Multimodality Imaging in Patients with Hypertrophic Cardiomyopathy and Atrial Fibrillation
Abstract
:1. Introduction
2. AF in HCM
3. Multimodality Imaging in HCM and AF
3.1. Transthoracic Echocardiography (TTE)
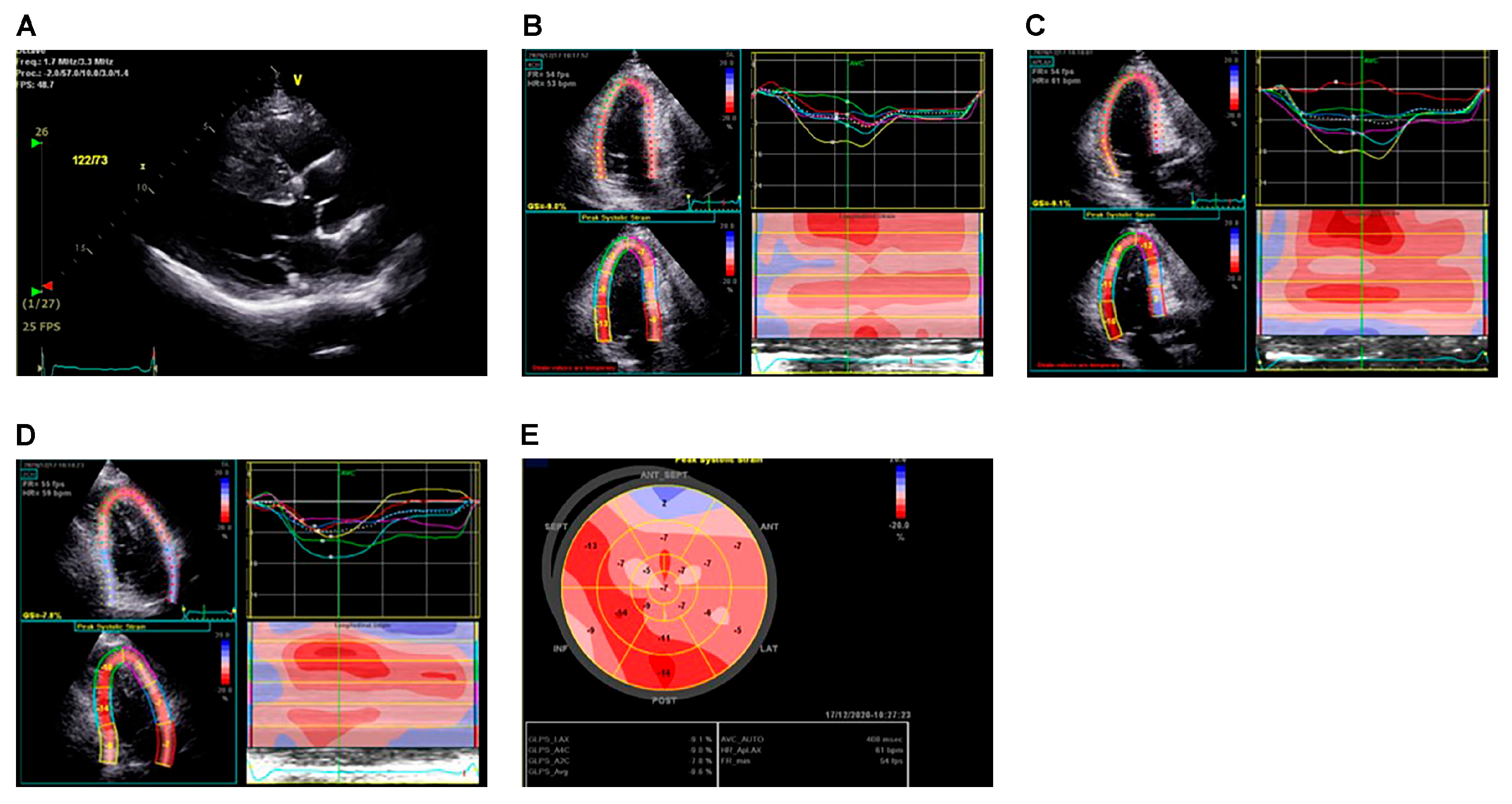
3.1.1. LA Assessment
3.1.2. LV Assessment
3.2. CMR
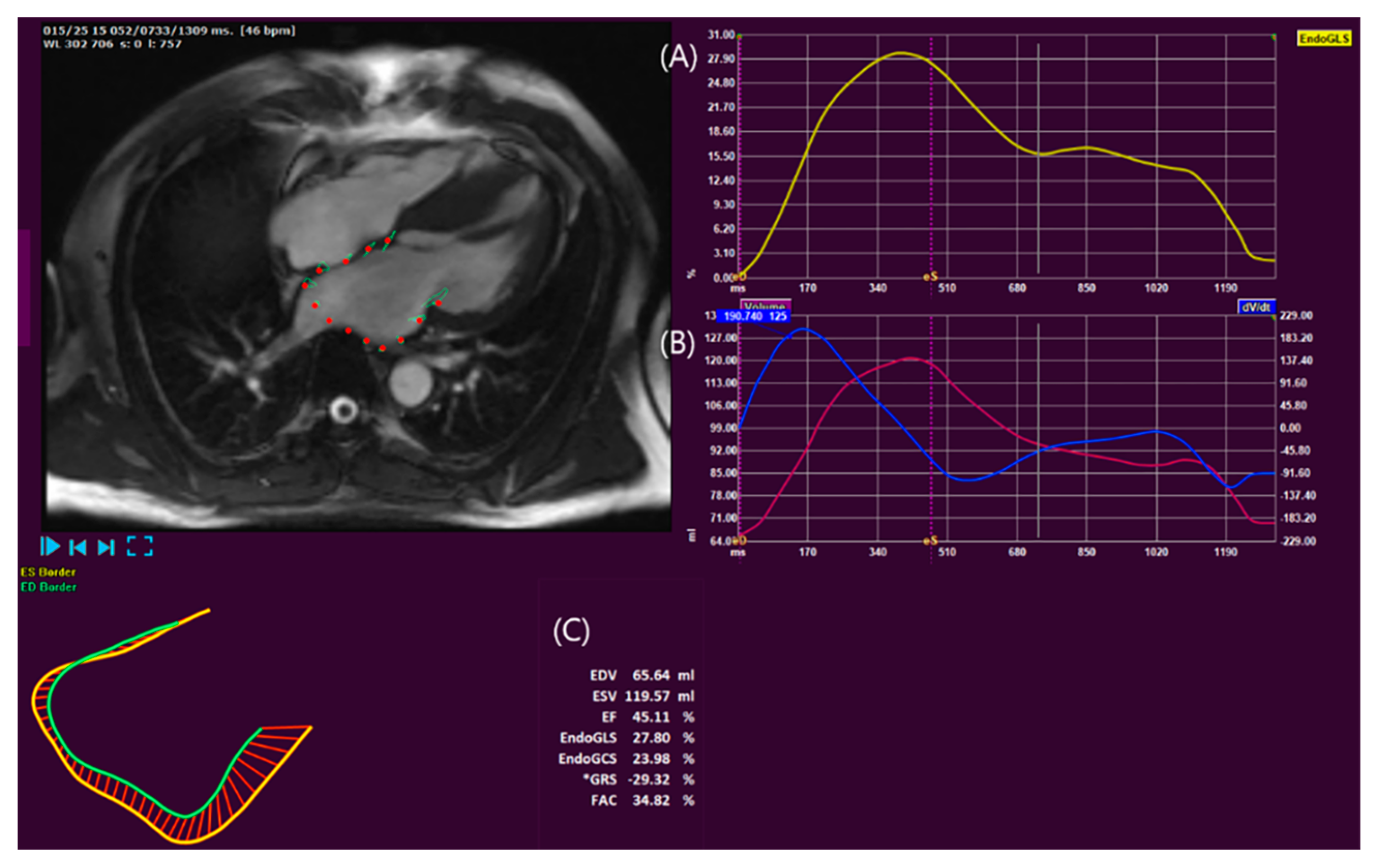
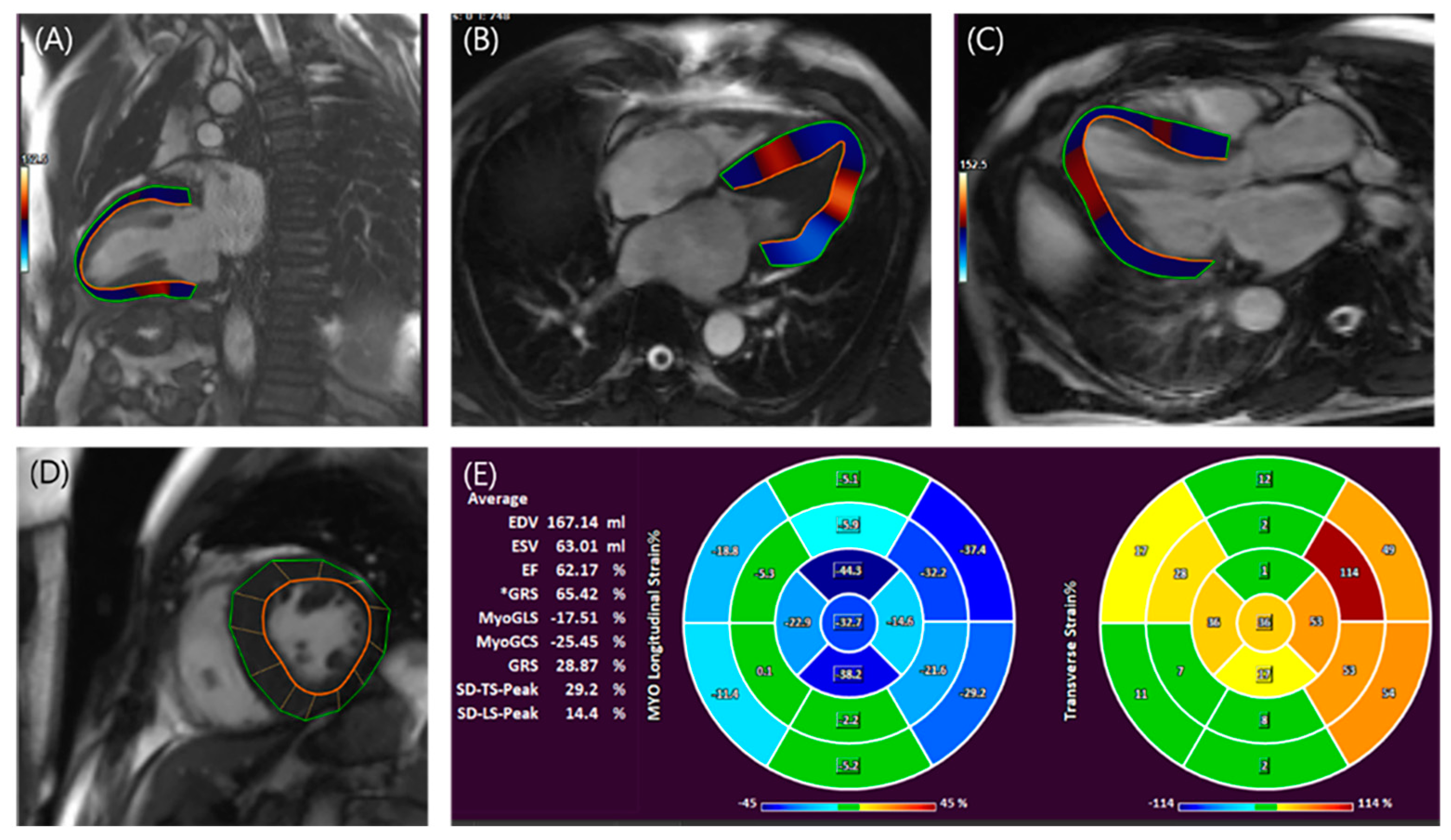
3.2.1. LA assessment
3.2.2. LV Assessment
4. The Genetic Influence on Atrial Myopathy and AF in Patients with HCM
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maron, B.J.; Gardin, J.M.; Flack, J.M.; Gidding, S.S.; Kurosaki, T.T.; Bild, D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995, 92, 785–789. [Google Scholar] [CrossRef]
- Noirclerc, N.; Huttin, O.; de Chillou, C.; Selton-Suty, C.; Fillipetti, L.; Sellal, J.M.; Bozec, E.; Donal, E.; Lamiral, Z.; Kobayashi, M.; et al. Cardiac Remodeling and Diastolic Dysfunction in Paroxysmal Atrial Fibrillation. J. Clin. Med. 2021, 10, 3894. [Google Scholar] [CrossRef] [PubMed]
- Gangu, K.; Bobba, A.; Chela, H.K.; Avula, S.; Basida, S.; Yadav, N. In-Hospital Mortality Rate and Predictors of 30-Day Readmission in Patients with Heart Failure Exacerbation and Atrial Fibrillation: A Cross-Sectional Study. Int. J. Heart Fail. 2022, 4, 145–153. [Google Scholar] [CrossRef]
- Raphael, C.E.; Liew, A.C.; Mitchell, F.; Kanaganayagam, G.S.; Di Pietro, E.; Newsome, S.; Owen, R.; Gregson, J.; Cooper, R.; Amin, F.R.; et al. Predictors and Mechanisms of Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2020, 136, 140–148. [Google Scholar] [CrossRef]
- Garg, L.; Gupta, M.; Sabzwari, S.R.A.; Agrawal, S.; Agarwal, M.; Nazir, T.; Gordon, J.; Bozorgnia, B.; Martinez, M.W. Atrial fibrillation in hypertrophic cardiomyopathy: Prevalence, clinical impact, and management. Heart Fail. Rev. 2019, 24, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-E.; Park, J.-K.; Uhm, J.-S.; Kim, J.Y.; Pak, H.-N.; Lee, M.-H.; Joung, B. Impact of atrial fibrillation on the clinical course of apical hypertrophic cardiomyopathy. Heart 2017, 103, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Burczak, D.R.; Julakanti, R.R.; Balla, A.K.; Scott, C.G.; Geske, J.B.; Ommen, S.R.; Nkomo, V.T.; Gersh, B.J.; Noseworthy, P.A.; Siontis, K.C. Risk of Left Atrial Thrombus in Patients with Hypertrophic Cardiomyopathy and Atrial Fibrillation. J. Am. Coll. Cardiol. 2023, 82, 278–279. [Google Scholar] [CrossRef]
- Psaty, B.M.; Manolio, T.A.; Kuller, L.H.; Kronmal, R.A.; Cushman, M.; Fried, L.P.; White, R.; Furberg, C.D.; Rautaharju, P.M. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997, 96, 2455–2461. [Google Scholar] [CrossRef]
- Vaziri, S.M.; Larson, M.G.; Benjamin, E.J.; Levy, D. Echocardiographic predictors of nonrheumatic atrial fibrillation. Fram. Heart Study Circ. 1994, 89, 724–730. [Google Scholar] [CrossRef]
- Ko, T. Left Atrium as an Active Component of the Pathophysiology in HCM. Int. Heart J. 2018, 59, 906–908. [Google Scholar] [CrossRef]
- Chung, H.; Kim, Y.; Park, C.H.; Kim, I.-S.; Kim, J.-Y.; Min, P.-K.; Yoon, Y.W.; Kim, T.H.; Lee, B.K.; Hong, B.-K.; et al. Contribution of sarcomere gene mutations to left atrial function in patients with hypertrophic cardiomyopathy. Cardiovasc. Ultrasound 2021, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-D.; Cho, D.-H.; Kim, M.-N.; Hwang, S.H.; Shim, J.; Choi, J.-I.; Kim, Y.-H.; Park, S.-M. Left Atrial Dysfunction, Fibrosis and the Risk of Thromboembolism in Patients with Paroxysmal and Persistent Atrial Fibrillation. Int. J. Heart Fail. 2022, 4, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Lee, J.M. Left Atrial Remodeling and Thromboembolic Risk in Patients with Atrial Fibrillation. Int. J. Heart Fail. 2022, 4, 26–28. [Google Scholar] [CrossRef]
- Szyguła-Jurkiewicz, B.; Szczurek-Wasilewicz, W.; Osadnik, T.; Frycz-Kurek, A.M.; Macioł-Skurk, K.; Małyszek-Tumidajewicz, J.; Skrzypek, M.; Romuk, E.; Gąsior, M.; Banach, M.; et al. Oxidative Stress Markers in Hypertrophic Cardiomyopathy. Medicina 2021, 58, 31. [Google Scholar] [CrossRef]
- Gasparova, I.; Kubatka, P.; Opatrilova, R.; Caprnda, M.; Filipova, S.; Rodrigo, L.; Malan, L.; Mozos, I.; Rabajdova, M.; Nosal, V.; et al. Perspectives and challenges of antioxidant therapy for atrial fibrillation. Naunyn Schmiedeberg’s Arch. Pharmacol. 2017, 390, 1–14. [Google Scholar] [CrossRef]
- Zamirian, M.; Sarmadi, T.; Aghasadeghi, K.; Kazemi, M.B. Liver cirrhosis prevents atrial fibrillation: A reality or just an illusion? J. Cardiovasc. Dis. Res. 2012, 3, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.L.; Aniskevich, S., 3rd; Logvinov, I.I.; Matcha, G.V.; Palmer, W.C.; Blackshear, J.L. Hypertrophic Cardiomyopathy in Liver Transplantation Patients. Transplant. Proc. 2018, 50, 1466–1469. [Google Scholar] [CrossRef]
- Csécs, I.; Yamaguchi, T.; Kheirkhahan, M.; Czimbalmos, C.; Fochler, F.; Kholmovski, E.G.; Morris, A.K.; Kaur, G.; Vago, H.; Merkely, B.; et al. Left atrial functional and structural changes associated with ablation of atrial fibrillation-Cardiac magnetic resonance study. Int. J. Cardiol. 2020, 305, 154–160. [Google Scholar] [CrossRef]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D.; Dominic, P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: A meta-analysis. Europace 2018, 20, 33–42. [Google Scholar] [CrossRef]
- Debonnaire, P.; Joyce, E.; Hiemstra, Y.; Mertens, B.J.; Atsma, D.E.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Left Atrial Size and Function in Hypertrophic Cardiomyopathy Patients and Risk of New-Onset Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2017, 10, e004052. [Google Scholar] [CrossRef]
- Hamatani, Y.; Ogawa, H.; Takabayashi, K.; Yamashita, Y.; Takagi, D.; Esato, M.; Chun, Y.-H.; Tsuji, H.; Wada, H.; Hasegawa, K.; et al. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci. Rep. 2016, 6, 31042. [Google Scholar] [CrossRef]
- Guttmann, O.P.; Pavlou, M.; O’Mahony, C.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; Garcia-Pavia, P.; et al. Predictors of atrial fibrillation in hypertrophic cardiomyopathy. Heart. (Br. Cardiac. Soc.) 2017, 103, 672–678. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Olivotto, I.; Cecchi, F.; Casey, S.A.; Dolara, A.; Traverse, J.H.; Maron, B.J. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001, 104, 2517–2524. [Google Scholar] [CrossRef]
- Tani, T.; Tanabe, K.; Ono, M.; Yamaguchi, K.; Okada, M.; Sumida, T.; Konda, T.; Fujii, Y.; Kawai, J.; Yagi, T.; et al. Left atrial volume and the risk of paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2004, 17, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Jang, J.-H.; Baek, Y.-S.; Kwon, S.-W.; Park, S.-D.; Woo, S.-I.; Kim, D.-H.; Kwan, J. Prognostic Impact of Left Atrial Minimal Volume on Clinical Outcome in Patients with Non-Obstructive Hypertrophic Cardiomyopathy. Int. Heart J. 2018, 59, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Fatema, K.; Barnes, M.E.; Bailey, K.R.; Abhayaratna, W.P.; Cha, S.; Seward, J.B.; Tsang, T.S. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: A prospective study. Eur. J. Echocardiogr. J. Work Group Echocardiogr. Eur. Soc. Cardiol. 2009, 10, 282–286. [Google Scholar] [CrossRef]
- Khan, H.R.; Yakupoglu, H.Y.; Kralj-Hans, I.; Haldar, S.; Bahrami, T.; Clague, J.; De Souza, A.; Hussain, W.; Jarman, J.; Jones, D.G.; et al. Left Atrial Function Predicts Atrial Arrhythmia Recurrence Following Ablation of Long-Standing Persistent Atrial Fibrillation. Circ. Cardiovasc. Imaging 2023, 16, e015352. [Google Scholar] [CrossRef]
- Shih, J.Y.; Tsai, W.C.; Huang, Y.Y.; Liu, Y.W.; Lin, C.C.; Huang, Y.S.; Tsai, L.M.; Lin, L.J. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2011, 24, 513–519. [Google Scholar] [CrossRef]
- Rosca, M.; Lancellotti, P.; Popescu, B.A.; Piérard, L.A. Left atrial function: Pathophysiology, echocardiographic assessment, and clinical applications. Heart 2011, 97, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; He, L.; Gao, L.; Lin, Y.; Xie, M.; Li, Y. Assessment of Left Atrial Structure and Function by Echocardiography in Atrial Fibrillation. Diagnostics 2022, 12, 1898. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.B.; Skaarup, K.G.; Hauser, R.; Johansen, N.D.; Lassen, M.C.H.; Jensen, G.B.; Schnohr, P.; Møgelvang, R.; Biering-Sørensen, T. Normal values and reference ranges for left atrial strain by speckle-tracking echocardiography: The Copenhagen City Heart Study. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Caputo, M.; Mondillo, S.; Ballo, P.; Palmerini, E.; Lisi, M.; Marino, E.; Galderisi, M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc. Ultrasound 2009, 7, 6. [Google Scholar] [CrossRef]
- Kuppahally, S.S.; Akoum, N.; Burgon, N.S.; Badger, T.J.; Kholmovski, E.G.; Vijayakumar, S.; Rao, S.N.; Blauer, J.; Fish, E.N.; DiBella, E.V.; et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: Relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ. Cardiovasc. Imaging 2010, 3, 231–239. [Google Scholar] [CrossRef]
- Vasquez, N.; Ostrander, B.T.; Lu, D.Y.; Ventoulis, I.; Haileselassie, B.; Goyal, S.; Greenland, G.V.; Vakrou, S.; Olgin, J.E.; Abraham, T.P.; et al. Low Left Atrial Strain Is Associated with Adverse Outcomes in Hypertrophic Cardiomyopathy Patients. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2019, 32, 593–603.e1. [Google Scholar] [CrossRef]
- Zegkos, T.; Ntelios, D.; Parcharidou, D.; Katranas, S.; Panagiotidis, T.; Papanastasiou, C.A.; Karagiannidis, E.; Rouskas, P.; Vassilikos, V.; Karvounis, H.; et al. The predictive value of left ventricular and left atrial mechanics for atrial fibrillation and heart failure in hypertrophic cardiomyopathy: A prospective cohort study. Int. J. Cardiovasc. Imaging 2021, 37, 2679–2690. [Google Scholar] [CrossRef]
- Siontis, K.C.; Geske, J.B.; Ong, K.; Nishimura, R.A.; Ommen, S.R.; Gersh, B.J. Atrial fibrillation in hypertrophic cardiomyopathy: Prevalence, clinical correlations, and mortality in a large high-risk population. J. Am. Heart Assoc. 2014, 3, e001002. [Google Scholar] [CrossRef]
- Captur, G.; Manisty, C.H.; Raman, B.; Marchi, A.; Wong, T.C.; Ariga, R.; Bhuva, A.; Ormondroyd, E.; Lobascio, I.; Camaioni, C.; et al. Maximal Wall Thickness Measurement in Hypertrophic Cardiomyopathy: Biomarker Variability and its Impact on Clinical Care. JACC Cardiovasc. Imaging 2021, 14, 2123–2134. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Saito, C.; Minami, Y.; Haruki, S.; Arai, K.; Ashihara, K.; Hagiwara, N. Prognostic Relevance of a Score for Identifying Diastolic Dysfunction according to the 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging Recommendations in Patients with Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2022, 35, 469–476. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Kopelen, H.A.; Quiñones, M.A. Assessment of left ventricular filling pressures by Doppler in the presence of atrial fibrillation. Circulation 1996, 94, 2138–2145. [Google Scholar] [CrossRef]
- McMahon, C.J.; Nagueh, S.F.; Pignatelli, R.H.; Denfield, S.W.; Dreyer, W.J.; Price, J.F.; Clunie, S.; Bezold, L.I.; Hays, A.L.; Towbin, J.A.; et al. Characterization of left ventricular diastolic function by tissue Doppler imaging and clinical status in children with hypertrophic cardiomyopathy. Circulation 2004, 109, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Okamatsu, H.; Ohara, T.; Kanzaki, H.; Nakajima, I.; Miyamoto, K.; Okamura, H.; Noda, T.; Aiba, T.; Kusano, K.; Kamakura, S.; et al. Impact of left ventricular diastolic dysfunction on outcome of catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. Circ. J. 2015, 79, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Ko, J.M. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005, 111, 920–925. [Google Scholar] [CrossRef]
- Harris, K.M.; Spirito, P.; Maron, M.S.; Zenovich, A.G.; Formisano, F.; Lesser, J.R.; Mackey-Bojack, S.; Manning, W.J.; Udelson, J.E.; Maron, B.J. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 2006, 114, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Marstrand, P.; Han, L.; Day, S.M.; Olivotto, I.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Wittekind, S.G.; Helms, A.; Saberi, S.; et al. Hypertrophic Cardiomyopathy with Left Ventricular Systolic Dysfunction: Insights from the SHaRe Registry. Circulation 2020, 141, 1371–1383. [Google Scholar] [CrossRef]
- Carasso, S.; Yang, H.; Woo, A.; Vannan, M.A.; Jamorski, M.; Wigle, E.D.; Rakowski, H. Systolic myocardial mechanics in hypertrophic cardiomyopathy: Novel concepts and implications for clinical status. J. Am. Soc. Echocardiogr. 2008, 21, 675–683. [Google Scholar] [CrossRef]
- Urheim, S.; Edvardsen, T.; Torp, H.; Angelsen, B.; Smiseth, O.A. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation 2000, 102, 1158–1164. [Google Scholar] [CrossRef]
- Ohki, T. Commentary on: Clark DJ, Lessio S, O'Donoghue M, Tsalamandris C, Schainfeld R, Rosenfield R. Mechanisms and predictors of carotid artery restenosis: A serial intravascular ultrasound study. J Am Coll Cardiol. 2006;47:2390–2396. Perspect. Vasc. Surg. Endovasc. Ther. 2007, 19, 199–201. [Google Scholar]
- Urtado, S.; Hergault, H.; Binsse, S.; Aidan, V.; Ouadahi, M.; Szymanski, C.; Mallet, S.; Hauguel-Moreau, M.; Carlier, R.Y.; Dubourg, O.; et al. Usefulness of Longitudinal Strain Adjusted to Regional Thickness in Hypertrophic Cardiomyopathy. J. Clin. Med. 2022, 11, 2089. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; DiMarco, J.P.; Kolm, P.; Ho, C.Y.; Desai, M.Y.; Kwong, R.Y.; Dolman, S.F.; Desvigne-Nickens, P.; Geller, N.; Kim, D.-Y.; et al. Predictors of Major Atrial Fibrillation Endpoints in the National Heart, Lung, and Blood Institute HCMR. JACC Clin. Electrophysiol. 2021, 7, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 142, e558–e631. [Google Scholar]
- Cau, R.; Bassareo, P.; Suri, J.S.; Pontone, G.; Saba, L. The emerging role of atrial strain assessed by cardiac MRI in different cardiovascular settings: An up-to-date review. Eur. Radiol. 2022, 32, 4384–4394. [Google Scholar] [CrossRef]
- Truong, V.T.; Palmer, C.; Wolking, S.; Sheets, B.; Young, M.; Ngo, T.N.M.; Taylor, M.; Nagueh, S.F.; Zareba, K.M.; Raman, S.; et al. Normal left atrial strain and strain rate using cardiac magnetic resonance feature tracking in healthy volunteers. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 446–453. [Google Scholar] [CrossRef]
- Bertelsen, L.; Diederichsen, S.Z.; Haugan, K.J.; Brandes, A.; Graff, C.; Krieger, D.; Kronborg, C.; Køber, L.; Højberg, S.; Vejlstrup, N.; et al. Left atrial volume and function assessed by cardiac magnetic resonance imaging are markers of subclinical atrial fibrillation as detected by continuous monitoring. Europace 2020, 22, 724–731. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, G.; Jiang, Y.; Song, L.; Zhao, S.; Lu, M. Quantification of left atrial function in patients with non-obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: A feasibility and reproducibility study. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2020, 22, 1–11. [Google Scholar] [CrossRef]
- Habibi, M.; Lima, J.A.; Ipek, E.G.; Zimmerman, S.L.; Zipunnikov, V.; Spragg, D.; Ashikaga, H.; Rickard, J.; Marine, J.E.; Berger, R.D.; et al. The association of baseline left atrial structure and function measured with cardiac magnetic resonance and pulmonary vein isolation outcome in patients with drug-refractory atrial fibrillation. Heart Rhythm. 2016, 13, 1037–1044. [Google Scholar] [CrossRef]
- Sivalokanathan, S.; Zghaib, T.; Greenland, G.V.; Vasquez, N.; Kudchadkar, S.M.; Kontari, E.; Lu, D.-Y.; Dolores-Cerna, K.; van der Geest, R.J.; Kamel, I.R.; et al. Hypertrophic Cardiomyopathy Patients with Paroxysmal Atrial Fibrillation Have a High Burden of Left Atrial Fibrosis by Cardiac Magnetic Resonance Imaging. JACC Clin. Electrophysiol. 2019, 5, 364–375. [Google Scholar] [CrossRef]
- Roh, S.-Y.; Lee, D.I.; Hwang, S.H.; Lee, K.-N.; Baek, Y.-S.; Iqbal, M.; Kim, D.-H.; Ahn, J.; Shim, J.; Choi, J.-I.; et al. Association of left atrial pressure with late gadolinium enhancement extent in patient who underwent catheter ablation for atrial fibrillation. Sci. Rep. 2020, 10, 16486. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri, K.; Franke, K.B.; Foo, F.S.; Stiles, M.K. Clinical utility of cardiac magnetic resonance imaging to assess the left atrium before catheter ablation for atrial fibrillation—A systematic review and meta-analysis. Int. J. Cardiol. 2021, 339, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Cavus, E.; Muellerleile, K.; Schellert, S.; Schneider, J.; Tahir, E.; Chevalier, C.; Jahnke, C.; Radunski, U.K.; Adam, G.; Kirchhof, P.; et al. CMR feature tracking strain patterns and their association with circulating cardiac biomarkers in patients with hypertrophic cardiomyopathy. Clin. Res. Cardiol. 2021, 110, 1757–1769. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Alexandridis, G.M.; Vassilikos, V.P. Fibrosis in hypertrophic cardiomyopathy: Role of novel echo techniques and multi-modality imaging assessment. Heart Fail. Rev. 2021, 26, 1297–1310. [Google Scholar] [CrossRef]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef]
- Vullaganti, S.; Levine, J.; Raiker, N.; Syed, A.A.; Collins, J.D.; Carr, J.C.; Bonow, R.O.; Choudhury, L. Fibrosis in Hypertrophic Cardiomyopathy Patients with and without Sarcomere Gene Mutations. Heart Lung Circ. 2021, 30, 1496–1501. [Google Scholar] [CrossRef]
- Chung, H.; Kim, Y.; Park, C.-H.; Kim, J.-Y.; Min, P.-K.; Yoon, Y.W.; Kim, T.H.; Lee, B.K.; Hong, B.-K.; Rim, S.-J.; et al. Effect of sarcomere and mitochondria-related mutations on myocardial fibrosis in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2021, 23, 18. [Google Scholar] [CrossRef]
- Yamaji, K.; Fujimoto, S.; Yutani, C.; Ikeda, Y.; Mizuno, R.; Hashimoto, T.; Nakamura, S. Does the progression of myocardial fibrosis lead to atrial fibrillation in patients with hypertrophic cardiomyopathy? Cardiovasc. Pathol. 2001, 10, 297–303. [Google Scholar] [CrossRef]
- Papavassiliu, T.; Germans, T.; Flüchter, S.; Doesch, C.; Suriyakamar, A.; Haghi, D.; Süselbeck, T.; Wolpert, C.; Dinter, D.; Schoenberg, S.O.; et al. CMR findings in patients with hypertrophic cardiomyopathy and atrial fibrillation. J. Cardiovasc. Magn. Reson. 2009, 11, 34. [Google Scholar] [CrossRef]
- Castelo, A.; Rosa, S.A.; Fiarresga, A.; Jalles, N.; Ferreira, V.V.; Brás, P.G.; Branco, L.M.; Oliveira, M.; Ferreira, R.C. Late gadolinium enhancement in the left ventricular wall is associated with atrial fibrillation in patients with hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2022, 38, 2733–2741. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, X.; Zheng, X.; Lu, J.; Wang, S.; Huang, X. Usefulness of Preoperative Transforming Growth Factor-Beta to Predict New Onset Atrial Fibrillation after Surgical Ventricular Septal Myectomy in Patients with Obstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2017, 120, 118–123. [Google Scholar] [CrossRef]
- Tian, H.; Cui, J.; Yang, C.; Hu, F.; Yuan, J.; Liu, S.; Yang, W.; Jiang, X.; Qiao, S. Left ventricular remodeling in hypertrophic cardiomyopathy patients with atrial fibrillation. BMC Cardiovasc. Disord. 2018, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Lee, A.S.; Liang, P.; Sanchez-Freire, V.; Nguyen, P.K.; Wang, L.; Han, L.; Yen, M.; Wang, Y.; Sun, N.; et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 2013, 12, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, X.; Zhao, K.; Yang, S.; Yang, K.; Yu, S.; Yin, G.; Dong, Z.; Song, Y.; Cui, C.; et al. Association between left atrial myopathy and sarcomere mutation in patients with hypertrophic cardiomyopathy: Insights into left atrial strain by MRI feature tracking. Eur. Radiol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
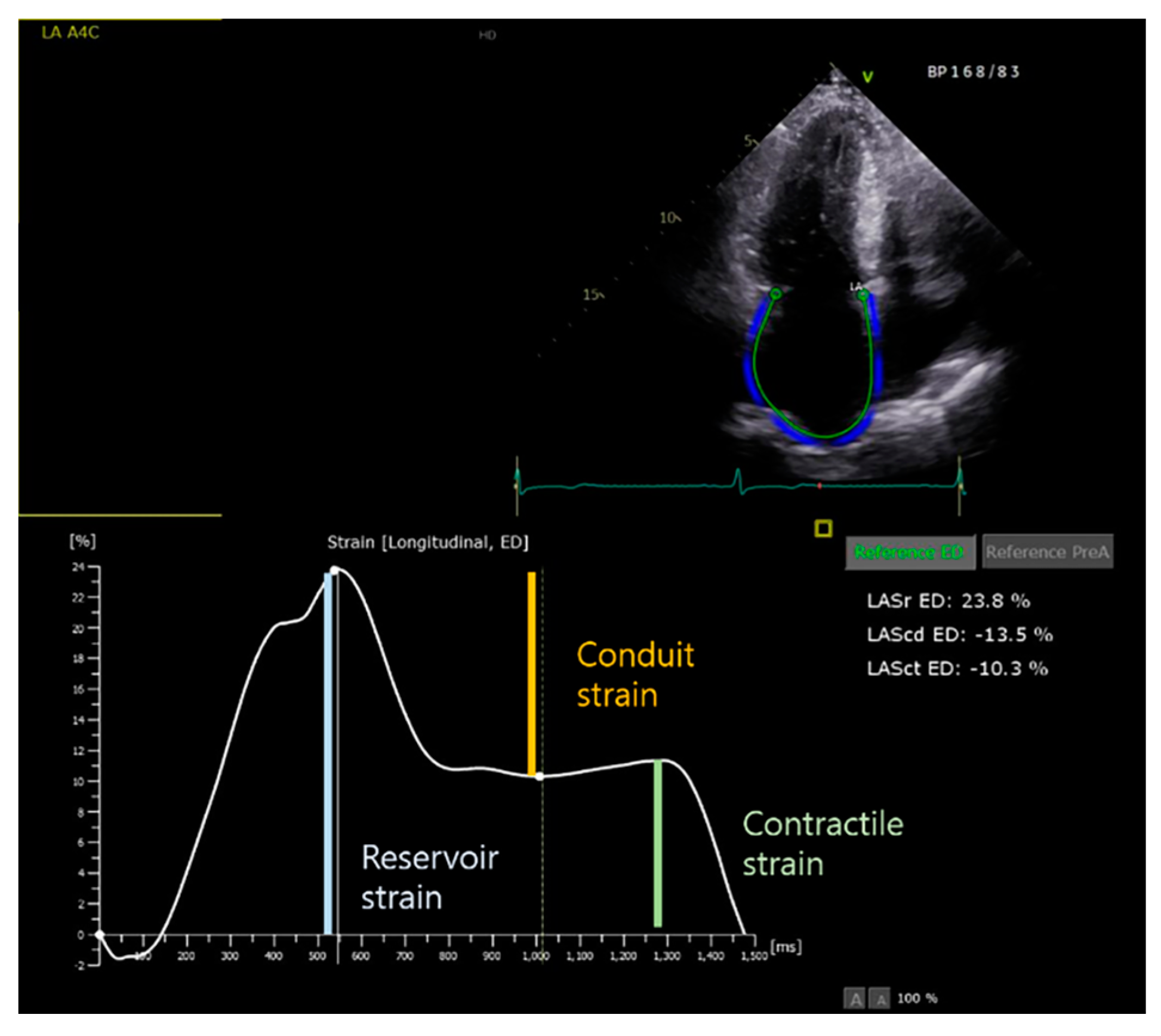
| LA total emptying fraction, % | (LAVmax − LAVmin) / LAVmax |
| LA reserve fraction, % | (LAVmax − LAVmin) / LAVmin |
| LA passive emptying (conduit) fraction, % | (LAVmax − LAVpreA) / LAVmax |
| LA active emptying (booster pump) fraction, % | (LAVpreA − LAVmin) / LAVpre-A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.; Choi, E.-Y. Multimodality Imaging in Patients with Hypertrophic Cardiomyopathy and Atrial Fibrillation. Diagnostics 2023, 13, 3049. https://doi.org/10.3390/diagnostics13193049
Chung H, Choi E-Y. Multimodality Imaging in Patients with Hypertrophic Cardiomyopathy and Atrial Fibrillation. Diagnostics. 2023; 13(19):3049. https://doi.org/10.3390/diagnostics13193049
Chicago/Turabian StyleChung, Hyemoon, and Eui-Young Choi. 2023. "Multimodality Imaging in Patients with Hypertrophic Cardiomyopathy and Atrial Fibrillation" Diagnostics 13, no. 19: 3049. https://doi.org/10.3390/diagnostics13193049
APA StyleChung, H., & Choi, E.-Y. (2023). Multimodality Imaging in Patients with Hypertrophic Cardiomyopathy and Atrial Fibrillation. Diagnostics, 13(19), 3049. https://doi.org/10.3390/diagnostics13193049





