Automated Intracranial Clot Detection: A Promising Tool for Vascular Occlusion Detection in Non-Enhanced CT
Abstract
:1. Introduction
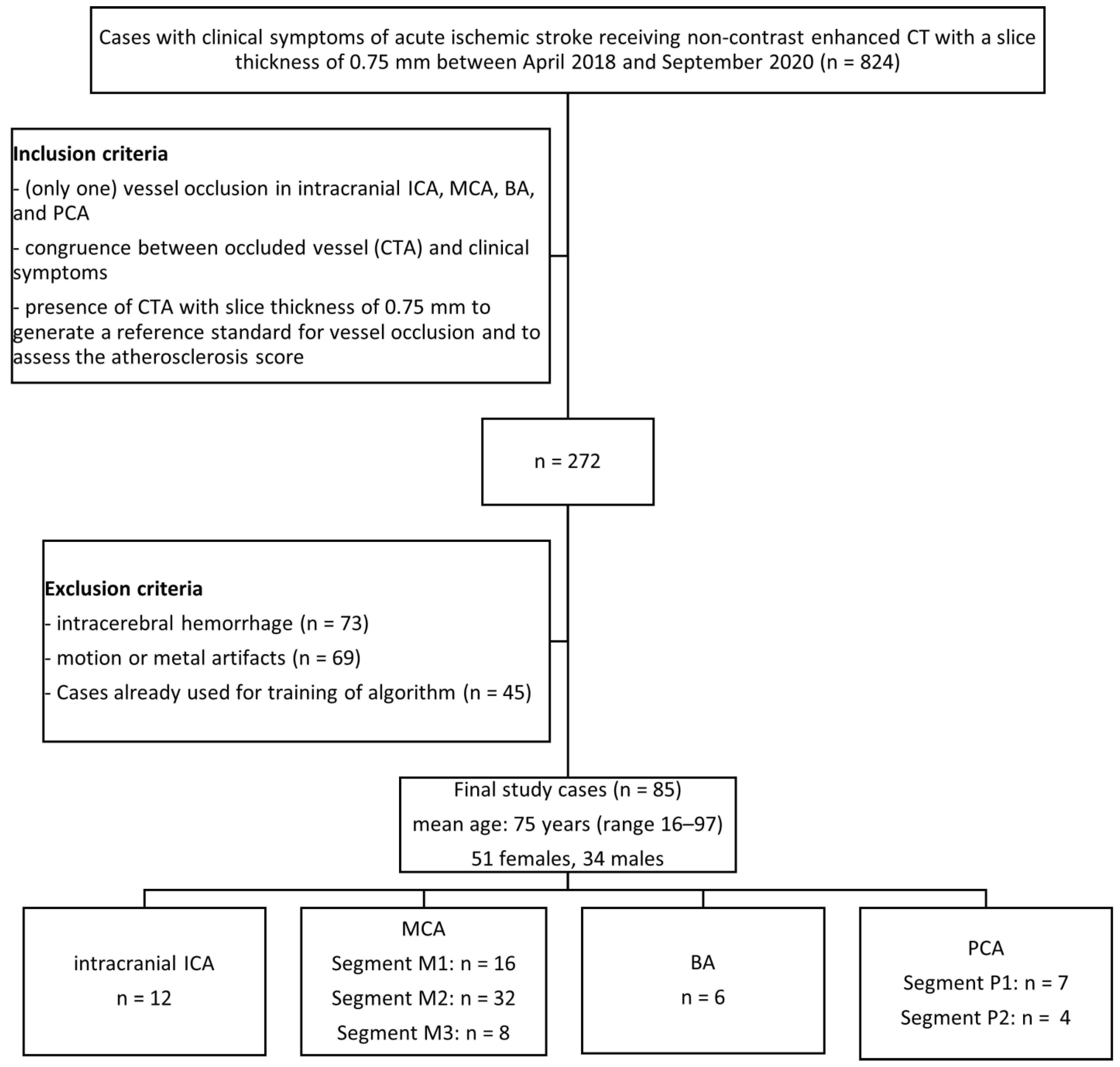
2. Materials and Methods
2.1. Study Design
2.2. CT Image Analysis
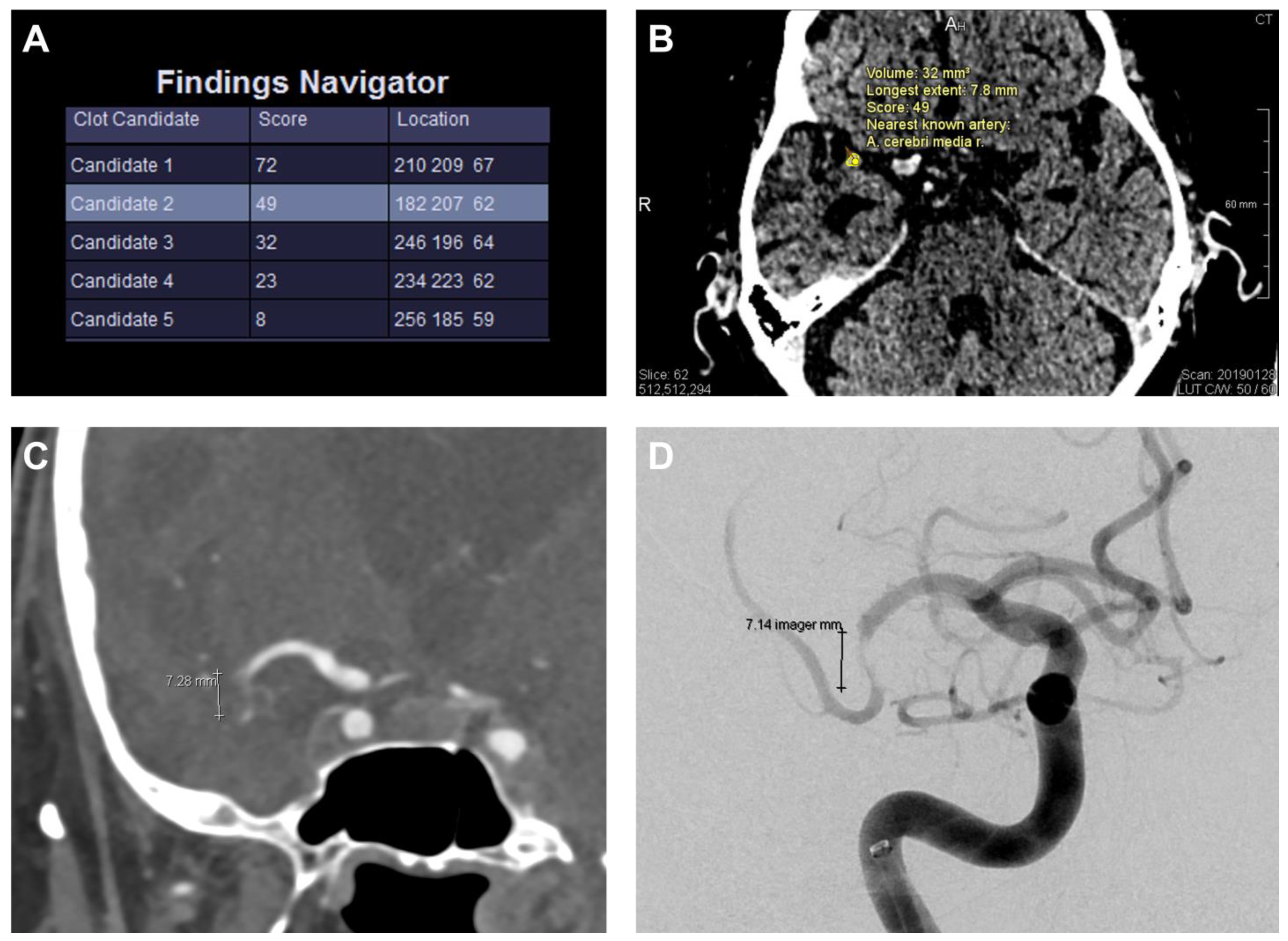
2.3. Automated Clot Detection Tool
2.4. Clinical Analysis
2.5. Standard of Reference
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Results of the NECT Analysis by the Readers
3.3. Results of the Automated Clot Detection by the Prototype
3.4. Head-to-Head Comparison of the Tool with the Human Readers
3.5. Clinical Use Case: Decision Support for ICA/MCA Recanalization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameter | ICA | MCA1 | MCA2 | MCA3 | BA | PCA1 | PCA2 | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Total number based on CTA | 12 | 16 | 32 | 8 | 6 | 7 | 2 | 85 [100%] | |
| Side [r/l] | basilar | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 6 [7%] |
| left | 7 | 8 | 14 | 7 | 0 | 2 | 1 | 40 [47%] | |
| right | 5 | 8 | 18 | 1 | 0 | 5 | 1 | 39 [46%] | |
| NIHSS: median (range) | 12 (0–23) | 11 (1–22) | 7 (2–21) | 5 (1–21) | 0 (32–11) | 9 (4–22) | 5 (2–19) | ||
| Time interval between symptom onset and CT scan | <4.5 h | 8 | 12 | 24 | 6 | 3 | 4 | 0 | 58 [68%] |
| >4.5 h | 4 | 1 | 2 | 0 | 1 | 3 | 1 | 12 [14%] | |
| uncertain | 0 | 3 | 6 | 2 | 2 | 0 | 1 | 15 [18%] | |
| Etiology of Stroke classified to TOAST criteria | large-artery atherosclerosis | 5 | 2 | 6 | 0 | 4 | 0 | 1 | 19 [22%] |
| cardioembolic | 5 | 7 | 20 | 3 | 1 | 4 | 1 | 41 [48%] | |
| other determined etiology | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 6 [7%] | |
| undetermined etiology | 1 | 6 | 4 | 3 | 1 | 3 | 0 | 19 [22%] | |
| Atherosclerosis Score published by Chen et al. [24] | none | 1 | 3 | 6 | 1 | 0 | 0 | 0 | 12 [14%] |
| mild | 3 | 10 | 5 | 2 | 4 | 1 | 0 | 26 [31%] | |
| mild to moderate | 2 | 3 | 14 | 5 | 0 | 2 | 0 | 26 [31%] | |
| moderate | 3 | 0 | 3 | 0 | 1 | 1 | 2 | 10 [12%] | |
| severe | 3 | 0 | 4 | 0 | 1 | 3 | 0 | 11 [13%] | |
| Therapy chosen | exclusively supportive | 3 | 0 | 7 | 4 | 3 | 3 | 1 | 22 [26%] |
| intravenous thrombolysis | 4 | 1 | 14 | 4 | 1 | 3 | 0 | 28 [33%] | |
| interventional thrombectomy | 4 | 5 | 1 | 0 | 1 | 0 | 0 | 11 [13%] | |
| thrombolysis and thrombectomy | 1 | 10 | 10 | 0 | 1 | 1 | 1 | 24 [28%] | |
| Location | Clot Candidate Score [Number (Mean ± Standard Deviation)] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Position 1 | Position 2 | Position 3 | Position 4 | Position 5 | ||||||
| ICA | 7 | (77 ± 7) | 3 | (53 ± 17) | 0 | (0 ± 0) | 1 | (23 ± 0) | 0 | (0 ± 0) |
| MCA1 | 11 | (67 ± 10) | 1 | (49 ± 0) | 0 | (0 ± 0) | 0 | (0 ± 0) | 2 | (9 ± 8) |
| MCA2 | 6 | (68 ± 18) | 9 | (32 ± 16) | 5 | (28 ± 16) | 2 | (22 ± 15) | 0 | (0 ± 0) |
| MCA3 | 1 | (33 ± 0) | 0 | (0 ± 0) | 0 | (0 ± 0) | 1 | (8 ± 0) | 1 | (15 ± 0) |
| BA | 2 | (49 ± 4) | 0 | (0 ± 0) | 1 | (29 ± 0) | 0 | (0 ± 0) | 1 | (9 ± 0) |
| PCA1 | 1 | (76 ± 0) | 0 | (0 ± 0) | 0 | (0 ± 0) | 0 | (0 ± 0) | 0 | (0 ± 0) |
| PCA2 | 0 | (0 ± 0) | 0 | (0 ± 0) | 1 | (13 ± 0) | 0 | (0 ± 0) | 0 | (0 ± 0) |
| Total | 28 | (68 ± 14) | 13 | (38 ± 18) | 7 | (26 ± 15) | 4 | (19 ± 11) | 4 | (10 ± 6) |
References
- Ingall, T. Stroke—Incidence, mortality, morbidity and risk. J. Insur. Med. 2004, 36, 143–152. [Google Scholar] [PubMed]
- Wardlaw, J.M.; Mielke, O. Early signs of brain infarction at CT: Observer reliability and outcome after thrombolytic treatment--systematic review. Radiology 2005, 235, 444–453. [Google Scholar] [CrossRef]
- Leys, D.; Pruvo, J.P.; Godefroy, O.; Rondepierre, P.; Leclerc, X. Prevalence and significance of hyperdense middle cerebral artery in acute stroke. Stroke 1992, 23, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Goyal, M.; Al Azri, F.; Lum, C. State-of-the-art imaging of acute stroke. Radiographics 2006, 26 (Suppl. S1), S75–S95. [Google Scholar] [CrossRef] [PubMed]
- Mair, G.; Boyd, E.V.; Chappell, F.M.; Kummer, R.v.; Lindley, R.I.; Sandercock, P.; Wardlaw, J.M. Sensitivity and specificity of the hyperdense artery sign for arterial obstruction in acute ischemic stroke. Stroke 2015, 46, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Liebeskind, D.S.; Sanossian, N.; Yong, W.H.; Starkman, S.; Tsang, M.P.; Moya, A.L.; Zheng, D.D.; Abolian, A.M.; Kim, D.; Ali, L.K.; et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011, 42, 1237–1243. [Google Scholar] [CrossRef]
- Moftakhar, P.; English, J.D.; Cooke, D.L.; Kim, W.T.; Stout, C.; Smith, W.S.; Dowd, C.F.; Higashida, R.T.; van Halbach, V.; Hetts, S.W. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke 2013, 44, 243–245. [Google Scholar] [CrossRef]
- Tan, I.Y.L.; Demchuk, A.M.; Hopyan, J.; Zhang, L.; Gladstone, D.; Wong, K.; Martin, M.; Symons, S.P.; Fox, A.J.; Aviv, R.I. CT angiography clot burden score and collateral score: Correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR. Am. J. Neuroradiol. 2009, 30, 525–531. [Google Scholar] [CrossRef]
- Winklhofer, S.; Vittoria De Martini, I.; Nern, C.; Blume, I.; Wegener, S.; Pangalu, A.; Valavanis, A.; Alkadhi, H.; Guggenberger, R. Dual-Energy Computed Tomography in Stroke Imaging: Technical and Clinical Considerations of Virtual Noncontrast Images for Detection of the Hyperdense Artery Sign. J. Comput. Assist. Tomogr. 2017, 41, 843–848. [Google Scholar] [CrossRef]
- Mair, G.; Kummer, R.v.; Morris, Z.; Heijne, A.v.; Bradey, N.; Cala, L.; Peeters, A.; Farrall, A.J.; Adami, A.; Potter, G.; et al. Effect of alteplase on the CT hyperdense artery sign and outcome after ischemic stroke. Neurology 2016, 86, 118–125. [Google Scholar] [CrossRef]
- Froehler, M.T.; Tateshima, S.; Duckwiler, G.; Jahan, R.; Gonzalez, N.; Vinuela, F.; Liebeskind, D.; Saver, J.L.; Villablanca, J.P. The hyperdense vessel sign on CT predicts successful recanalization with the Merci device in acute ischemic stroke. J. NeuroInterventional Surg. 2013, 5, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Weyland, C.S.; Papanagiotou, P.; Schmitt, N.; Joly, O.; Bellot, P.; Mokli, Y.; Ringleb, P.A.; Kastrup, A.; Möhlenbruch, M.A.; Bendszus, M.; et al. Hyperdense Artery Sign in Patients with Acute Ischemic Stroke-Automated Detection With Artificial Intelligence-Driven Software. Front. Neurol. 2022, 13, 807145. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, K.; Shibata, M.; Nakajima, H.; Mizutani, A.; Kitano, Y.; Seguchi, M.; Yamasaki, M.; Kobayashi, K.; Sano, T.; Mori, G.; et al. Erythrocyte-Rich Thrombus Is Associated with Reduced Number of Maneuvers and Procedure Time in Patients with Acute Ischemic Stroke Undergoing Mechanical Thrombectomy. Cerebrovasc. Dis. Extra 2018, 8, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Gersh, K.C.; Nagaswami, C.; Weisel, J.W. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb. Haemost. 2009, 102, 1169–1175. [Google Scholar] [CrossRef]
- Chueh, J.Y.; Wakhloo, A.K.; Hendricks, G.H.; Silva, C.F.; Weaver, J.P.; Gounis, M.J. Mechanical characterization of thromboemboli in acute ischemic stroke and laboratory embolus analogs. AJNR. Am. J. Neuroradiol. 2011, 32, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.M.; Zevallos, C.B.; Dandapat, S.; Ume, K.L.; Weber, M.; Dajles, A.; Quispe-Orozco, D.; Farooqui, M.; Ortega-Gutierrez, S. Quantitative Assessment of Hyperdense Sign Measured by Hounsfield Units is Associated with Unsuccessful Mechanical Thrombectomy. Clin. Neuroradiol. 2021, 31, 1111–1119. [Google Scholar] [CrossRef]
- Kimura, K.; Iguchi, Y.; Shibazaki, K.; Watanabe, M.; Iwanaga, T.; Aoki, J. M1 susceptibility vessel sign on T2* as a strong predictor for no early recanalization after IV-t-PA in acute ischemic stroke. Stroke 2009, 40, 3130–3132. [Google Scholar] [CrossRef]
- Bastianello, S.; Pierallini, A.; Colonnese, C.; Brughitta, G.; Angeloni, U.; Antonelli, M.; Fantozzi, L.M.; Fieschi, C.; Bozzao, L. Hyperdense middle cerebral artery CT sign. Neuroradiology 1991, 33, 207–211. [Google Scholar] [CrossRef]
- Novotná, J.; Kadlecová, P.; Czlonkowska, A.; Brozman, M.; Švigelj, V.; Csiba, L.; Kõrv, J.; Demarin, V.; Vilionskis, A.; Mikulík, R. Hyperdense cerebral artery computed tomography sign is associated with stroke severity rather than stroke subtype. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, 2533–2539. [Google Scholar] [CrossRef]
- Löber, P.; Stimpel, B.; Syben, C.; Maier, A.; Ditt, H.; Schramm, P.; Raczkowski, B.; Kemmling, A. Automatic Thrombus Detection in Non-Enhanced Computed Tomography Images in Patients with Acute Ischemic Stroke; The Eurographics Association: Saarbrücken, Germany, 2017. [Google Scholar]
- Takahashi, N.; Lee, Y.; Tsai, D.-Y.; Matsuyama, E.; Kinoshita, T.; Ishii, K. An automated detection method for the MCA dot sign of acute stroke in unenhanced CT. Radiol. Phys. Technol. 2014, 7, 79–88. [Google Scholar] [CrossRef]
- Tolhuisen, M.L.; Ponomareva, E.; Boers, A.M.M.; Jansen, I.G.H.; Koopman, M.S.; Sales Barros, R.; Berkhemer, O.A.; van Zwam, W.H.; van der Lugt, A.; Majoie Charles, B.L.M.; et al. A Convolutional Neural Network for Anterior Intra-Arterial Thrombus Detection and Segmentation on Non-Contrast Computed Tomography of Patients with Acute Ischemic Stroke. Appl. Sci. 2020, 10, 4861. [Google Scholar] [CrossRef]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Lam, W.W.M.; Ng, H.K.; Fan, Y.-H.; Wong, K.S. The frequency and determinants of calcification in intracranial arteries in Chinese patients who underwent computed tomography examinations. Cerebrovasc. Dis. 2006, 21, 91–97. [Google Scholar] [CrossRef]
- Woodcock, R.J.; Goldstein, J.H.; Kallmes, D.F.; Cloft, H.J.; Phillips, C.D. Angiographic correlation of CT calcification in the carotid siphon. AJNR. Am. J. Neuroradiol. 1999, 20, 495–499. [Google Scholar] [PubMed]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sporns, P.B.; Fiehler, J.; Ospel, J.; Safouris, A.; Hanning, U.; Fischer, U.; Goyal, M.; McTaggart, R.; Brehm, A.; Psychogios, M. Expanding indications for endovascular thrombectomy-how to leave no patient behind. Ther. Adv. Neurol. Disord. 2021, 14, 1756286421998905. [Google Scholar] [CrossRef]
- Barber, P.A.; Demchuk, A.M.; Hudon, M.E.; Pexman, J.H.; Hill, M.D.; Buchan, A.M. Hyperdense sylvian fissure MCA “dot” sign: A CT marker of acute ischemia. Stroke 2001, 32, 84–88. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Farrall, A.J.; Perry, D.; Kummer, R.v.; Mielke, O.; Moulin, T.; Ciccone, A.; Hill, M. Factors influencing the detection of early CT signs of cerebral ischemia: An internet-based, international multiobserver study. Stroke 2007, 38, 1250–1256. [Google Scholar] [CrossRef]
- Aoki, J.; Kimura, K.; Shibazaki, K.; Sakamoto, Y. DWI-ASPECTS as a predictor of dramatic recovery after intravenous recombinant tissue plasminogen activator administration in patients with middle cerebral artery occlusion. Stroke 2013, 44, 534–537. [Google Scholar] [CrossRef]
- Flacke, S.; Urbach, H.; Keller, E.; Träber, F.; Hartmann, A.; Textor, J.; Gieseke, J.; Block, W.; Folkers, P.J.; Schild, H.H. Middle cerebral artery (MCA) susceptibility sign at susceptibility-based perfusion MR imaging: Clinical importance and comparison with hyperdense MCA sign at CT. Radiology 2000, 215, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Weisstanner, C.; Gratz, P.P.; Schroth, G.; Verma, R.K.; Köchl, A.; Jung, S.; Arnold, M.; Gralla, J.; Zubler, C.; Hsieh, K.; et al. Thrombus imaging in acute stroke: Correlation of thrombus length on susceptibility-weighted imaging with endovascular reperfusion success. Eur. Radiol. 2014, 24, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Kim, K.; Yoo, J.; Kim, Y.D.; Nam, H.S.; Kim, E.Y. Computed Tomography-Based Thrombus Imaging for the Prediction of Recanalization after Reperfusion Therapy in Stroke. J. Stroke 2017, 19, 40–49. [Google Scholar] [CrossRef]
- Al Kasab, S.; Almallouhi, E.; Harvey, J.; Turner, N.; Debenham, E.; Caudill, J.; Holmstedt, C.A.; Switzer, J.A. Door in door out and transportation times in 2 telestroke networks. Neurol. Clin. Pract. 2019, 9, 41–47. [Google Scholar] [CrossRef]
- Amukotuwa, S.A.; Straka, M.; Dehkharghani, S.; Bammer, R. Fast Automatic Detection of Large Vessel Occlusions on CT Angiography. Stroke 2019, 50, 3431–3438. [Google Scholar] [CrossRef] [PubMed]
- Maiora, J.; Papakostas, G.A.; Kaburlasos, V.G.; Grana, M. A proposal of Texture Features for interactive CTA Segmentation by Active Learning. Stud. Health Technol. Inform. 2014, 207, 311–320. [Google Scholar]
- Zohios, C.; Kossioris, G.; Papaharilaou, Y. Geometrical methods for level set based abdominal aortic aneurysm thrombus and outer wall 2D image segmentation. Comput. Methods Programs Biomed. 2012, 107, 202–217. [Google Scholar] [CrossRef]
- Amukotuwa, S.A.; Straka, M.; Smith, H.; Chandra, R.V.; Dehkharghani, S.; Fischbein, N.J.; Bammer, R. Automated Detection of Intracranial Large Vessel Occlusions on Computed Tomography Angiography: A Single Center Experience. Stroke 2019, 50, 2790–2798. [Google Scholar] [CrossRef]
- Castaigne, P.; Lhermitte, F.; Gautier, J.C.; Escourolle, R.; Derouesné, C. Internal carotid artery occlusion. A study of 61 instances in 50 patients with post-mortem data. Brain A J. Neurol. 1970, 93, 231–258. [Google Scholar] [CrossRef]
- Puig, J.; Pedraza, S.; Demchuk, A.; Daunis-I-Estadella, J.; Termes, H.; Blasco, G.; Soria, G.; Boada, I.; Remollo, S.; Baños, J.; et al. Quantification of thrombus hounsfield units on noncontrast CT predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. AJNR. Am. J. Neuroradiol. 2012, 33, 90–96. [Google Scholar] [CrossRef]
- Hanning, U.; Sporns, P.B.; Psychogios, M.N.; Jeibmann, A.; Minnerup, J.; Gelderblom, M.; Schulte, K.; Nawabi, J.; Broocks, G.; Meyer, L.; et al. Imaging-based prediction of histological clot composition from admission CT imaging. J. NeuroInterventional Surg. 2021, 13, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.; Nie, X.; Xu, Y.; Liu, C.; Zhao, X.; Wang, Y. Predictive value of thrombus susceptibility for cardioembolic stroke by quantitative susceptibility mapping. Quant. Imaging Med. Surg. 2022, 12, 550–557. [Google Scholar] [CrossRef] [PubMed]
- De Bruijne, M.; van Ginneken, B.; Viergever, M.A.; Niessen, W.J. Interactive segmentation of abdominal aortic aneurysms in CTA images. Med. Image Anal. 2004, 8, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Lalys, F.; Yan, V.; Kaladji, A.; Lucas, A.; Esneault, S. Generic thrombus segmentation from pre- and post-operative CTA. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-W.; Kim, Y.-C.; Cha, J.; Choi, E.-H.; Kim, B.M.; Seo, W.-K.; Kim, G.-M.; Bang, O.Y. Characterization of clot composition in acute cerebral infarct using machine learning techniques. Ann. Clin. Transl. Neurol. 2019, 6, 739–747. [Google Scholar] [CrossRef]
- Qiu, W.; Kuang, H.; Nair, J.; Assis, Z.; Najm, M.; McDougall, C.; McDougall, B.; Chung, K.; Wilson, A.T.; Goyal, M.; et al. Radiomics-Based Intracranial Thrombus Features on CT and CTA Predict Recanalization with Intravenous Alteplase in Patients with Acute Ischemic Stroke. AJNR. Am. J. Neuroradiol. 2019, 40, 39–44. [Google Scholar] [CrossRef]
- Topcuoglu, M.A.; Arsava, E.M.; Akpinar, E. Clot characteristics on computed tomography and response to thrombolysis in acute middle cerebral artery stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2015, 24, 1363–1372. [Google Scholar] [CrossRef]
- Mannel, R.K.; Sandhu, S.J.; Silliman, S.L. Multiplanar computed tomography reconstruction to aid in recognition of the middle cerebral artery "Dot" sign: The sagittal string sign. SAGE Open Med. Case Rep. 2017, 5, 2050313X17748864. [Google Scholar] [CrossRef]
- Kaesmacher, J.; Mosimann, P.J.; Giarrusso, M.; El-Koussy, M.; Zibold, F.; Piechowiak, E.; Dobrocky, T.; Meier, R.; Jung, S.; Bellwald, S.; et al. Multivessel Occlusion in Patients Subjected to Thrombectomy: Prevalence, Associated Factors, and Clinical Implications. Stroke 2018, 49, 1355–1362. [Google Scholar] [CrossRef]

| Parameter | ICA | MCA1 | MCA2 | MCA3 | BA | PCA1 | PCA2 | Total |
|---|---|---|---|---|---|---|---|---|
| Ground truth (CTA) | 12 | 16 | 32 | 8 | 6 | 7 | 4 | 85 [100%] |
| True positive | ||||||||
| Tool | 11 | 14 | 22 | 3 | 4 | 1 | 1 | 56 [66%] |
| Reader 1 | 5 | 7 | 14 | 5 | 5 | 3 | 0 | 39 [46%] |
| Reader 2 | 5 | 10 | 3 | 0 | 2 | 0 | 0 | 20 [24%] |
| False positive | ||||||||
| Tool | 1 | 2 | 10 | 5 | 2 | 6 | 3 | 29 [34%] |
| Reader 1 | 1 | 1 | 6 | 2 | 0 | 1 | 1 | 12 [14%] |
| Reader 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 [1%] |
| Sensitivity in % | ||||||||
| Tool | 92 | 88 | 69 | 38 | 67 | 14 | 25 | 66 |
| Reader 1 | 42 | 44 | 44 | 63 | 83 | 43 | 0 | 46 |
| Reader 2 | 42 | 63 | 9 | 0 | 33 | 0 | 0 | 24 |
| Positive predictive value in % | ||||||||
| Tool | 92 | 88 | 69 | 38 | 67 | 14 | 25 | 66 |
| Reader 1 | 83 | 88 | 70 | 71 | 100 | 75 | 0 | 76 |
| Reader 2 | 83 | 100 | 100 | n/a | 100 | n/a | n/a | 95 |
| Location | ASPECT Score | Volume [mm3] | Longest Extent [mm] | CCS [Mean ± Standard Deviation] |
|---|---|---|---|---|
| Median (Range) | Mean (Range) | Mean (Range) | All Positions | |
| ICA | 8 (2–10) | 159 (33–443) | 19 (9–37) | 65 ± 20 |
| MCA1 | 1 (0–9) | 159 (1–365) | 17 (1–41) | 58 ± 23 |
| MCA2 | 9 (2–10) | 74 (2–332) | 12 (2–36) | 40 ± 24 |
| MCA3 | 9 (0–10) | 7 (6–8) | 4 (3–5) | 19 ± 13 |
| BA | 9 (7–10) | 68 (13–137) | 10 (4–21) | 27 ± 22 |
| PCA1 | 10 (7–10) | 9 (9–9) | 3 (3–3) | 38 ± 54 |
| PCA2 | 8 (4–9) | 20 (20–20) | 6 (6–6) | 13 ± 0 |
| Total | 8 (0–10) | 106 (1–443) | 14 (1–41) | 46 ± 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, R.; Bier, G.; Wilke, V.; Wilke, C.; Taubmann, O.; Ditt, H.; Hempel, J.-M.; Ernemann, U.; Horger, M.; Gohla, G. Automated Intracranial Clot Detection: A Promising Tool for Vascular Occlusion Detection in Non-Enhanced CT. Diagnostics 2023, 13, 2863. https://doi.org/10.3390/diagnostics13182863
Schwarz R, Bier G, Wilke V, Wilke C, Taubmann O, Ditt H, Hempel J-M, Ernemann U, Horger M, Gohla G. Automated Intracranial Clot Detection: A Promising Tool for Vascular Occlusion Detection in Non-Enhanced CT. Diagnostics. 2023; 13(18):2863. https://doi.org/10.3390/diagnostics13182863
Chicago/Turabian StyleSchwarz, Ricarda, Georg Bier, Vera Wilke, Carlo Wilke, Oliver Taubmann, Hendrik Ditt, Johann-Martin Hempel, Ulrike Ernemann, Marius Horger, and Georg Gohla. 2023. "Automated Intracranial Clot Detection: A Promising Tool for Vascular Occlusion Detection in Non-Enhanced CT" Diagnostics 13, no. 18: 2863. https://doi.org/10.3390/diagnostics13182863
APA StyleSchwarz, R., Bier, G., Wilke, V., Wilke, C., Taubmann, O., Ditt, H., Hempel, J.-M., Ernemann, U., Horger, M., & Gohla, G. (2023). Automated Intracranial Clot Detection: A Promising Tool for Vascular Occlusion Detection in Non-Enhanced CT. Diagnostics, 13(18), 2863. https://doi.org/10.3390/diagnostics13182863






