Head and Neck Low Grade Chondrosarcoma—A Rare Entity
Abstract
1. Introduction
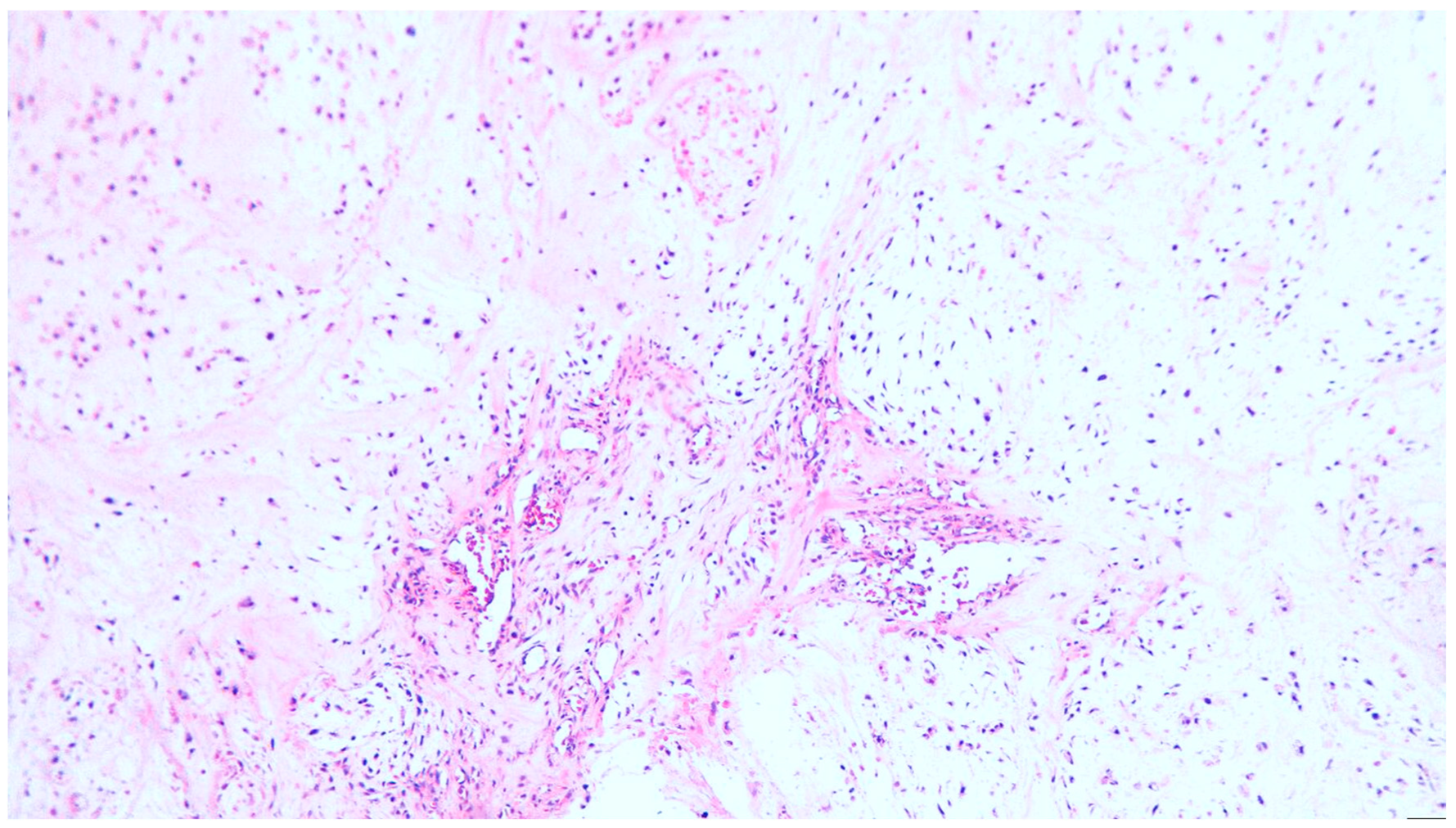
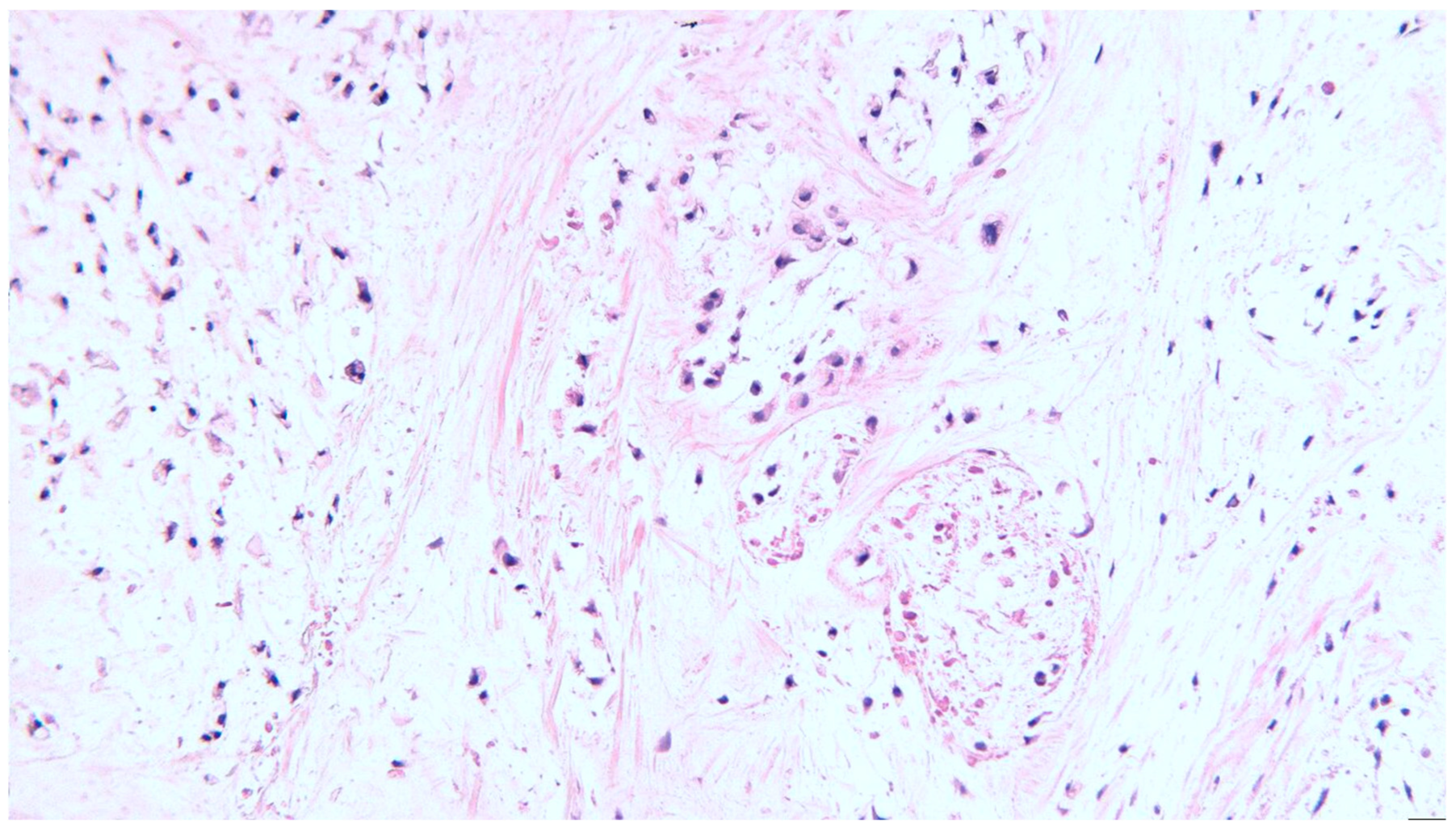
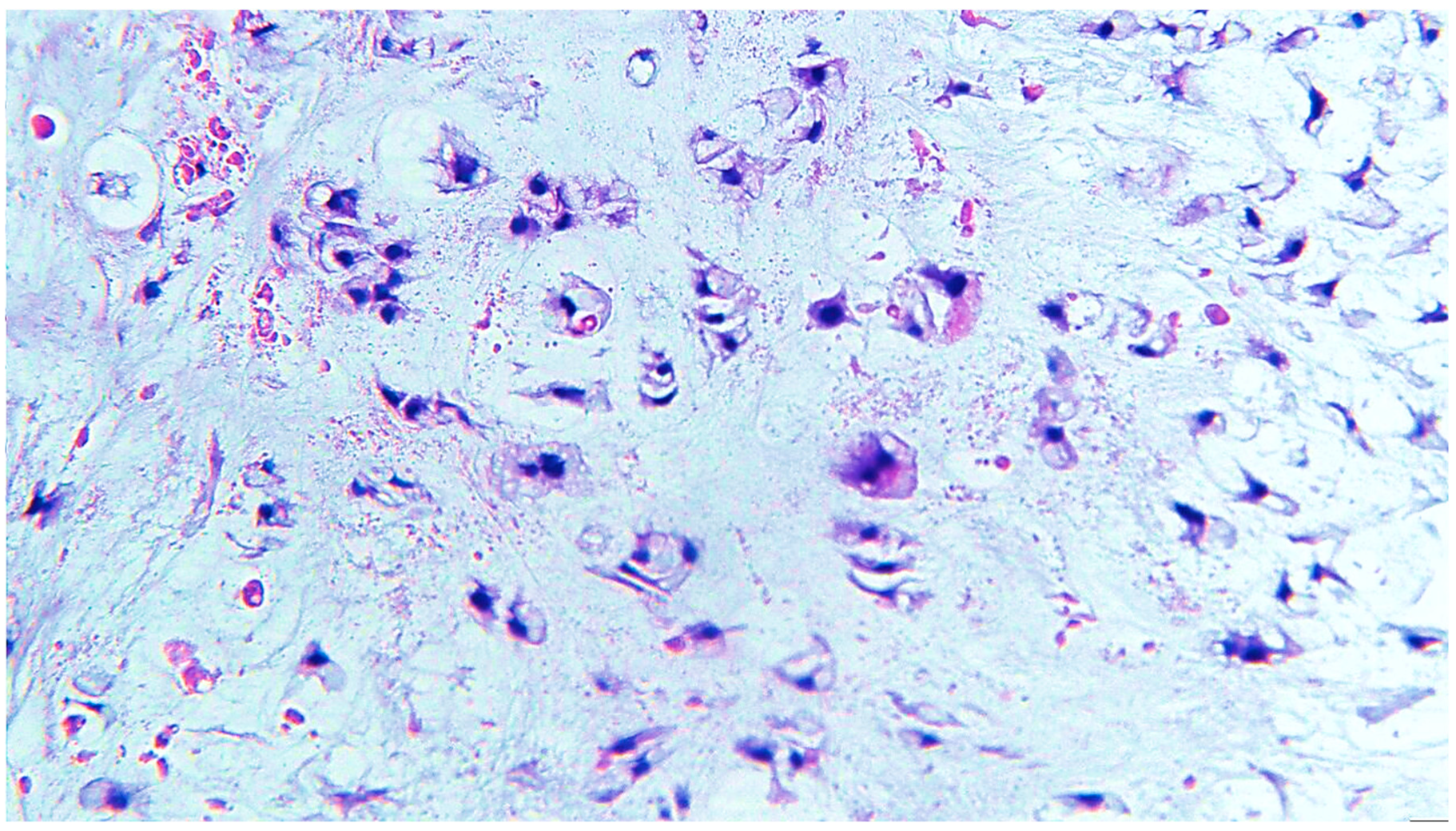
2. Clinical Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.Y.; Lim, Y.C.; Song, M.H.; Seok, J.Y.; Lee, W.S.; Choi, E.C. Chondrosarcoma of the head and neck. Yonsei Med. J. 2005, 46, 228–232. [Google Scholar] [CrossRef]
- Quevedo, F.C.; Quevedo, F.B.; Neto, J.C.; Ferreira, E.N.; Carraro, D.M.; Soares, F.A. Case report: Chondrosarcoma of the head and neck. Hum. Pathol. Case Rep. 2017, 7, 4–7. [Google Scholar] [CrossRef][Green Version]
- Evans, H.L.; Ayala, A.G.; Romsdahl, M.M. Prognostic factors in chondrosarcoma of bone. A clinic-pathologic analysis with emphasis on histologic grading. Cancer 1977, 40, 818–831. [Google Scholar] [CrossRef]
- Obeso, S.; Llorente, J.L.; Díaz-Molina, J.P.; Sánchez-Fernández, R.; Rodrigo, J.P.; Suárez, C. Surgical treatment of head and neck chondrosarcomas. Tratamiento quirúrgico de los condrosarcomas de cabeza y cuello. Acta Otorrinolaringol. 2010, 61, 262–271. [Google Scholar] [CrossRef]
- Catanzano, A.A.; Kerr, D.L.; Lazarides, A.L.; Dial, B.L.; Lane, W.O.; Blazer, D.G.; Larrier, N.A.; Kirsch, D.G.; Brigman, B.E.; Eward, W.C. Revisiting the Role of Radiation Therapy in Chondrosarcoma: A National Cancer Database Study. Sarcoma 2019, 2019, 4878512. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Rodrigo, J.P.; Triantafyllou, A.; Hunt, J.L.; Fernández-Miranda, J.C.; Strojan, P.; de Bree, R.; Rinaldo, A.; Takes, R.P.; Ferlito, A. Chondrosarcomas of the head and neck. Eur. Arch. Oto-Rhino-Laryngol. 2013, 271, 2601–2609. [Google Scholar] [CrossRef]
- Horta, M.; Fernandes, L.; Borges, A. Chondrosarcoma of the hyoid bone: An atypical site of a sarcoma of the head and the neck. BMJ Case Rep. 2015, 2015, bcr2015212291. [Google Scholar] [CrossRef]
- Zhang, I.; Zaorsky, N.G.; Abraham, J.A.; Tuluc, M.; Curry, J.M.; Bar-Ad, V. Chondrosarcoma of the hyoid bone: Case report and review of current management options. Head Neck 2014, 36, E65–E72. [Google Scholar] [CrossRef]
- Kalavrezos, N.; Sinha, D. Head and neck sarcomas in adulthood: Current trends and evolving management concepts. Br. J. Oral Maxillofac. Surg. 2020, 58, 890–897. [Google Scholar] [CrossRef]
- Vučković, L.; Klisic, A.; Filipović, A.; Popović, M.; Ćulafić, T. Low-grade chondrosarcoma of the larynx: A case report. World J. Clin. Cases 2021, 9, 7805–7810. [Google Scholar] [CrossRef]
- Ram, H.; Kumar, S.; Singh, S.N.; Kumar, P.; Singh, G.; Ganguly, R.; Sagar, M.; Howlader, D. Head and Neck Sarcomas-clinicopathological Findings, Treatment Modalities and Its Outcome—A Retrospective Study. Ann. Maxillofac. Surg. 2021, 11, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.-P.; Liu, J.-H.; Hou, F.; Liu, H.; Xu, W.-J. Low-grade chondrosarcoma of the cricoid cartilage: A case report and review of the literature. Skelet. Radiol. 2017, 46, 1597–1601. [Google Scholar] [CrossRef]
- Pino Rivero, V.; Keituqwa Yáñez, T.; González Palomino, A.; Trinidad Ramos, G.; Marqués Rebollo, L.; Gómez de Tejadae Romero, R.; Blasco Huelva, A. Condrosarcoma laríngeo de bajo grado de malignidad. A propósito de un caso localizado en cartílago tiroides. [Laryngeal chondrosarcoma of low grade of malignancy. Report of a case located on thyroid cartilage]. Otorrinolaringol Ibero Am. 2006, 33, 249–256. (In Spanish) [Google Scholar]
- Burkey, B.B.; Hoffman, H.T.; Baker, S.R.; Thornton, A.F.; McClatchey, K.D. Chondrosarcoma of the Head and Neck. Laryngoscope 1990, 100, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Tejani, M.A.; Galloway, T.J.; Lango, M.; Ridge, J.A.; Von Mehren, M. Head and Neck Sarcomas: A Comprehensive Cancer Center Experience. Cancers 2013, 5, 890–900. [Google Scholar] [CrossRef]
- Elktaibi, A.; Rharrassi, I.; Hammoune, N.; Darouassi, Y.; Hanine, M.A.; Ammar, H. A Rare Case of Malignant Tumor of the Larynx with Good Prognosis: Laryngeal Chondrosarcoma. Case Rep. Oncol. Med. 2019, 2019, 9468194. [Google Scholar] [CrossRef]
- MacNeil, S.D. Non-squamous Laryngeal Cancer. Otolaryngol. Clin. North Am. 2023, 56, 345–359. [Google Scholar] [CrossRef]
- Kremenevski, N.; Schlaffer, S.-M.; Coras, R.; Kinfe, T.M.; Graillon, T.; Buchfelder, M. Skull Base Chordomas and Chondrosarcomas. Neuroendocrinology 2020, 110, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Asioli, S.; Ruengwanichayakun, P.; Zoli, M.; Guaraldi, F.; Sollini, G.; Greco, P.; Facco, C.; Gibertoni, D.; Jiménez, B.V.; Benini, S.; et al. Association of Clinicopathological Features with Outcome in Chondrosarcomas of the Head and Neck. Otolaryngol. Neck Surg. 2020, 164, 807–814. [Google Scholar] [CrossRef]
- Tallegas, M.; Miquelestorena-Standley, E.; Labit-Bouvier, C.; Badoual, C.; Francois, A.; Gomez-Brouchet, A.; Aubert, S.; Collin, C.; Tallet, A.; de Pinieux, G. IDH mutation status in a series of 88 head and neck chondrosarcomas: Different profile between tumors of the skull base and tumors involving the facial skeleton and the laryngotracheal tract. Hum. Pathol. 2018, 84, 183–191. [Google Scholar] [CrossRef]
- Álvarez-Calderón-Iglesias, O.; Pérez-Sayáns, M.; Hurtado-Ruzza, R.; Lorenzo-Pouso, A.I.; Chamorro-Petronacci, C.M. Survival outcomes in laryngeal chondrosarcoma: A systematic review. Acta Otorhinolaryngol. Ital. 2022, 42, 502–515. [Google Scholar] [CrossRef]
- Jones, A.J.; Alwani, M.; Summerlin, D.J.; Sim, M.W. Head and Neck Juxtacortical Chondrosarcoma: A Systematic Review. Arch. Otorhinolaryngol.-Head Neck Surg. 2019, 3, 4. [Google Scholar] [CrossRef]
- Finn, D.G.; Goepfert, H.; Batsakis, J.G. Chondrosarcoma of the head and neck. Laryngoscope 1984, 94, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Pellini, R.; Mercante, G.; Marchese, C.; Terenzi, V.; Sperduti, I.; Manciocco, V.; Ruscito, P.; Cristalli, G.; Marchesi, P.; Pichi, B.; et al. Predictive factors for postoperative wound complications after neck dissection. Acta Otorhinolaryngol. Ital. 2013, 33, 16–22. [Google Scholar]
- Eisbruch, A.; Schwartz, M.; Rasch, C.; Vineberg, K.; Damen, E.; Van As, C.J.; Marsh, R.; Pameijer, F.A.; Balm, A.J. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: Which anatomic structures are affected and can they be spared by IMRT? Int. J. Radiat. Oncol. Biol Phys. 2004, 60, 1425–1439. [Google Scholar] [CrossRef]
- Petkar, I.; Rooney, K.; Roe, J.W.G.; Patterson, J.M.; Bernstein, D.; Tyler, J.M.; Emson, M.A.; Morden, J.P.; Mertens, K.; Miles, E.; et al. DARS: A phase III randomised multicentre study of dysphagia- optimised intensity- modulated radiotherapy (Do-IMRT) versus standard intensity- modulated radiotherapy (S-IMRT) in head and neck cancer. BMC Cancer 2016, 16, 770. [Google Scholar] [CrossRef] [PubMed]
- Christianen, M.E.; Verdonck-de Leeuw, I.M.; Doornaert, P.; Chouvalova, O.; Steenbakkers, R.J.; Koken, P.W.; Leemans, C.R.; Oosting, S.F.; Roodenburg, J.L.; van der Laan, B.F.; et al. Patterns of Long-Term Swallowing Dysfunction after Definitive Radiotherapy or Chemoradiation. Radiother. Oncol. Oct. 2015, 117, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Kumar, P.; Chauhan, A.K.; Kumar, P.; Nigam, J.; Navitha, S.; Upadhyay, P. Comparison of Dosimetric Parameters in Dysphagia Aspiration-Related Structures and Clinical Correlation in Head and Neck Cancer Patients Treated with Radiotherapy. Cureus 2022, 14, e26765. [Google Scholar] [CrossRef] [PubMed]
- Denaro, N.; Merlano, M.C.; Russi, E.G. Dysphagia in Head and Neck Cancer Patients: Pretreatment Evaluation, Predictive Factors, and Assessment during Radio-Chemotherapy, Recommendations. Clin. Exp. Otorhinolaryngol. 2013, 6, 117–126. [Google Scholar] [CrossRef]
- Madan, R.; Kairo, A.K.; Sharma, A.; Roy, S.; Singh, S.; Singh, L.; Kaur, J.; Mohanti, B.K.; Bhasker, S.; Upadhyay, A.D.; et al. Aspiration pneumonia related deaths in head and neck cancer patients: A retrospective analysis of risk factors from a tertiary care centre in North India. J. Laryngol. Otol. 2015, 129, 710–714. [Google Scholar] [CrossRef]
- Christianen, M.E.; Schilstra, C.; Beetz, I.; Muijs, C.T.; Chouvalova, O.; Burlage, F.R.; Doornaert, P.; Koken, P.W.; Leemans, C.R.; Rinkel, R.N.; et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: Results of a prospective observational study. Radiother. Oncol. 2012, 105, 107–114. [Google Scholar] [CrossRef]
- Stieb, S.; Lee, A.; van Dijk, L.V.; Frank, S.; Fuller, C.D.; Blanchard, P. NTCP Modeling of Late Effects for Head and Neck Cancer: A Systematic Review. Int. J. Part. Ther. 2021, 8, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.A.; Arul Ponni, T.R. Dose to swallowing structures and dysphagia in head and neck Intensity Modulated Radiation Therapy—A long term prospective analysis. Rep. Pract. Oncol. Radiother. 2019, 24, 654–659. [Google Scholar] [CrossRef]
- Lindblom, U.; Nilsson, P.; Gärskog, O.; Kjellen, E.; Laurell, G.; Wahlberg, P.; Zackrisson, B.; Jäghagen, E.L. Aspiration as a late complication after accelerated versus conventional radiotherapy in patients with head and neck cancer. Acta Oto-Laryngol. 2016, 136, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Boero, I.J.; Hwang, L.; Le, Q.-T.; Moiseenko, V.; Sanghvi, P.R.; Cohen, E.E.W.; Mell, L.K.; Murphy, J.D. Aspiration pneumonia after concurrent chemoradiotherapy for head and neck cancer. Cancer 2014, 121, 1303–1311. [Google Scholar] [CrossRef]
- Liu, H.C.; Williamson, C.W.; Zou, J.; Todd, J.R.; Nelson, T.J.; Hill, L.M.; Linnemeyer, K.E.; Henderson, G.; Madgula, P.; Faung, B.; et al. Quantitative prediction of aspiration risk in head and neck cancer patients treated with radiation therapy. Oral Oncol. 2023, 136, 106247. [Google Scholar] [CrossRef]
- Zaheen, A.; Stanbrook, M.B. Accidental barium bronchography. Can. Med. Assoc. J. 2017, 189, E670. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Main Objective | Article Type | Number of Cases | Results/Conclusions | Reference |
|---|---|---|---|---|
| To investigate clinical characteristics and treatment outcome of head and neck chondrosarcoma | Case series | 8 | Primary tumor sites were sinus, mastoid, jugular foramen and thyroid cartilage. A total of 50% of cases benefited from adjuvant radiotherapy. Three cases relapsed locally and two were treated for recurrence. One case operated with positive margins was associated with early death during the follow-up period | Lee et al., 2005 [1] |
| Description of a case of head and neck chondrosarcoma with aggressive behavior | Case report | 1 | A large number of molecular abnormalities and chromosomal instability is described in a case of grade III invasive chondrosarcoma of the maxillary sinus that recurs after adjuvant treatment. | Quevedo et al., 2007 [2] |
| To summarize diagnostic, pathological, clinical and evolutionary data of head and neck chondrosarcoma. | Review | Not specified | Conservative surgery should be the option for low-grade tumors; adjuvant radiotherapy should be considered in cases with higher local recurrence risk. OS is usually considered favorable | Coca-Pelaz A. et al., 2014 [6] |
| Description of an atypical case of hyoid bone chondrosarcoma | Case report | 1 | Painless palpable lump with growth of approximately 10 months. Considered extremely rare with only 20 cases in the literature. | Horta et al., 2015 [7] |
| Case report and review of current management options of a hyoid bone chondrosarcoma | Case report | 1 | Complete surgical removal is the recommended treatment, but radiotherapy can be used as adjuvant treatment. | Zhang et al., 2014 [8] |
| Description of chondrosarcoma of the larynx | Case report | 1 | Chondrosarcoma of the larynx should be considered as a differential diagnosis with chondroma for laryngeal submucosal tumors. The possible malignant transformation of the chondroma should also be taken into account. | Vučković et al., 2021 [10] |
| Clear cell chondrosarcoma of head and neck therapeutic diagnosis and outcomes presentation as a very rare entity. | Review | Not specified | Nasal tumor is associated with ballooning of the septum, and maxillary tumor does not involve the mucosa. Chondroblastic osteosarcoma differential diagnosis is essential for jaw tumors. Laryngeal localization tends to recur. | Mokhtari et al., 2012 [12] |
| Presentation of a case of low-grade chondrosarcoma of the cricoid cartilage with atypical onset 12-day history of vomiting | Case report and review of the literature | 1 | Tracheal cartilaginous ring can be associated with intraluminal and extraluminal extension. This subtype is rare and has a benign behavior. If the resection is incomplete, there is an increased risk of recurrence. In this case, adjuvant radiotherapy is necessary. | Gao et al., 2017 [13] |
| Report of a case of laryngeal chondrosarcoma located on thyroid cartilage | Case report | 1 | Chondrosarcoma of the larynx appears especially in the 6th–7th decades of life. The majority (70–75%) are located at the level of the cricoid and at the level of the thyroid cartilage (10–20%). | Pino Rivero et al., 2006 [14] |
| Retrospectively analyzes paranasal sinuses, mandible, temporal bone and larynx chondrosarcoma | Retrospective review | 40 cases | The OS is 70% with a median follow-up of 3.5 years. Sinonasal chondrosarcoma is associated with the worst prognosis. | Burkey et al., 1990 [15] |
| Presentation of a case of laryngeal chondrosarcoma with a good prognosis. | Case report | 1 | Surrey with preservation of the larynx was associated with a favorable response and absence of recurrence 5 years after treatment. | Elktaibi et al., 2019 [17] |
| Evaluation of clinical data, imaging, histopathology and management outcomes for head and neck juxtacortical chondrosarcoma (HNJCS) | Case report and systematic review | 9 | One out of nine cases relapsed locally 4 years after the initial treatment. Most cases were low-grade tumors. Adjuvant treatment was delivered in 4 out of 9 cases. | Jones at al., 2019 [18] |
| Analysis of the correlations of the degree of histological differentiation with tumor parameters | Review | 23 cases | Histological grade is correlated with survival; grade II and III tumors are more extensive and grow rapidly; there is no correlation between tumor size and histological grade. | Finn et al., 1984 [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mireștean, C.C.; Simionescu, C.E.; Iancu, R.I.; Stan, M.C.; Iancu, D.P.T.; Bădulescu, F. Head and Neck Low Grade Chondrosarcoma—A Rare Entity. Diagnostics 2023, 13, 3026. https://doi.org/10.3390/diagnostics13193026
Mireștean CC, Simionescu CE, Iancu RI, Stan MC, Iancu DPT, Bădulescu F. Head and Neck Low Grade Chondrosarcoma—A Rare Entity. Diagnostics. 2023; 13(19):3026. https://doi.org/10.3390/diagnostics13193026
Chicago/Turabian StyleMireștean, Camil Ciprian, Cristiana Eugenia Simionescu, Roxana Irina Iancu, Mihai Cosmin Stan, Dragoș Petru Teodor Iancu, and Florinel Bădulescu. 2023. "Head and Neck Low Grade Chondrosarcoma—A Rare Entity" Diagnostics 13, no. 19: 3026. https://doi.org/10.3390/diagnostics13193026
APA StyleMireștean, C. C., Simionescu, C. E., Iancu, R. I., Stan, M. C., Iancu, D. P. T., & Bădulescu, F. (2023). Head and Neck Low Grade Chondrosarcoma—A Rare Entity. Diagnostics, 13(19), 3026. https://doi.org/10.3390/diagnostics13193026






