. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined Nivolumab and Ipilimumab versus Ipilimumab Alone in Patients with Advanced Melanoma: 2-Year Overall Survival Outcomes in a Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.S.; Dercle, L.; Goldmacher, G.V.; Yang, H.; Connors, D.; Tang, Y.; Karovic, S.; Zhao, B.; Carvajal, R.D.; Robert, C.; et al. Comparing RECIST 1.1 and IRECIST in Advanced Melanoma Patients Treated with Pembrolizumab in a Phase II Clinical Trial. Eur. Radiol. 2021, 31, 1853–1862. [Google Scholar] [CrossRef]
- Humbert, O.; Chardin, D. Dissociated Response in Metastatic Cancer: An Atypical Pattern Brought Into the Spotlight With Immunotherapy. Front. Oncol. 2020, 10, 566297. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.-C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Eigentler, T.K.; Keilholz, U.; Hauschild, A.; Kirkwood, J.M. Systematic Review of Medical Treatment in Melanoma: Current Status and Future Prospects. Oncologist 2011, 16, 5–24. [Google Scholar] [CrossRef]
- Huang, A.C.; Zappasodi, R. A Decade of Checkpoint Blockade Immunotherapy in Melanoma: Understanding the Molecular Basis for Immune Sensitivity and Resistance. Nat. Immunol. 2022, 23, 660–670. [Google Scholar] [CrossRef]
- Ralli, M.; Botticelli, A.; Visconti, I.C.; Angeletti, D.; Fiore, M.; Marchetti, P.; Lambiase, A.; de Vincentiis, M.; Greco, A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020, 2020, 9235638. [Google Scholar] [CrossRef]
- Gide, T.N.; Wilmott, J.S.; Scolyer, R.A.; Long, G.V. Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Metastatic Melanoma. Clin. Cancer Res. 2018, 24, 1260–1270. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Smyth, M.J.; Teng, M.W. Improved Mouse Models to Assess Tumour Immunity and IrAEs after Combination Cancer Immunotherapies. Clin. Transl. Immunol. 2014, 3, e22. [Google Scholar] [CrossRef] [PubMed]
- Dercle, L.; Sun, S.; Seban, R.-D.; Mekki, A.; Sun, R.; Tselikas, L.; Hans, S.; Bernard-Tessier, A.; Bouvier, F.M.; Aide, N.; et al. Emerging and Evolving Concepts in Cancer Immunotherapy Imaging. Radiology 2023, 306, e239003. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus Ipilimumab or Nivolumab Alone versus Ipilimumab Alone in Advanced Melanoma (CheckMate 067): 4-Year Outcomes of a Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Park, H.; Malone, D.C.; Wang, C.-Y.; Wilson, D.L.; Yeh, Y.-M.; Van Boemmel-Wegmann, S.; Lo-Ciganic, W.-H. Immune Checkpoint Inhibitors and Immune-Related Adverse Events in Patients With Advanced Melanoma: A Systematic Review and Network Meta-Analysis. JAMA Netw. Open 2020, 3, e201611. [Google Scholar] [CrossRef]
- Marabelle, A.; Andtbacka, R.; Harrington, K.; Melero, I.; Leidner, R.; de Baere, T.; Robert, C.; Ascierto, P.A.; Baurain, J.-F.; Imperiale, M.; et al. Starting the Fight in the Tumor: Expert Recommendations for the Development of Human Intratumoral Immunotherapy (HIT-IT). Ann. Oncol. 2018, 29, 2163–2174. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.I.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2018, 174, 1031–1032. [Google Scholar] [CrossRef]
- Rager, T.; Eckburg, A.; Patel, M.; Qiu, R.; Gantiwala, S.; Dovalovsky, K.; Fan, K.; Lam, K.; Roesler, C.; Rastogi, A.; et al. Treatment of Metastatic Melanoma with a Combination of Immunotherapies and Molecularly Targeted Therapies. Cancers 2022, 14, 3779. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Valenti, F.; Falcone, I.; Ungania, S.; Desiderio, F.; Giacomini, P.; Bazzichetto, C.; Conciatori, F.; Gallo, E.; Cognetti, F.; Ciliberto, G.; et al. Precision Medicine and Melanoma: Multi-Omics Approaches to Monitoring the Immunotherapy Response. Int. J. Mol. Sci. 2021, 22, 3837. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Chen, D.S. Immune Escape to PD-L1/PD-1 Blockade: Seven Steps to Success (or Failure). Ann. Oncol. 2016, 27, 1492–1504. [Google Scholar] [CrossRef]
- Hegde, P.S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016, 22, 1865–1874. [Google Scholar] [CrossRef]
- Dercle, L.; McGale, J.; Sun, S.; Marabelle, A.; Yeh, R.; Deutsch, E.; Mokrane, F.-Z.; Farwell, M.; Ammari, S.; Schoder, H.; et al. Artificial Intelligence and Radiomics: Fundamentals, Applications, and Challenges in Immunotherapy. J. ImmunoTherapy Cancer 2022, 10, e005292. [Google Scholar] [CrossRef]
- Guerrisi, A.; Russillo, M.; Loi, E.; Ganeshan, B.; Ungania, S.; Desiderio, F.; Bruzzaniti, V.; Falcone, I.; Renna, D.; Ferraresi, V.; et al. Exploring CT Texture Parameters as Predictive and Response Imaging Biomarkers of Survival in Patients With Metastatic Melanoma Treated With PD-1 Inhibitor Nivolumab: A Pilot Study Using a Delta-Radiomics Approach. Front. Oncol. 2021, 11, 704607. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Mao, L.-L.; Zhou, Z.-G.; Si, L.; Zhu, H.-T.; Chen, X.; Zhou, M.-J.; Sun, Y.-S.; Guo, J. Pilot Study of CT-Based Radiomics Model for Early Evaluation of Response to Immunotherapy in Patients With Metastatic Melanoma. Front. Oncol. 2020, 10, 1524. [Google Scholar] [CrossRef]
- Dittrich, D.; Pyka, T.; Scheidhauer, K.; Lütje, S.; Essler, M.; Bundschuh, R.A. Textural Features in FDG-PET/CT Can Predict Outcome in Melanoma Patients to Treatment with Vemurafenib and Ipililumab. Nuklearmedizin 2020, 59, 228–234. [Google Scholar] [CrossRef]
- Schraag, A.; Klumpp, B.; Afat, S.; Gatidis, S.; Nikolaou, K.; Eigentler, T.K.; Othman, A.E. Baseline Clinical and Imaging Predictors of Treatment Response and Overall Survival of Patients with Metastatic Melanoma Undergoing Immunotherapy. Eur. J. Radiol. 2019, 121, 108688. [Google Scholar] [CrossRef]
- Brendlin, A.S.; Peisen, F.; Almansour, H.; Afat, S.; Eigentler, T.; Amaral, T.; Faby, S.; Calvarons, A.F.; Nikolaou, K.; Othman, A.E. A Machine Learning Model Trained on Dual-Energy CT Radiomics Significantly Improves Immunotherapy Response Prediction for Patients with Stage IV Melanoma. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef]
- Aoude, L.G.; Wong, B.Z.Y.; Bonazzi, V.F.; Brosda, S.; Walters, S.B.; Koufariotis, L.T.; Naeini, M.M.; Pearson, J.V.; Oey, H.; Patel, K.; et al. Radiomics Biomarkers Correlate with CD8 Expression and Predict Immune Signatures in Melanoma Patients. Mol. Cancer Res. 2021, 19, 950–956. [Google Scholar] [CrossRef]
- Bonnin, A.; Durot, C.; Barat, M.; Djelouah, M.; Grange, F.; Mulé, S.; Soyer, P.; Hoeffel, C. CT Texture Analysis as a Predictor of Favorable Response to Anti-PD1 Monoclonal Antibodies in Metastatic Skin Melanoma. Diagn. Interv. Imaging 2022, 103, 97–102. [Google Scholar] [CrossRef]
- Dercle, L.; Zhao, B.; Gönen, M.; Moskowitz, C.S.; Firas, A.; Beylergil, V.; Connors, D.E.; Yang, H.; Lu, L.; Fojo, T.; et al. Early Readout on Overall Survival of Patients With Melanoma Treated With Immunotherapy Using a Novel Imaging Analysis. JAMA Oncol. 2022, 8, 385–392. [Google Scholar] [CrossRef]
- Flaus, A.; Habouzit, V.; de Leiris, N.; Vuillez, J.-P.; Leccia, M.-T.; Simonson, M.; Perrot, J.-L.; Cachin, F.; Prevot, N. Outcome Prediction at Patient Level Derived from Pre-Treatment 18F-FDG PET Due to Machine Learning in Metastatic Melanoma Treated with Anti-PD1 Treatment. Diagnostics 2022, 12, 388. [Google Scholar] [CrossRef]
- Sun, R.; Limkin, E.J.; Vakalopoulou, M.; Dercle, L.; Champiat, S.; Han, S.R.; Verlingue, L.; Brandao, D.; Lancia, A.; Ammari, S.; et al. A Radiomics Approach to Assess Tumour-Infiltrating CD8 Cells and Response to Anti-PD-1 or Anti-PD-L1 Immunotherapy: An Imaging Biomarker, Retrospective Multicohort Study. Lancet Oncol. 2018, 19, 1180–1191. [Google Scholar] [CrossRef]
- Trebeschi, S.; Drago, S.G.; Birkbak, N.J.; Kurilova, I.; Cǎlin, A.M.; Delli Pizzi, A.; Lalezari, F.; Lambregts, D.M.J.; Rohaan, M.W.; Parmar, C.; et al. Predicting Response to Cancer Immunotherapy Using Noninvasive Radiomic Biomarkers. Ann. Oncol. 2019, 30, 998–1004. [Google Scholar] [CrossRef]
- Sun, R.; Sundahl, N.; Hecht, M.; Putz, F.; Lancia, A.; Rouyar, A.; Milic, M.; Carré, A.; Battistella, E.; Alvarez Andres, E.; et al. Radiomics to Predict Outcomes and Abscopal Response of Patients with Cancer Treated with Immunotherapy Combined with Radiotherapy Using a Validated Signature of CD8 Cells. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Bilen, M.A.; Martini, D.J.; Liu, Y.; Shabto, J.M.; Brown, J.T.; Williams, M.; Khan, A.I.; Speak, A.; Lewis, C.; Collins, H.; et al. Combined Effect of Sarcopenia and Systemic Inflammation on Survival in Patients with Advanced Stage Cancer Treated with Immunotherapy. Oncologist 2020, 25, e528–e535. [Google Scholar] [CrossRef]
- Wang, J.; Cao, L.; Xu, S. Sarcopenia Affects Clinical Efficacy of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer Patients: A Systematic Review and Meta-Analysis. Int. Immunopharmacol. 2020, 88, 106907. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.-J.; Wienke, A. Role of Sarcopenia in Advanced Malignant Cutaneous Melanoma Treated with Immunotherapy: A Meta-Analysis. Oncology 2022, 100, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.; Jogiat, U.; Baracos, V.E.; McCall, M.; Eurich, D.T.; Sawyer, M.B. CT-Based Assessment of Body Composition and Skeletal Muscle in Melanoma: A Systematic Review. Clin. Nutr. ESPEN 2021, 45, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Umemura, Y.; Wang, D.; Peck, K.K.; Flynn, J.; Zhang, Z.; Fatovic, R.; Anderson, E.S.; Beal, K.; Shoushtari, A.N.; Kaley, T.; et al. DCE-MRI Perfusion Predicts Pseudoprogression in Metastatic Melanoma Treated with Immunotherapy. J. Neurooncol. 2020, 146, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ayati, N.; Sadeghi, R.; Kiamanesh, Z.; Lee, S.T.; Zakavi, S.R.; Scott, A.M. The Value of 18F-FDG PET/CT for Predicting or Monitoring Immunotherapy Response in Patients with Metastatic Melanoma: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 428–448. [Google Scholar] [CrossRef]
- Seban, R.-D.; Nemer, J.S.; Marabelle, A.; Yeh, R.; Deutsch, E.; Ammari, S.; Moya-Plana, A.; Mokrane, F.-Z.; Gartrell, R.D.; Finkel, G.; et al. Prognostic and Theranostic 18F-FDG PET Biomarkers for Anti-PD1 Immunotherapy in Metastatic Melanoma: Association with Outcome and Transcriptomics. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2298–2310. [Google Scholar] [CrossRef]
- Sun, R.; Lerousseau, M.; Briend-Diop, J.; Routier, E.; Roy, S.; Henry, T.; Ka, K.; Jiang, R.; Temar, N.; Carré, A.; et al. Radiomics to Evaluate Interlesion Heterogeneity and to Predict Lesion Response and Patient Outcomes Using a Validated Signature of CD8 Cells in Advanced Melanoma Patients Treated with Anti-PD1 Immunotherapy. J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef]
- Sun, R.; Henry, T.; Laville, A.; Carré, A.; Hamaoui, A.; Bockel, S.; Chaffai, I.; Levy, A.; Chargari, C.; Robert, C.; et al. Imaging Approaches and Radiomics: Toward a New Era of Ultraprecision Radioimmunotherapy? J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef]
- Wan, B.; Ganier, C.; Du-Harpur, X.; Harun, N.; Watt, F.M.; Patalay, R.; Lynch, M.D. Applications and Future Directions for Optical Coherence Tomography in Dermatology. Br. J. Dermatol. 2021, 184, 1014–1022. [Google Scholar] [CrossRef]
- Turani, Z.; Fatemizadeh, E.; Blumetti, T.; Daveluy, S.; Moraes, A.F.; Chen, W.; Mehregan, D.; Andersen, P.E.; Nasiriavanaki, M. Optical Radiomic Signatures Derived from Optical Coherence Tomography Images Improve Identification of Melanoma. Cancer Res. 2019, 79, 2021–2030. [Google Scholar] [CrossRef]
- Freise, A.C.; Wu, A.M. In Vivo Imaging with Antibodies and Engineered Fragments. Mol. Immunol. 2015, 67, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, F.; Huang, Y.; Yin, N.; Chu, J.; Ma, Y.; Pettigrew, R.I.; Hamilton, D.J.; Martin, D.R.; Li, Z. Dynamic Tumor-Specific MHC-II Immuno-PET Predicts the Efficacy of Checkpoint Inhibitor Immunotherapy in Melanoma. J. Nucl. Med. 2022, 63, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Bridgwater, C.; Geller, A.; Hu, X.; Burlison, J.A.; Zhang, H.-G.; Yan, J.; Guo, H. 89Zr-Labeled Anti-PD-L1 Antibody Fragment for Evaluating In Vivo PD-L1 Levels in Melanoma Mouse Model. Cancer Biother. Radiopharm. 2020, 35, 549–557. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGale, J.; Hama, J.; Yeh, R.; Vercellino, L.; Sun, R.; Lopci, E.; Ammari, S.; Dercle, L. Artificial Intelligence and Radiomics: Clinical Applications for Patients with Advanced Melanoma Treated with Immunotherapy. Diagnostics 2023, 13, 3065. https://doi.org/10.3390/diagnostics13193065
McGale J, Hama J, Yeh R, Vercellino L, Sun R, Lopci E, Ammari S, Dercle L. Artificial Intelligence and Radiomics: Clinical Applications for Patients with Advanced Melanoma Treated with Immunotherapy. Diagnostics. 2023; 13(19):3065. https://doi.org/10.3390/diagnostics13193065
Chicago/Turabian StyleMcGale, Jeremy, Jakob Hama, Randy Yeh, Laetitia Vercellino, Roger Sun, Egesta Lopci, Samy Ammari, and Laurent Dercle. 2023. "Artificial Intelligence and Radiomics: Clinical Applications for Patients with Advanced Melanoma Treated with Immunotherapy" Diagnostics 13, no. 19: 3065. https://doi.org/10.3390/diagnostics13193065
APA StyleMcGale, J., Hama, J., Yeh, R., Vercellino, L., Sun, R., Lopci, E., Ammari, S., & Dercle, L. (2023). Artificial Intelligence and Radiomics: Clinical Applications for Patients with Advanced Melanoma Treated with Immunotherapy. Diagnostics, 13(19), 3065. https://doi.org/10.3390/diagnostics13193065







