Risk of Subsequent Primary Cancer in Thyroid Cancer Survivors: A Nationwide Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Outcomes (Subsequent Primary Cancers) and Covariates
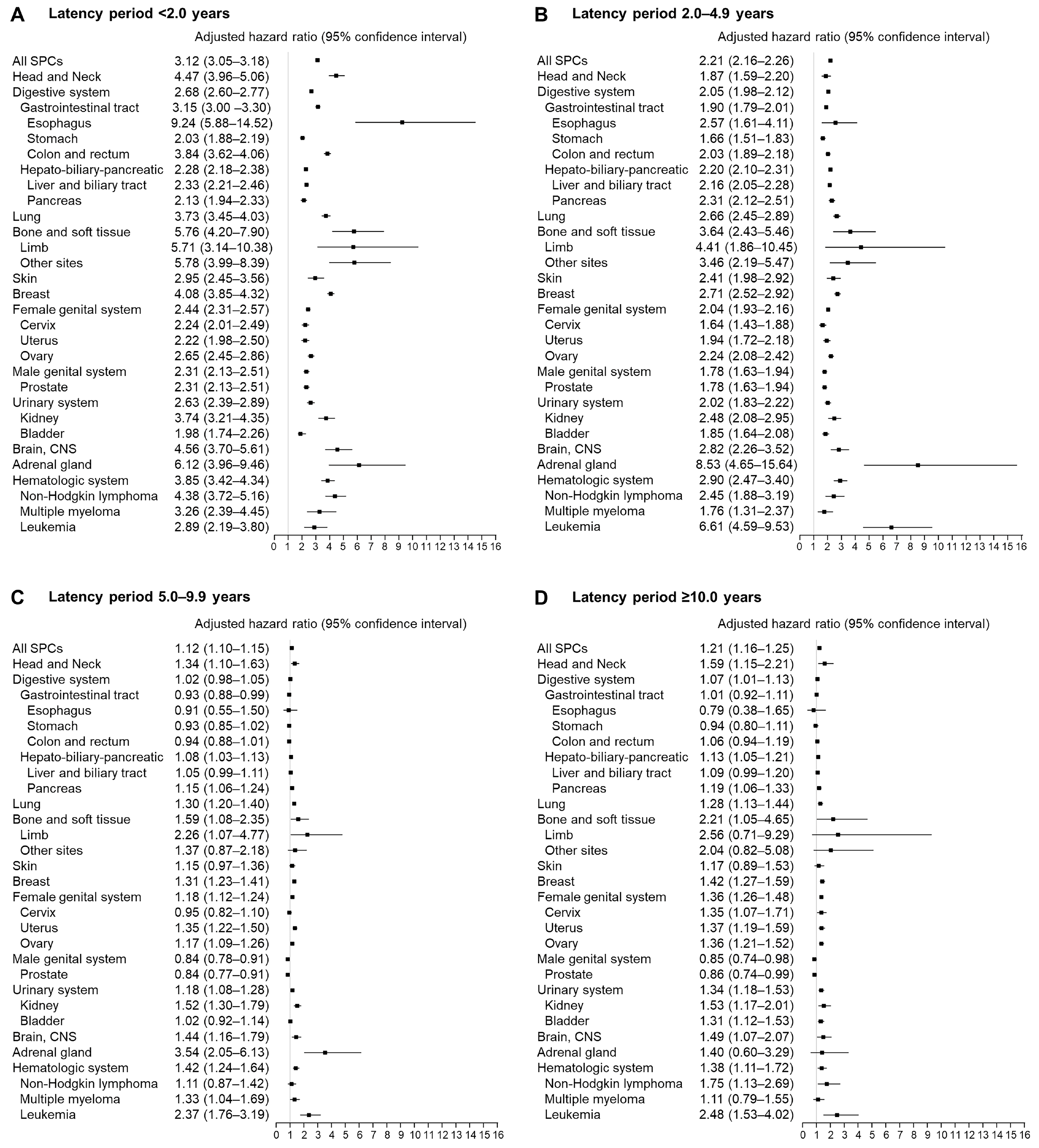
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, M.J.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kim, H.J.; Kong, H.J.; Im, J.S.; Seo, H.G. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2019. Cancer Res. Treat. 2022, 54, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Lim, J.; Oh, C.M.; Ryu, J.; Jung, K.W.; Chung, J.H.; Won, Y.J.; Kim, S.W. Elevated risks of subsequent primary malignancies in patients with thyroid cancer: A nationwide, population-based study in Korea. Cancer 2015, 121, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Rubino, C.; de Vathaire, F.; Dottorini, M.E.; Hall, P.; Schvartz, C.; Couette, J.E.; Dondon, M.G.; Abbas, M.T.; Langlois, C.; Schlumberger, M. Second primary malignancies in thyroid cancer patients. Br. J. Cancer 2003, 89, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Ronckers, C.M.; McCarron, P.; Ron, E. Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int. J. Cancer 2005, 117, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.P.; Chen, J.; Hitchcock, Y.J.; Szabo, A.; Shrieve, D.C.; Tward, J.D. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2008, 93, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Bi, X.; Pan, D.; Chen, Y.; Carling, T.; Ma, S.; Udelsman, R.; Zhang, Y. The risk of second cancers after diagnosis of primary thyroid cancer is elevated in thyroid microcarcinomas. Thyroid 2013, 23, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.V.; Lee, J.H.; Bove-Fenderson, E.A.; Park, S.Y.; Mannstadt, M.; Lee, S. Incidence of Hypoparathyroidism After Thyroid Cancer Surgery in South Korea, 2007–2016. JAMA 2019, 322, 2441–2443. [Google Scholar] [CrossRef] [PubMed]
- Kyoung, D.-S.; Kim, H.-S. Understanding and Utilizing Claim Data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) Database for Research. J. Lipid Atheroscler. 2022, 11, 103. [Google Scholar] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, B.E.; Ganz, P.A.; Rowe, K.; Snyder, J.; Wan, Y.; Deshmukh, V.; Newman, M.; Fraser, A.; Smith, K.; Herget, K.; et al. Aging-Related Disease Risks among Young Thyroid Cancer Survivors. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Saeed, O.; Goldberg, A.S.; Farooq, S.; Fazelzad, R.; Goldstein, D.P.; Tsang, R.W.; Brierley, J.D.; Ezzat, S.; Thabane, L.; et al. A Systematic Review and Meta-Analysis of Subsequent Malignant Neoplasm Risk After Radioactive Iodine Treatment of Thyroid Cancer. Thyroid 2018, 28, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Marti, J.L.; Jain, K.S.; Morris, L.G. Increased risk of second primary malignancy in pediatric and young adult patients treated with radioactive iodine for differentiated thyroid cancer. Thyroid 2015, 25, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Silva-Vieira, M.; Carrilho Vaz, S.; Esteves, S.; Ferreira, T.C.; Limbert, E.; Salgado, L.; Leite, V. Second Primary Cancer in Patients with Differentiated Thyroid Cancer: Does Radioiodine Play a Role? Thyroid 2017, 27, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Boffetta, P. Multiple primary cancers as clues to environmental and heritable causes of cancer and mechanisms of carcinogenesis. IARC Sci. Publ. 2004, 157, 289–297. [Google Scholar]
- Schonfeld, S.J.; Merino, D.M.; Curtis, R.E.; Berrington de González, A.; Herr, M.M.; Kleinerman, R.A.; Savage, S.A.; Tucker, M.A.; Morton, L.M. Risk of Second Primary Bone and Soft-Tissue Sarcomas Among Young Adulthood Cancer Survivors. JNCI Cancer Spectr. 2019, 3, pkz043. [Google Scholar] [CrossRef] [PubMed]
- Cappagli, V.; Caldarella, A.; Manneschi, G.; Piaggi, P.; Bottici, V.; Agate, L.; Molinaro, E.; Bianchi, F.; Elisei, R. Nonthyroidal second primary malignancies in differentiated thyroid cancer patients: Is the incidence increased comparing to the general population and could it be a radioiodine therapy consequence? Int. J. Cancer 2020, 147, 2838–2846. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, H.; Park, S.; Joo, J.; Kim, I.J.; Kim, B.H. Risk factors for second primary malignancies following thyroid cancer: A nationwide cohort study. Eur. J. Endocrinol. 2022, 186, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef] [PubMed]
- Larouche, V.; Akirov, A.; Thomas, C.M.; Krzyzanowska, M.K.; Ezzat, S. A primer on the genetics of medullary thyroid cancer. Curr. Oncol. 2019, 26, 389–394. [Google Scholar] [CrossRef] [PubMed]
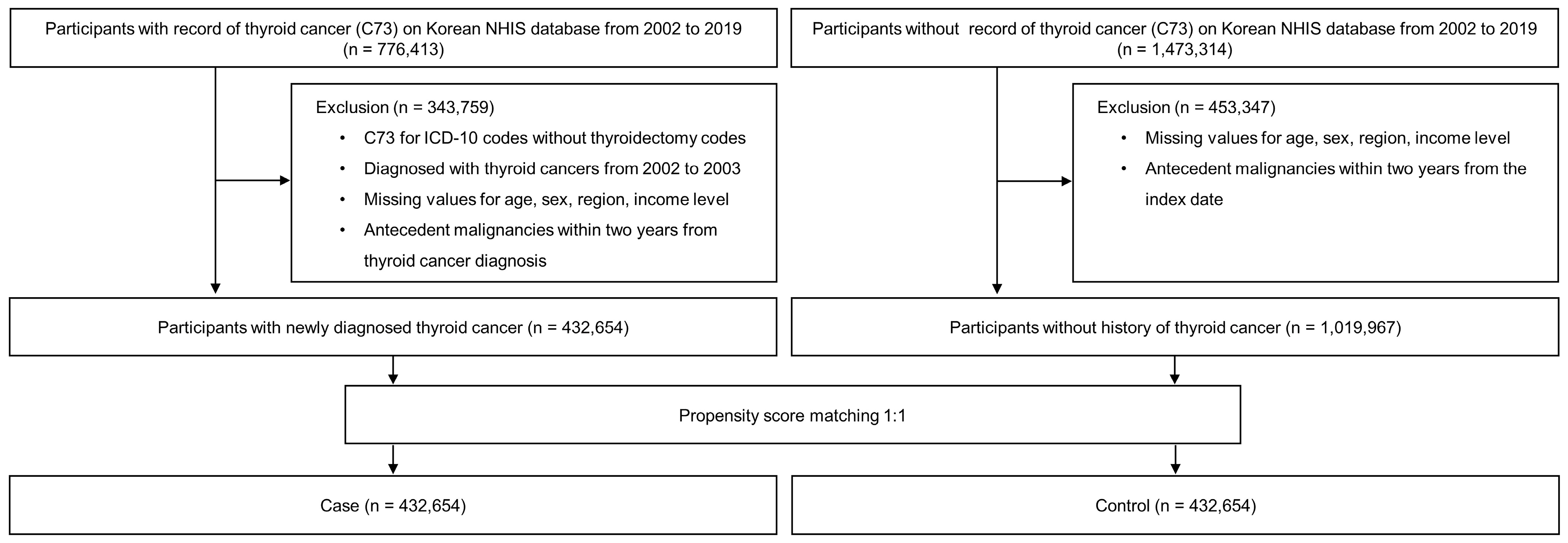

| Overall | Case | Control | p-Value | |
|---|---|---|---|---|
| Total number, n | 865,308 | 432,654 | 432,654 | |
| Age (years), mean ± SD | 48.6 ± 12.2 | 48.6 ± 12.2 | 48.6 ± 12.2 | 0.930 |
| Sex, n (%) | 1.000 | |||
| Male | 164,939 (19.1) | 82,470 (19.1) | 82,469 (19.1) | |
| Female | 700,369 (80.9) | 350,184 (80.9) | 350,185 (80.9) | |
| Region of residence, n (%) | 0.890 | |||
| Urban | 422,549 (48.8) | 211,307 (48.8) | 211,242 (48.8) | |
| Rural | 442,759 (51.2) | 221,347 (51.2) | 221,412 (51.2) | |
| Income level, n (%) | 1.000 | |||
| 1 (lowest) | 133,490 (15.5) | 66,745 (15.4) | 66,745 (15.4) | |
| 2 | 114,044 (13.2) | 57,022 (13.2) | 57,022 (13.2) | |
| 3 | 140,750 (16.3) | 70,375 (16.3) | 70,375 (16.3) | |
| 4 | 193,973 (22.4) | 96,986 (22.4) | 96,987 (22.4) | |
| 5 (highest) | 283,051 (32.7) | 141,526 (32.7) | 141,525 (32.7) | |
| CCI score, n (%) | <0.001 | |||
| 0 | 314,134 (51.8) | 119,726 (27.7) | 194,408 (44.9) | |
| 1 | 112,833 (13.0) | 56,715 (13.1) | 56,118 (13.0) | |
| 2 | 73,239 (8.5) | 38,655 (8.9) | 34,584 (8.0) | |
| 3 | 53,871 (6.2) | 30,388 (7.0) | 23,483 (5.4) | |
| ≥4 | 311,231 (36.0) | 187,170 (43.3) | 124,061 (28.7) | |
| Subgroup a | ||||
| n (%) | 258,585 | 141,952 | 116,633 | |
| Obesity b | <0.001 | |||
| Underweight | 7324 (2.8) | 3472 (2.4) | 3852 (3.3) | |
| Normal | 100,905 (39.0) | 52,170 (36.8) | 48,735 (41.8) | |
| Overweight | 63,497 (24.6) | 34,679 (24.4) | 28,818 (24.7) | |
| Obesity I | 75,443 (29.2) | 44,316 (31.2) | 31,127 (26.7) | |
| Obesity II | 11,416 (4.4) | 7315 (5.2) | 4101 (3.5) | |
| Smoking | <0.001 | |||
| Never | 211,094 (81.6) | 116,839 (82.3) | 94,255 (80.8) | |
| Former | 22,508 (8.7) | 13,408 (9.4) | 9100 (7.8) | |
| Current | 24,983 (9.7) | 11,705 (8.2) | 13,278 (11.4) |
| Type of SPC | Case (n = 432,654) | Control (n = 432,654) | Crude HR (95% CI) | Adjusted HR a (95% CI) |
|---|---|---|---|---|
| All SPCs | 78,584 (18.2) | 49,979 (11.6) | 1.84 (1.82–1.86) | 1.62 (1.60–1.64) |
| Head and Neck (C00–C14, C30–C32) | 2070 (0.5) | 853 (0.2) | 2.68 (2.47–2.90) | 2.27 (2.10–2.47) |
| Digestive system (C15–C26) | 31,518 (7.3) | 22,066 (5.1) | 1.67 (1.64–1.64) | 1.43 (1.41–1.46) |
| Gastrointestinal tract (C15–C20) | 14,204 (3.3) | 8889 (2.1) | 1.83 (1.78–1.88) | 1.57 (1.53–1.62) |
| Esophagus (C15) | 283 (0.1) | 103 (0.0) | 3.09 (2.47–3.88) | 2.53 (2.01–3.18) |
| Stomach (C16) | 4095 (1.0) | 3258 (0.8) | 1.44 (1.38–1.51) | 1.22 (1.17–1.28) |
| Colon and rectum (C18–C20) | 9686 (2.2) | 5429(1.3) | 2.04 (1.97–2.11) | 1.77 (1.71–1.83) |
| Hepato-biliary-pancreatic (C22–C25) | 16,548 (3.8) | 12,432 (2.9) | 1.58 (1.54–1.61) | 1.35 (1.32–1.38) |
| Liver and biliary tract (C22–C24) | 11,483 (2.7) | 8618 (1.9) | 1.56 (1.51–1.60) | 1.35 (1.31–1.39) |
| Pancreas (C25) | 5065 (1.2) | 3814 (0.9) | 1.62 (1.55–1.69) | 1.35 (1.29–1.41) |
| Lung (C34) | 6613 (1.5) | 3682 (0.9) | 2.12 (2.04–2.21) | 1.82 (1.74–1.89) |
| Bone and soft tissue (C40–C41) | 420 (0.1) | 138 (0.0) | 3.40 (2.81–4.13) | 2.96 (2.43–3.59) |
| Limb (C40) | 118 (0.03) | 36 (0.0) | 3.76 (2.58–5.47) | 3.32 (2.27–4.86) |
| Other sites (C41) | 302 (0.07) | 102 (0.0) | 3.28 (2.61–4.11) | 2.83 (2.25–3.55) |
| Skin (C43–44) | 1096 (0.3) | 731 (0.2) | 1.79 (1.63–1.97) | 1.56 (1.42–1.72) |
| Breast (C50) | 10,458 (2.4) | 5173 (1.2) | 2.32 (2.24–2.40) | 2.16 (2.09–2.24) |
| Female genital system (C51–C58) | 10,810 (2.5) | 8312 (1.9) | 1.55 (1.51–1.60) | 1.50 (1.45–1.54) |
| Cervix (C53) | 1975 (0.5) | 1518 (0.4) | 1.48 (1.39–1.59) | 1.37 (1.28–1.47) |
| Uterus (C54–C55) | 2689 (0.6) | 2123 (0.5) | 1.56 (1.48–1.65) | 1.49 (1.40–1.58) |
| Ovary (C56–C57) | 6090 (1.4) | 4615 (1.1) | 1.57 (1.51–1.63) | 1.55 (1.49–1.61) |
| Male genital system (C60–C63) | 4549 (1.1) | 3815 (0.9) | 1.40 (1.40–1.46) | 1.18 (1.13–1.23) |
| Prostate (C61) | 4478 (1.0) | 3764 (0.9) | 1.40 (1.37–1.45) | 1.17 (1.12–1.23) |
| Urinary system (C64–C68) | 4177 (1.0) | 2940 (0.7) | 1.71 (1.63–1.79) | 1.46 (1.39–1.54) |
| Kidney (C64) | 1633 (0.4) | 821 (0.2) | 2.31 (2.12–2.51) | 1.97 (1.80–2.14) |
| Bladder (C67) | 2292 (0.5) | 1940 (0.5) | 1.45 (1.63–1.54) | 1.25 (1.17–1.33) |
| Eye, orbit (C69) | 73 (0.0) | 41 (0.0) | 2.14 (1.46–3.16) | 1.96 (1.32–2.91) |
| Brain, CNS (C70–C72) | 968 (0.2) | 468 (0.1) | 2.41 (2.16–2.70) | 2.15 (1.92–2.40) |
| Adrenal gland (C74) | 278 (0.1) | 68 (0.0) | 4.63 (3.55–6.04) | 4.07 (3.11–5.32) |
| Hematologic system (C81–C96) | 2479 (0.6) | 1135 (0.3) | 2.52 (2.35–2.71) | 2.15 (2.00–2.31) |
| Hodgkin Disease (C81) | 54 (0.0) | 20 (0.0) | 3.05 (1.82–5.11) | 2.79 (1.65–4.70) |
| Non-Hodgkin lymphoma (C82–C86) | 1165 (0.3) | 440 (0.1) | 2.93 (2.62–3.27) | 2.45 (2.19–2.74) |
| Multiple myeloma (C90) | 494 (0.1) | 340 (0.1) | 1.80 (1.57–2.07) | 1.48 (1.28–1.70) |
| Leukemia (C91–C95) | 547 (0.1) | 209 (0.1) | 3.07 (2.61–3.60) | 2.72 (2.31–3.20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Lee, S.J.; Lee, M.H.; Choi, J.H.; Han, H.W.; Song, Y.S. Risk of Subsequent Primary Cancer in Thyroid Cancer Survivors: A Nationwide Population-Based Study. Diagnostics 2023, 13, 2903. https://doi.org/10.3390/diagnostics13182903
Kim M-S, Lee SJ, Lee MH, Choi JH, Han HW, Song YS. Risk of Subsequent Primary Cancer in Thyroid Cancer Survivors: A Nationwide Population-Based Study. Diagnostics. 2023; 13(18):2903. https://doi.org/10.3390/diagnostics13182903
Chicago/Turabian StyleKim, Min-Su, Sang Jun Lee, Myeong Hoon Lee, Jay Hyug Choi, Hyun Wook Han, and Young Shin Song. 2023. "Risk of Subsequent Primary Cancer in Thyroid Cancer Survivors: A Nationwide Population-Based Study" Diagnostics 13, no. 18: 2903. https://doi.org/10.3390/diagnostics13182903
APA StyleKim, M.-S., Lee, S. J., Lee, M. H., Choi, J. H., Han, H. W., & Song, Y. S. (2023). Risk of Subsequent Primary Cancer in Thyroid Cancer Survivors: A Nationwide Population-Based Study. Diagnostics, 13(18), 2903. https://doi.org/10.3390/diagnostics13182903






