Pre-Existing Interstitial Lung Abnormalities in Patients with Head and Neck Squamous Cell Carcinoma and Their Follow Up after Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Analysis of Imaging Data
2.3. Statistical Analysis
2.4. Institutional Rewiev Board Statement
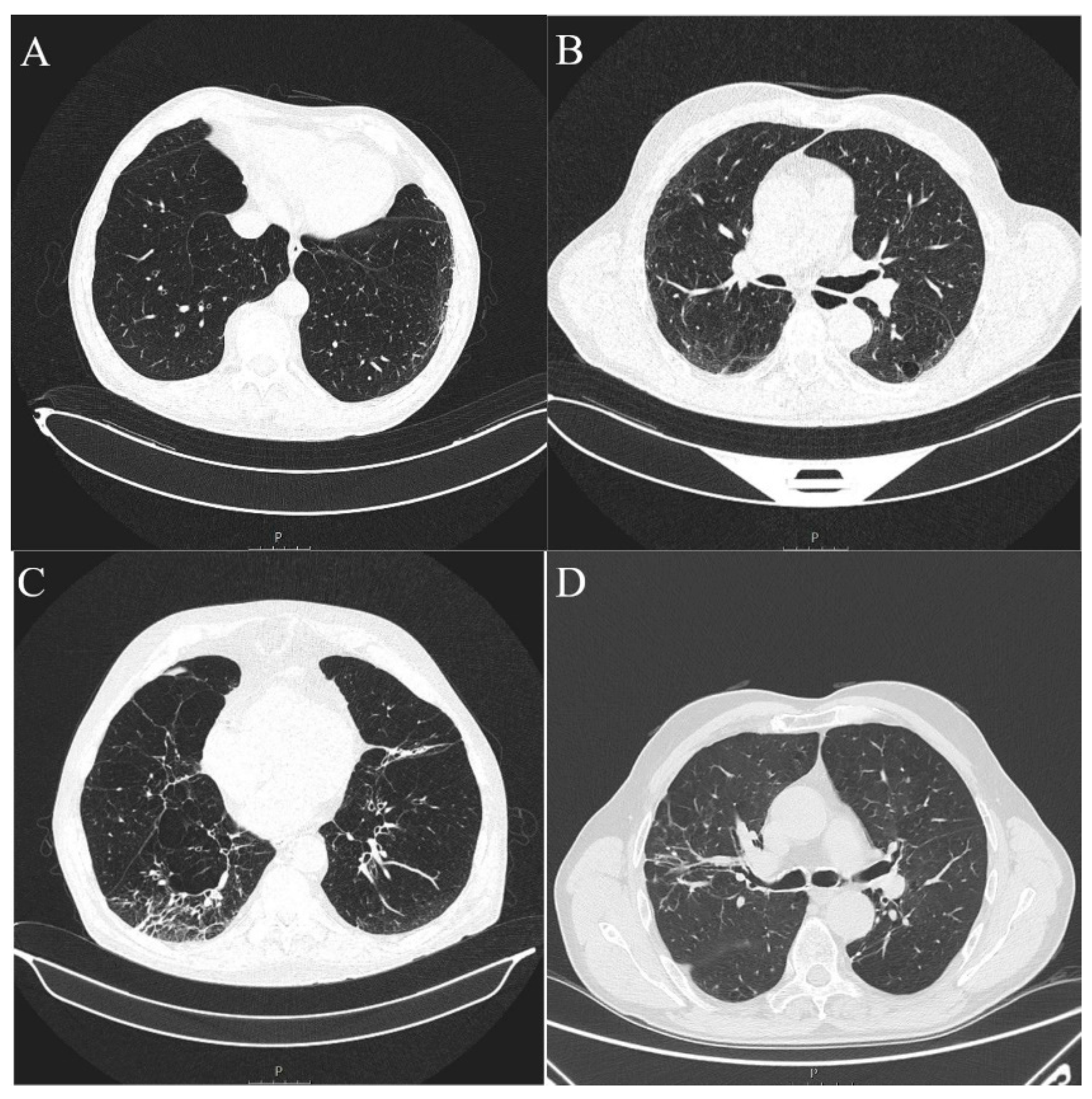
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Strojan, P.; Ferlito, A.; Medina, J.E.; Woolgar, J.A.; Rinaldo, A.; Robbins, K.T.; Fagan, J.J.; Mendenhall, W.M.; Paleri, V.; Silver, C.E.; et al. Contemporary management of lymph node metastases from an unknown primary to the neck: I. A review of diagnostic approaches. Head Neck 2013, 35, 123–132. [Google Scholar] [CrossRef]
- Civantos, F.J.; Vermorken, J.B.; Shah, J.P.; Rinaldo, A.; Suárez, C.; Kowalski, L.P.; Rodrigo, J.P.; Olsen, K.; Strojan, P.; Mäkitie, A.A.; et al. Metastatic Squamous Cell Carcinoma to the Cervical Lymph Nodes from an Unknown Primary Cancer: Management in the HPV Era. Front. Oncol. 2020, 10, 593164. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.P.; Saha, S.; Kraninger, J.L.; Swick, A.D.; Yu, M.; Lambert, P.F.; Kimple, R. Prevalence of Human Papillomavirus in Oro-pharyngeal Cancer: A Systematic Review. Cancer J. 2015, 21, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Schiebler, M.L.; Lynch, D.A.; Hatabu, H. Interstitial Lung Abnormalities: State of the Art. Radiology 2021, 301, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Hatabu, H.; Hunninghake, G.M.; Richeldi, L.; Brown, K.K.; Wells, A.U.; Remy-Jardin, M.; Verschakelen, J.; Nicholson, A.G.; Beasley, M.B.; Christiani, D.C.; et al. Interstitial lung abnormalities detected incidentally on CT: A Position Paper from the Fleischner Society. Lancet Respir. Med. 2020, 8, 726–737. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Hatabu, H.; Hunninghake, G.M.; Lynch, D.A. Interstitial Lung Abnormality: Recognition and Perspectives. Radiology 2019, 291, 1–3. [Google Scholar] [CrossRef]
- Araki, T.; Putman, R.K.; Hatabu, H.; Gao, W.; Dupuis, J.; Latourelle, J.C.; Nishino, M.; Zazueta, O.E.; Kurugol, S.; Ross, J.C.; et al. Development and Progression of Interstitial Lung Abnormalities in the Framingham Heart Study. Am. J. Respir. Crit. Care Med. 2016, 194, 1514–1522. [Google Scholar] [CrossRef]
- Tan, Y.; Jia, D.; Lin, Z.; Guo, B.; He, B.; Lu, C.; Xiao, C.; Liu, Z.; Zhao, N.; Bian, Z.; et al. Potential Metabolic Biomarkers to Identify Interstitial Lung Abnormalities. Int. J. Mol. Sci. 2016, 17, 1148. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Quintero, B.; Buendía-Roldán, I.; Ramírez-Salazar, E.G.; Balderas-Martínez, Y.I.; Ramírez-Rodríguez, S.L.; Martínez-Espinosa, K.; Selman, M. Circulating microRNA Signature Associated to Interstitial Lung Abnormalities in Respiratory Asymptomatic Subjects. Cells 2020, 9, 1556. [Google Scholar] [CrossRef] [PubMed]
- Putman, R.K.; Gudmundsson, G.; Axelsson, G.T.; Hida, T.; Honda, O.; Araki, T.; Yanagawa, M.; Nishino, M.; Miller, E.R.; Eiriksdottir, G.; et al. Imaging Patterns Are Associated with Interstitial Lung Abnormality Progression and Mortality. Am. J. Respir. Crit. Care Med. 2019, 200, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Hunninghake, G.M.; Hatabu, H.; Okajima, Y.; Gao, W.; Dupuis, J.; Latourelle, J.C.; Nishino, M.; Araki, T.; Zazueta, O.E.; Kurugol, S.; et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N. Engl. J. Med. 2013, 368, 2192–2200. [Google Scholar] [CrossRef]
- Buendia-Roldan, I.; Ponce-Gallegos, M.A.; Lara-Beltrán, D.; Del Ángel-Pablo, A.D.; Pérez-Rubio, G.; Mejía, M.; Selman, M.; Falfán-Valencia, R. The HLA-DRB1*07 Allele Is Associated with Interstitial Lung Abnormalities (ILA) and Subpleural Location in a Mexican Mestizo Pop-ulation. Biomolecules 2022, 12, 1662. [Google Scholar] [CrossRef]
- Hoyer, N.; Wille, M.M.; Thomsen, L.H.; Wilcke, T.; Dirksen, A.; Pedersen, J.H.; Saghir, Z.; Ashraf, H.; Shaker, S.B. Interstitial lung abnormalities are associated with increased mortality in smokers. Respir. Med. 2018, 136, 77–82. [Google Scholar] [CrossRef]
- Brown, S.-A.W.; Padilla, M.; Mhango, G.; Powell, C.; Salvatore, M.; Henschke, C.; Yankelevitz, D.; Sigel, K.; De-Torres, J.P.; Wisnivesky, J. Interstitial Lung Abnormalities and Lung Cancer Risk in the National Lung Screening Trial. Chest 2019, 156, 1195–1203. [Google Scholar] [CrossRef]
- Im, Y.; Park, H.Y.; Shin, S.; Shin, S.H.; Lee, H.; Ahn, J.H.; Sohn, I.; Cho, J.H.; Kim, H.K.; Zo, J.I.; et al. Prevalence of and risk factors for pulmonary complications after curative resection in otherwise healthy elderly patients with earlystage lung cancer. Respir. Res. 2019, 20, 136. [Google Scholar] [CrossRef]
- Li, F.; Zhou, Z.; Wu, A.; Cai, Y.; Wu, H.; Chen, M.; Liang, S. Preexisting radiological interstitial lung abnormalities are a risk factor for severe radiation pneumonitis in patients with small-cell lung cancer after thoracic radiation therapy. Radiat. Oncol. 2018, 13, 82. [Google Scholar] [CrossRef]
- Ito, M.; Katano, T.; Okada, H.; Sakuragi, A.; Minami, Y.; Abe, S.; Adachi, S.; Oshima, Y.; Ohashi, W.; Kubo, A.; et al. Subpleural fibrotic interstitial lung abnormalities are implicated in non-small cell lung cancer radiotherapy outcomes. Radiol. Oncol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Masuda, T.; Yamaguchi, K.; Sakamoto, S.; Horimasu, Y.; Nakashima, T.; Miyamoto, S.; Tsutani, Y.; Iwamoto, H.; Fujitaka, K.; et al. Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer. Respir. Investig. 2019, 57, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Li, Y.Z.; Jin, G.Y.; Chae, K.J.; Han, Y.M. Quantitative Assessment of Airway Changes in Fibrotic Interstitial Lung Abnormality Patients by Chest CT According to Cumulative Cigarette Smoking. Tomography 2022, 8, 1024–1032. [Google Scholar] [CrossRef]
- Choi, W.I.; Dauti, S.; Kim, H.J.; Park, S.H.; Park, J.S.; Lee, C.W. Risk factors for interstitial lung disease: A 9-year Nationwide population-based study. BMC Pulm. Med. 2018, 18, 96. [Google Scholar] [CrossRef]
- Tseng, S.C.; Hino, T.; Hatabu, H.; Park, H.; Sanford, N.N.; Lin, G.; Nishino, M.; Mamon, H. Interstitial Lung Abnormalities in Patients with Locally Advanced Esophageal Cancer: Prevalence, Risk Factors, and Clinical Implications. J. Comput. Assist. Tomogr. 2022, 46, 871–877. [Google Scholar] [CrossRef]
- Mackintosh, J.A.; Marshall, H.M.; Slaughter, R.; Reddy, T.; Yang, I.A.; Bowman, R.V.; Fong, K.M. Interstitial lung abnormalities in the Queensland Lung Cancer Screening Study: Prevalence and progression over 2 years of surveillance. Intern. Med. J. 2019, 49, 843–849. [Google Scholar] [CrossRef]
- Jin, G.Y.; Lynch, D.; Chawla, A.; Garg, K.; Tammemagi, M.C.; Sahin, H.; Misumi, S.; Kwon, K.S. Interstitial lung abnormalities in a CT lung cancer screening population: Prevalence and progression rate. Radiology 2013, 268, 563–571. [Google Scholar] [CrossRef]
- Nemec, S.F.; Bankier, A.A.; Eisenberg, R.L. Lower Lobe—Predominant Diseases of the Lung. Am. J. Roentgenol. 2013, 200, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, H.; Richeldi, L.; Zhu, M.; Huang, Y.; Xiong, X.; Liao, J.; Zhu, W.; Mao, L.; Xu, L.; et al. Reticulation Is a Risk Factor of Progressive Subpleural Nonfibrotic Interstitial Lung Abnormalities. Am. J. Respir. Crit. Care Med. 2022, 206, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Chae, K.J.; Suh, Y.J.; Jeong, W.G.; Lee, T.; Kim, Y.-H.; Jin, G.Y.; Jeong, Y.J. Prevalence and Long-term Outcomes of CT Interstitial Lung Abnormalities in a Health Screening Cohort. Radiology 2023, 306, e221172. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, E.; Meena, P.; Ramalingam, V.; Sahoo, R.; Rastogi, S.; Thulkar, S. Chemotherapy-induced pulmonary complications in cancer: Significance of clinicoradiological correlation. Indian. J. Radiol. Imaging 2020, 30, 20–26. [Google Scholar] [CrossRef]
- Patel, A.S.; Miller, E.; Regis, S.M.; Hunninghake, G.M.; Price, L.L.; Gawlik, M.; McKee, A.B.; Rieger-Christ, K.M.; Pinto-Plata, V.; Liesching, T.N.; et al. Interstitial lung abnormalities in a large clinical lung cancer screening cohort: Association with mortality and ILD diagnosis. Respir. Res. 2023, 24, 49. [Google Scholar] [CrossRef]
- Daido, W.; Masuda, T.; Imano, N.; Matsumoto, N.; Hamai, K.; Iwamoto, Y.; Takayama, Y.; Ueno, S.; Sumii, M.; Shoda, H.; et al. Pre-Existing Interstitial Lung Abnormalities Are Independent Risk Factors for Interstitial Lung Disease during Durvalumab Treatment after Chemoradiotherapy in Patients with Locally Advanced Non-Small-Cell Lung Cancer. Cancers 2022, 14, 6236. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Wang, X.; Zhou, J.; Wei, Q.; Jiang, R. Association of pre-existing lung interstitial changes with immune-related pneumonitis in patients with non-small lung cancer receiving immunotherapy. Support. Care Cancer 2022, 30, 6515–6524. [Google Scholar] [CrossRef]
- Murata, D.; Azuma, K.; Matama, G.; Zaizen, Y.; Matsuo, N.; Murotani, K.; Tokito, T.; Hoshino, T. Clinical significance of interstitial lung abnormalities and immune checkpoint inhibitor-induced interstitial lung disease in patients with non-small cell lung cancer. Thorac. Cancer 2023, 14, 73–80. [Google Scholar] [CrossRef]
- Jeong, W.G.; Kim, Y.-H.; Ahn, S.-J.; Jeong, J.-U.; Lee, B.C.; Cho, I.J.; Kim, Y.-H. Effect of Interstitial Lung Abnormality on Concurrent Chemoradiotherapy-treated Stage III Non-small Cell Lung Cancer Patients. Anticancer Res. 2023, 43, 1797–1807. [Google Scholar] [CrossRef]
- Colombi, D.; Petrini, M.; Morelli, N.; Silva, M.; Milanese, G.; Sverzellati, N.; Michieletti, E. Are Interstitial Lung Abnormalities a Prognostic Factor of Worse Outcome in COVID-19 Pneumonia? J. Thorac. Imaging 2023, 38, 137–144. [Google Scholar] [CrossRef]
- Warheit-Niemi, H.I.; Edwards, S.J.; SenGupta, S.; Parent, C.A.; Zhou, X.; O’dwyer, D.N.; Moore, B.B. Fibrotic lung disease inhibits immune responses to staphylococcal pneumonia via impaired neutrophil and macrophage function. JCI Insight 2022, 7, e152690. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, J.; Bari, S.; Kirtane, K.; Chung, C.H. Recent Advances and Future Directions in Clinical Management of Head and Neck Squamous Cell Carcinoma. Cancers 2021, 18, 338. [Google Scholar] [CrossRef] [PubMed]


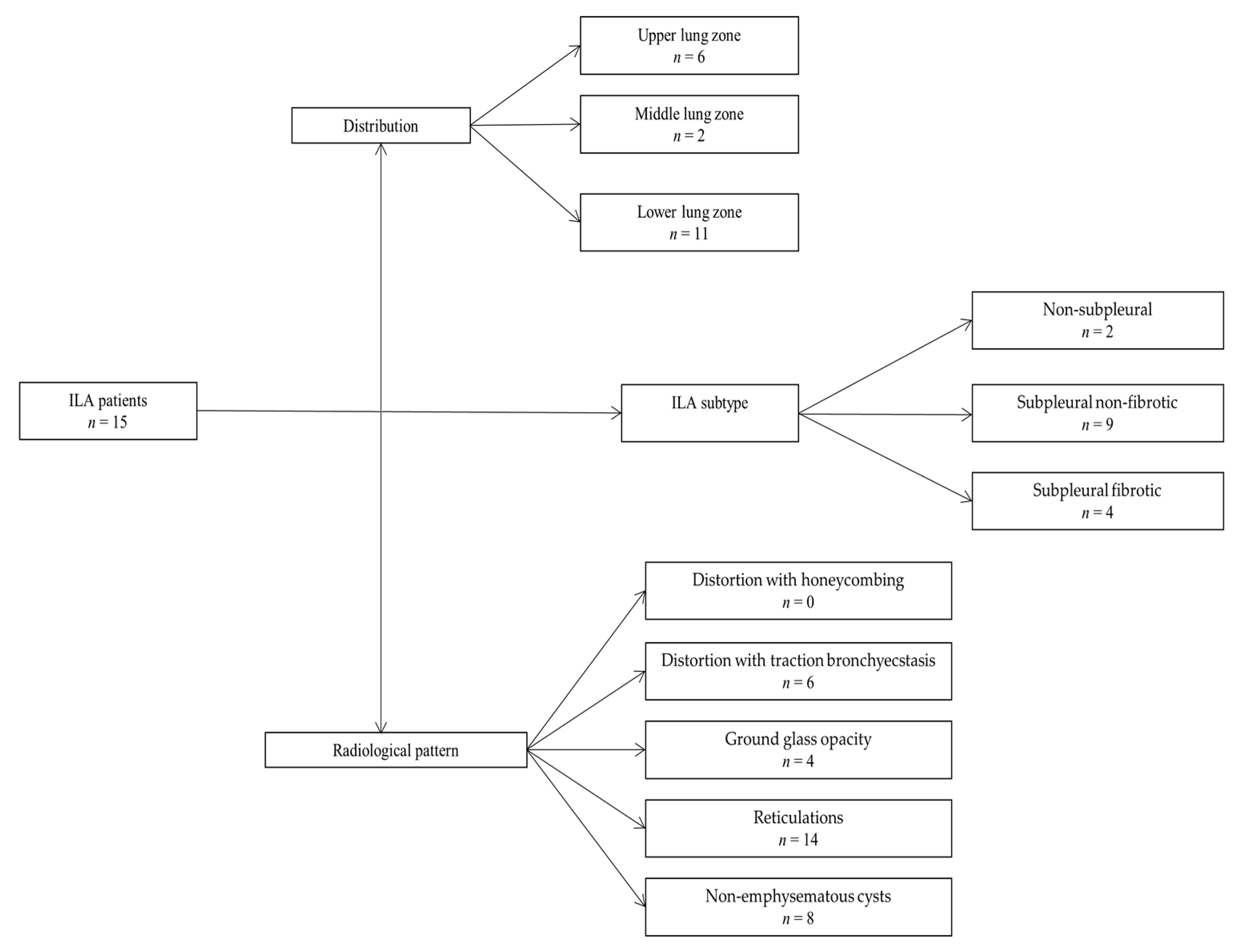


| ILA (n = 15) | Non-ILA (n = 98) | p Value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Age (years, IQR) | 75 (65–75) | 67 (61–71.25) | 0.0300 * |
| Sex (Male) | 14 (93.33) | 86 (87.76) | 0.5283 # |
| Alcohol consumption | 2 (13.33) | 11 (11.22) | 0.8115 # |
| Tobacco smoking statusNumber of cigarettes per day, n = 49 (IQR) | 12 (80.00) 30 (20–50) | 89 (90.82) 40 (20–40) | 0.1979 # 0.3878 † |
| Earlier localized malignancy | 2 (13.33) | 8 (8.16) | 0.6198 # |
| Earlier metastatic malignancy | 0 (0) | 2 (2.04) | 0.5767 # |
| Diabetes mellitus | 2 (13.33) | 8 (81.63) | 0.6198 # |
| Arterial hypertension | 7 (46.67) | 19 (19.39) | 0.0418 # |
| COPD | 2 (13.33) | 8 (8.16) | 0.6198 # |
| Region of cancer | |||
| Oral cavity | 3 (20.00) | 34 (34.69) | 0.3781 # |
| Pharynx | 5 (33.33) | 29 (29.59) | 0.7684 # |
| Larynx | 7 (46.67) | 33 (33.67) | 0.3886 # |
| Salivary glands | 0 | 1 (1.02) | 1 # |
| Unknown origin | 0 | 3 (30.61) | 1 # |
| ILA (n = 15) | Non-ILA (n = 98) | p Value * | |
|---|---|---|---|
| n (%) | n (%) | ||
| Centrilobular emphysema | 7 (46.67) | 27 (27.55) | 0.1429 |
| Paraseptal emphysema | 8 (53.33) | 28 (28.57) | 0.0744 |
| Centrilobular nodules | 5 (33.33) | 16 (16.33) | 0.1504 |
| Pleural plaques | 2 (13.33) | 0 (0) | 0.0165 |
| Coronary calcifications Lung metastasis | 13 (86.67) 2 (13.33) | 78 (79.59) 7 (7.14) | 0.7315 0.341 |
| Lower Zone | Middle Zone | Upper Zone | p Value * | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Present | 11 (73.33) | 2 (13.33) | 6 (40.00) | 0.0045 |
| Non-subpleural | Subpleural fibrotic | Subpleural non-fibrotic | ||
| n (%) | n (%) | n (%) | ||
| Present | 2 (13.33) | 4 (26.67) | 9 (60.00) | 0.0354 |
| Distortion with Honeycombing | Distortion with Traction Bronchiectasis | Ground Glass Opacity | Non-Emphysematous Cysts | Reticulations | p Value * | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Present | 0 (0.00) | 6 (40.00) | 4 (26.67) | 8 (53.33) | 14 (93.33) | <0.0001 |
| Upper Zone | Middle Zone | Lower Zone | p Value * | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Arterial hypertension | 1 (12.5) | 1 (12.5) | 6 (75) | 0.0338 |
| Diabetes mellitus | 1 (33.33) | 1 (33.33) | 1 (33.33) | 1 |
| COPD | 0 (0) | 0 (0) | 2 (100) | 0.3182 |
| ILA (n = 6 of 11 Follow-Up MSCT in the ILA Group) | Non-ILA (n = 15 of 54 Follow-Up MSCT in Non-ILA Group) | p Value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Age (years, IQR) | 74 (70–74) | 70 (63–77) | 0.1584 # |
| Sex (male) | 5 (83.33) | 12 (80.00) | 1 * |
| Radiation-induced lung fibrosis | 5 (83.33) | 4 (26.67) | 0.0464 * |
| Region of cancer | |||
| Oral cavity | 2 (33.33) | 5 (33.33) | 1 * |
| Pharynx | 1 (16.67) | 2 (13.33) | 1 * |
| Larynx | 3 (50.00) | 8 (53.33) | 1 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuković, D.; Budimir Mršić, D.; Jerković, K.; Benzon, B.; Tadić, T. Pre-Existing Interstitial Lung Abnormalities in Patients with Head and Neck Squamous Cell Carcinoma and Their Follow Up after Therapy. Diagnostics 2023, 13, 2908. https://doi.org/10.3390/diagnostics13182908
Vuković D, Budimir Mršić D, Jerković K, Benzon B, Tadić T. Pre-Existing Interstitial Lung Abnormalities in Patients with Head and Neck Squamous Cell Carcinoma and Their Follow Up after Therapy. Diagnostics. 2023; 13(18):2908. https://doi.org/10.3390/diagnostics13182908
Chicago/Turabian StyleVuković, Danica, Danijela Budimir Mršić, Kristian Jerković, Benjamin Benzon, and Tade Tadić. 2023. "Pre-Existing Interstitial Lung Abnormalities in Patients with Head and Neck Squamous Cell Carcinoma and Their Follow Up after Therapy" Diagnostics 13, no. 18: 2908. https://doi.org/10.3390/diagnostics13182908
APA StyleVuković, D., Budimir Mršić, D., Jerković, K., Benzon, B., & Tadić, T. (2023). Pre-Existing Interstitial Lung Abnormalities in Patients with Head and Neck Squamous Cell Carcinoma and Their Follow Up after Therapy. Diagnostics, 13(18), 2908. https://doi.org/10.3390/diagnostics13182908







