Single Quasi–Symmetrical LED with High Intensity and Wide Beam Width Using Diamond–Shaped Mirror Refraction Method for Surgical Fluorescence Microscope Applications
Abstract
:1. Introduction
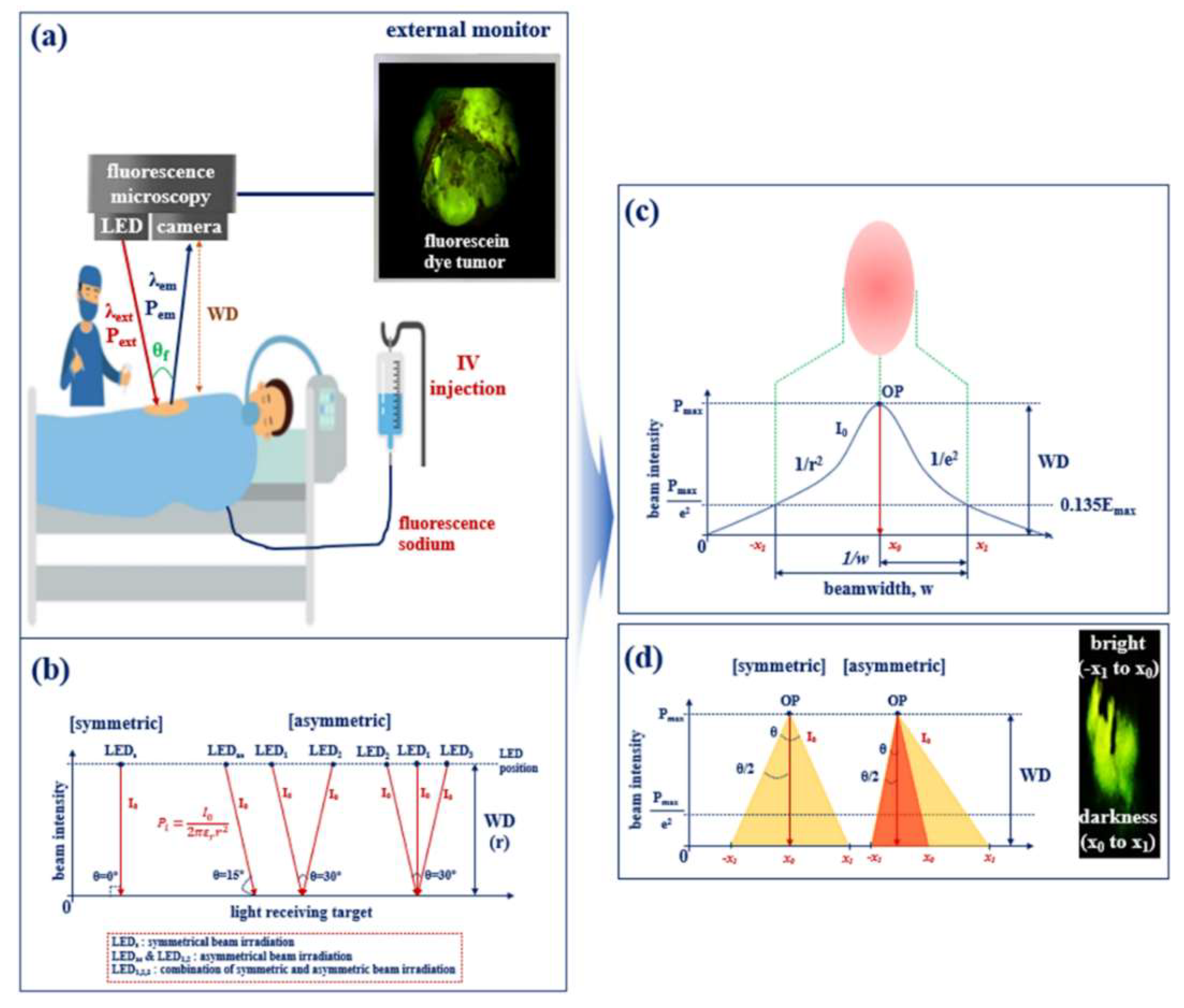
2. Method for Increasing the Beam Width and Emission Power
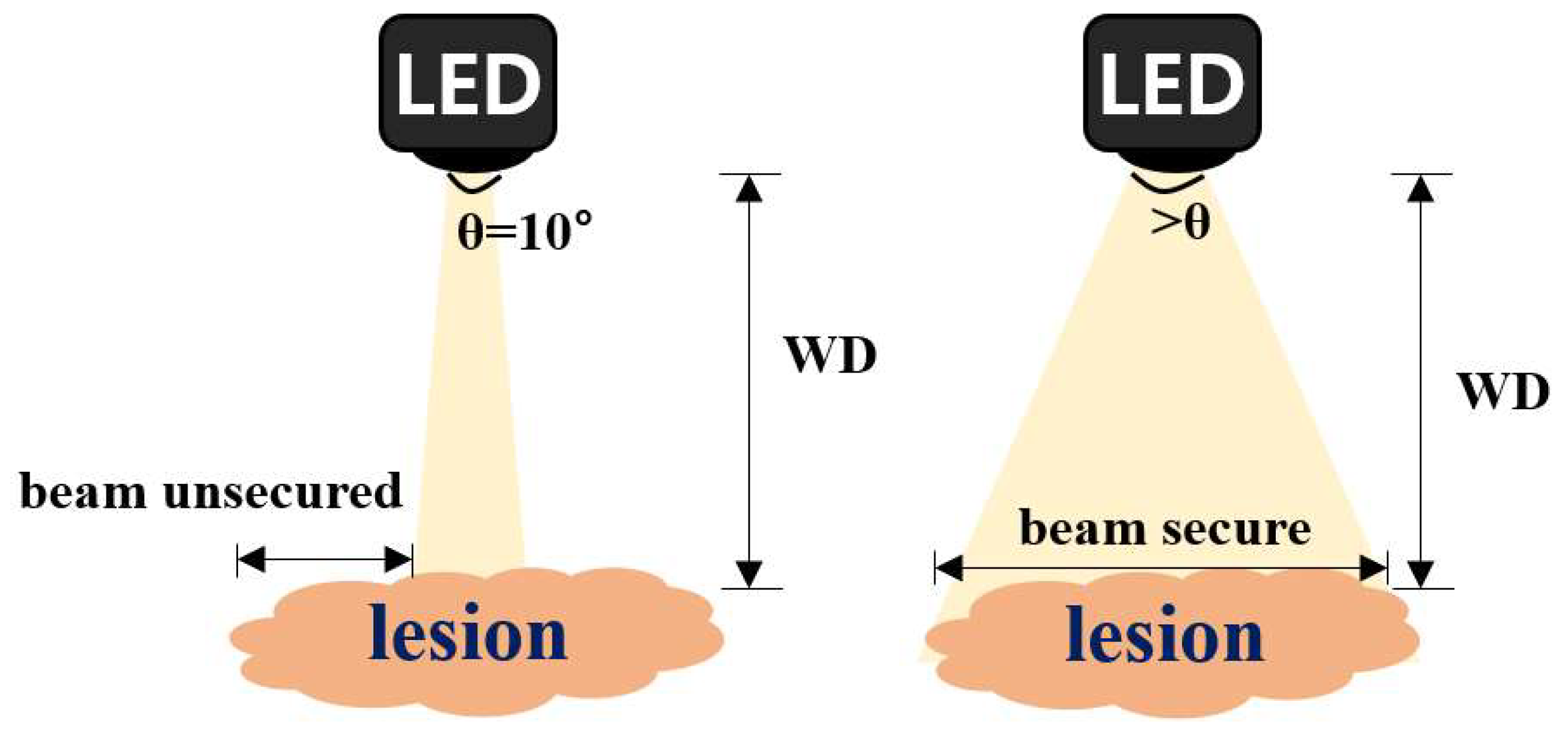
2.1. Examining the Limit of Fluorescence
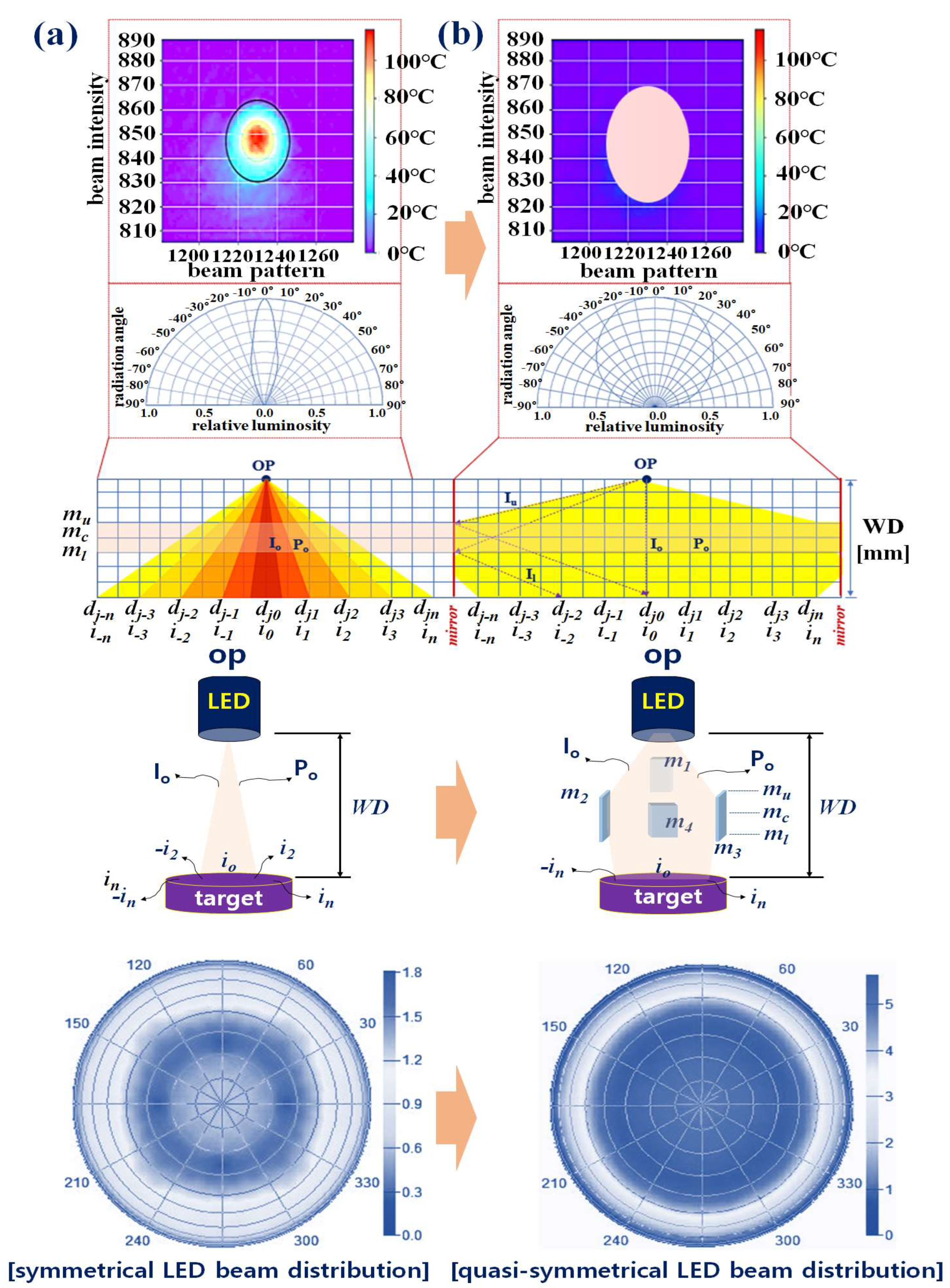
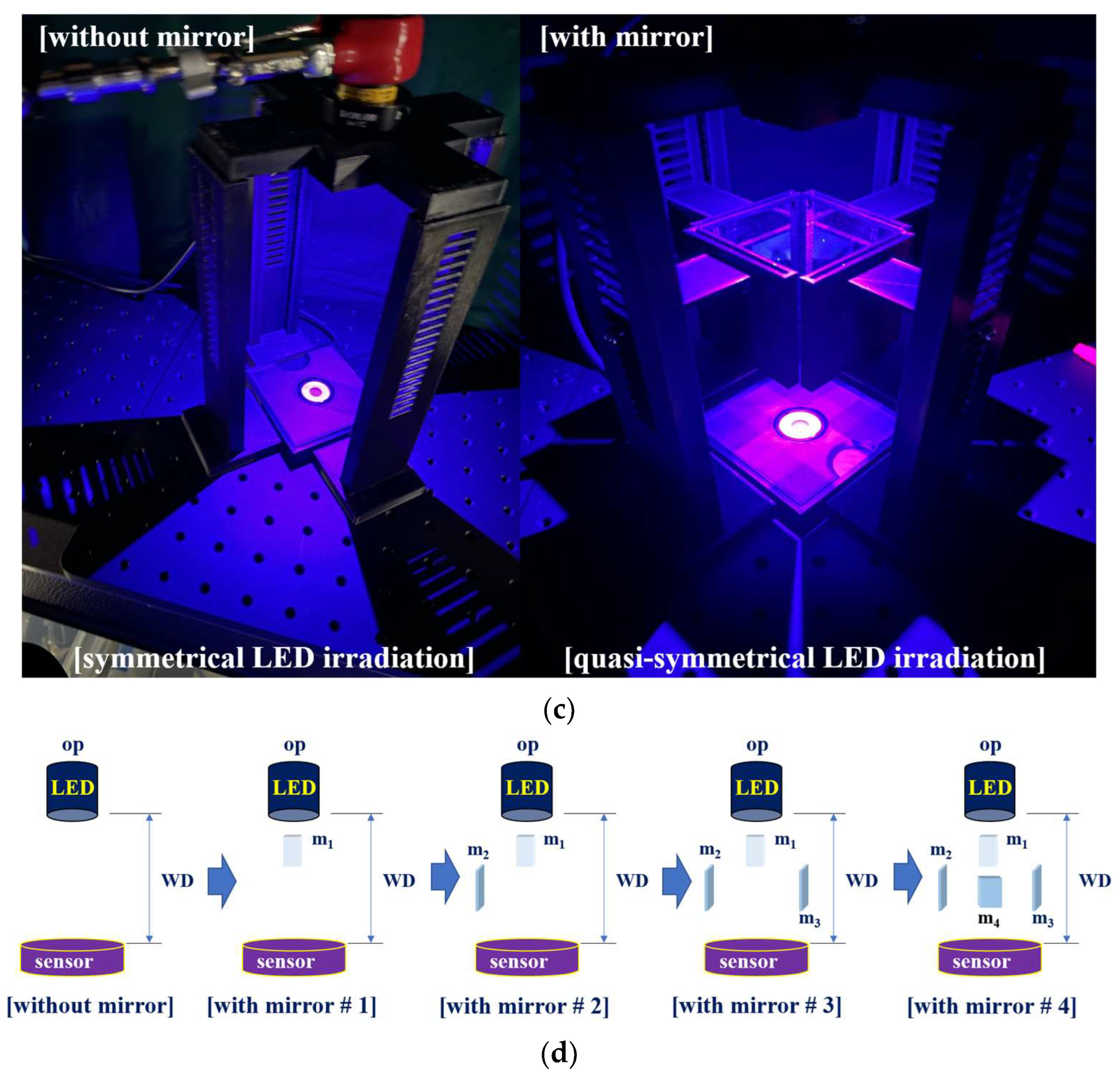
2.2. Quasi–Symmetrical Single LED Divergence
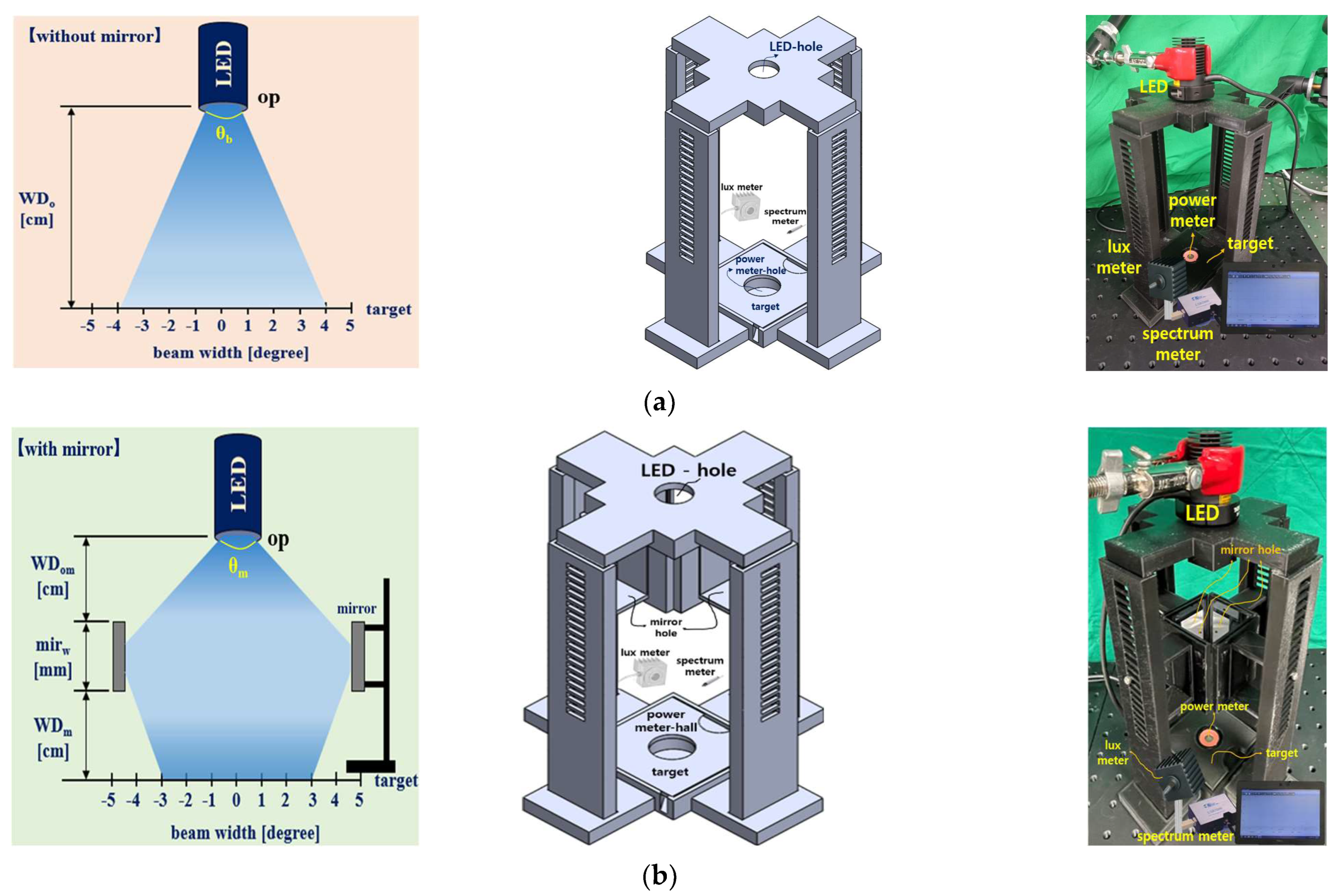
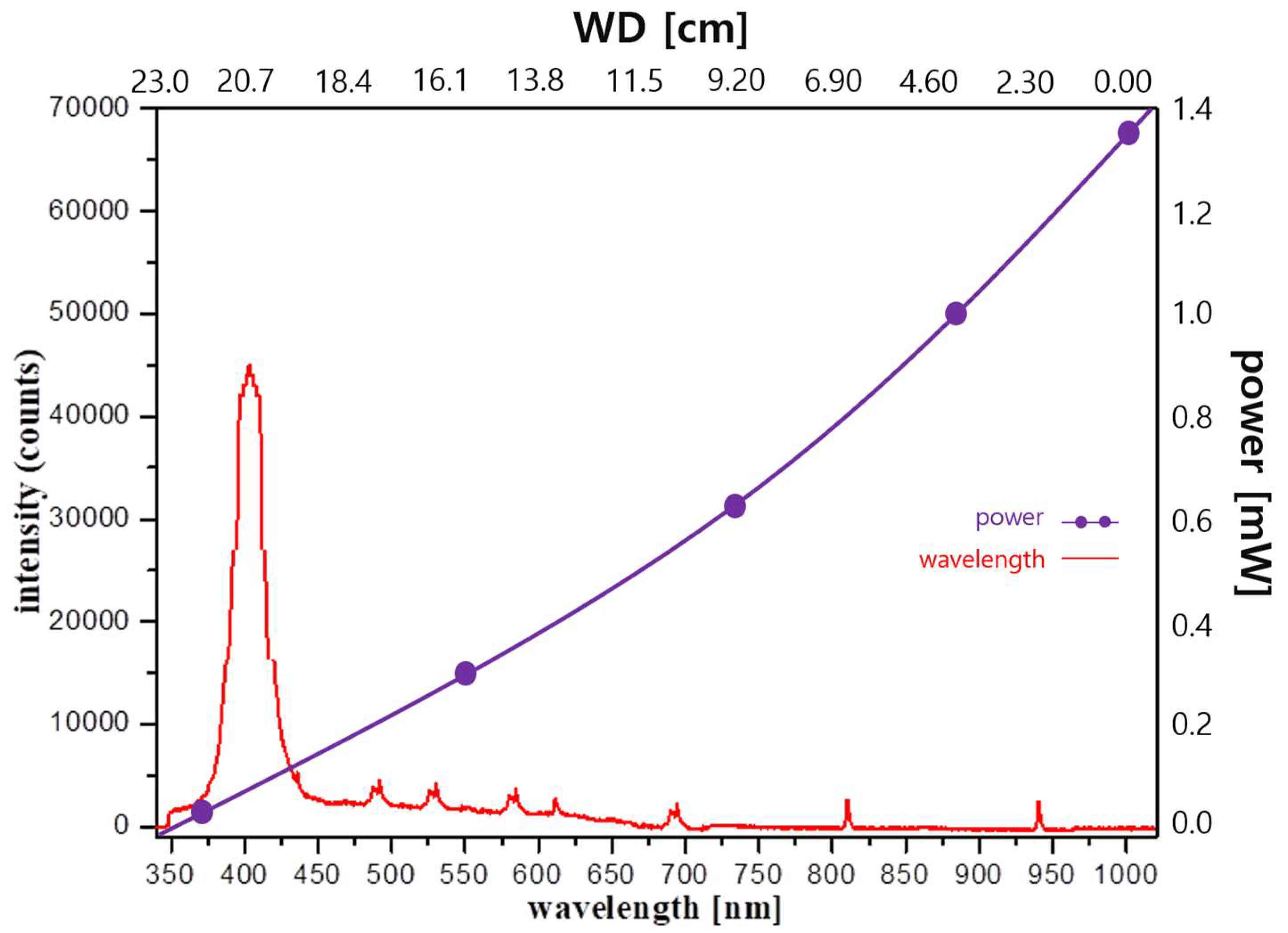
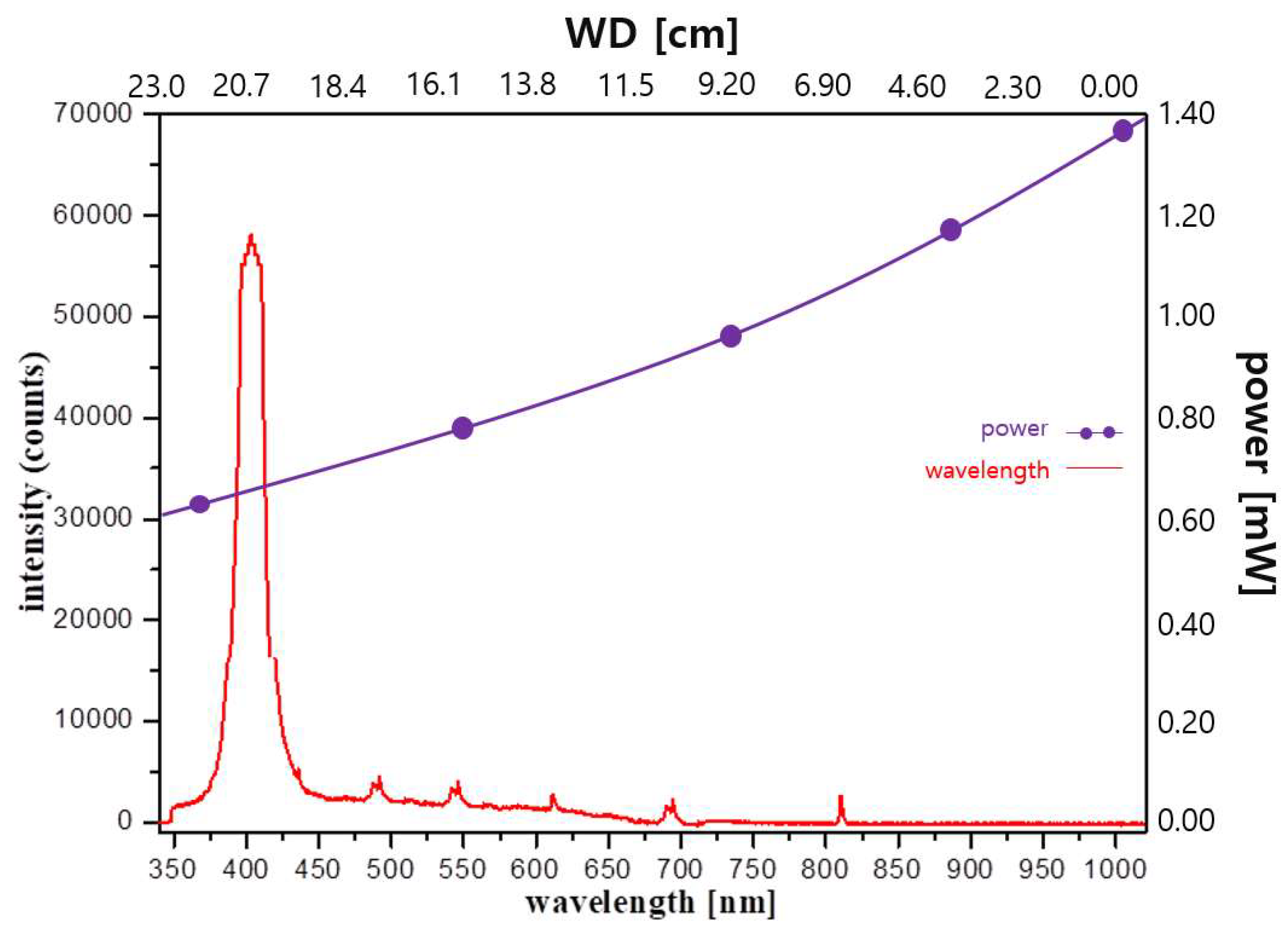
3. Experimental Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, M.S.; Cha, H.J.; Lee, S.; Han, L.; Park, W.; Ahn, J.S.; Park, S.C. Deep–learning–based cerebral artery semantic segmentation in neurosurgical operating microscope vision using indocyanine green fluorescence videoangiography. Front. Neurorobot. 2022, 12, 735177. [Google Scholar] [CrossRef]
- Seki, Y.; Kajikawa, A.; Yamamoto, T.; Takeuchi, T.; Terashima, T.; Kurogi, N. Real–time indocyanine green videolymphography navigation for lymphaticovenular anastomosis. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Papayan, G.; Akopov, A. Potential of indocyanine green near–infrared fluorescence imaging in experimental and clinical practice. Photodiagn. Photodyn. Ther. 2018, 24, 292–299. [Google Scholar] [CrossRef]
- Cavallo, C.; Gandhi, S.; Zhao, X.; Belykh, E.; Valli, D.; Nakaji, P.; Preul, M.C.; Lawton, M.T. Applications of microscope–integrated indocyanine green videoangiography in cerebral revascularization procedures. Front. Surg. 2019, 6, 59. [Google Scholar] [CrossRef]
- Takemura, N.; Ito, K.; Inagaki, F.; Mihara, F.; Kokudo, N. Added value of indocyanine green fluorescence imaging in liver surgery. Hepatobiliary Pancreat. Dis. Int. 2022, 21, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Yamamoto, T. Identification of lymph vessels using an indocyanine green camera–integrated operative microscope for lymphovenous anastomosis in the treatment of secondary lymphedema. J. Vasc. Surg. Venous Lymphat. Disord. 2023, 11, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Z.; Fang, M.; Mikesell, L.; Steenwinkel Tessa, E.; Wan, S.; Phillips, T.; Luck Rudy, L.; Werner, T.; Liu, H. A FRET–based near–infrared fluorescent probe for ratiometric detection of cysteine in mitochondria. ChemBioChem 2019, 20, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.; Yan, Q.; Qiu, X.; Cheng, Y.; Wang, B.; Tan, X.; Fang, M.; Luck, R.L.; Liu, H. Near–infrared fluorescent probe based on cyanine scaffold for sensitive detection of uranyl ions in living cells and water samples. Microchem. J. 2022, 180, 107619. [Google Scholar] [CrossRef]
- Siegenthaler, F.; Imboden, S.; Knabben, L.; Mohr, S.; Papadia, A.; Mueller Michael, D. Exploratory study of the clinical value of near–infrared sentinel lymph node mapping with indocyanine green in vulvar cancer patients. Front. Oncol. 2021, 22, 652458. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Wang, J.; Liu, P.; Pan, Y.; Han, C.; Pei, J. Real–time in situ navigation system with indocyanine green fluorescence for sentinel lymph node biopsy in patients with breast cancer. Front. Oncol. 2021, 11, 621914. [Google Scholar] [CrossRef]
- Richter, J.C.O.; Haj-Hosseini, N.; Hallbeck, M.; Wårdell, K. Combination of hand–held probe and microscopy for fluorescence guided surgery in the brain tumor marginal zone. Photodiagn. Photodyn. Ther. 2017, 18, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Mangraviti, A.; Volpin, F.; Cha, J.; Cunningham, S.I.; Raje, K.; Jason Brooke, M.; Brem, H.; Olivi, A.; Huang, J.; Tyler, B.M.; et al. Intraoperative laser speckle contrast imaging for real–time visualization of cerebral blood flow in cerebrovascular surgery: Results from pre–clinical studies. Sci. Rep. 2020, 10, 7614. [Google Scholar] [CrossRef]
- Keeler, R. The evolution of the ophthalmic surgical microscope. Hist. Ophthal. Intern. 2015, 1, 35–66. [Google Scholar]
- Barkana, Y.; Belkin, M. Laser eye injuries. Surv. Ophthalmol. 2000, 44, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.A.; Henary, M.; El Fakhri, G.; Choi, H.S. Tissue–specific near–infrared fluorescence imaging. Acc. Chem. Res. 2016, 49, 1731–1740. [Google Scholar] [CrossRef]
- Wens, E.A.; Lee, S.; Choi, J.; Henary, M.; Choi, H.S. NIR fluorescent small molecules for intraoperative imaging. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2015, 7, 828–838. [Google Scholar]
- Van der Poel, H.G.; Buckle, T.; Brouwer, O.R.; Olmos, R.A.V.; van Leeuwen, F.W. Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: Clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur. Urol. 2011, 60, 826–833. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Klop, W.M.C.; Buckle, T.; Vermeeren, L.; Brekel Michiel, W.M.V.D.; Balm Alfons, J.M.; Nieweg Omgo, E.; Valdés Olmos Renato, A.; Leeuwen Fijs, W.B.V. Feasibility of sentinel node biopsy in head and neck melanoma using a hybrid radioactive and fluorescent tracer. Ann. Surg. Oncol. 2012, 19, 1988–1994. [Google Scholar] [CrossRef]
- Grootendorst, M.R.; Cariati, M.; Pinder, S.; Kothari, A.; Douek, M.; Kovacs, T.; Hamed, H.; Pawa, A.; Nimmo, F.; Owen, J.; et al. Intraoperative assessment of tumor resection margins in breast–conserving surgery using 18F–FDG Cerenkov luminescence imaging: A first–in–human feasibility study. J. Nucl. Med. 2017, 58, 891–898. [Google Scholar] [CrossRef]
- Nolan, R.M.; Adie, S.G.; Marjanovic, M.; Chaney, E.J.; South Fredrick, A.; Monroy Guillermo, L.; Shemonski Nathan, D.; Erickson-Bhatt Sarah, J.; Shelton Ryan, L.; Bower Andrew, J.; et al. Intraoperative optical coherence tomography for assessing human lymph nodes for metastatic cancer. BMC Cancer 2016, 16, 144. [Google Scholar] [CrossRef]
- Dima, A.; Gateau, J.; Claussen, J.; Wilhelm, D.; Ntziachristos, V. Optoacoustic imaging of blood perfusion: Techniques for intraoperative tissue viability assessment. J. Biophotonics 2013, 6, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Phillips, A.; Adar, T.; Hui, A. Ocular injury in cosmetic laser treatments of the face. J. Clin. Aesthet. Dermatol. 2018, 11, 15–18. [Google Scholar] [PubMed]
- Pomykala, A.; Szelag, A. Reduction of power consumption and CO2 emissions as a result of putting into service high–speed trains: Polish case. Energies 2022, 15, 4206. [Google Scholar] [CrossRef]
- Cosco, E.D.; Lim, I.; Sletten, E.M. Photophysical Prop. Indocyanine Green. Shortwave Infrared Reg. Chem. Phot. Chem. 2021, 5, 727–734. [Google Scholar]
- Liu, R.; Xu, Y.; Xu, K.; Dai, Z. Current trends and key considerations in the clinical translation of targeted fluorescent probes for intraoperative navigation. Aggregate 2021, 2, e23. [Google Scholar] [CrossRef]
- Borlan, R.; Focsan, M.; Maniu, D.; Astilean, S. Interventional NIR fluorescence imaging of cancer: Review on next generation of dye–loaded protein–based nanoparticles for real–time feedback during cancer surgery. Int. J. Nanomed. 2021, 12, 2147–2171. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, X.; Zhang, B.; Xie, G.; Zhou, Z.; Yang, X.; Ying, L.; Mei, Y.; Fan, W.; Lon, Z.; et al. Optical gain at 637 nm wavelength in polymer waveguide amplifier under commercial LED Pumping for planar photonic Integration. Adv. Opt. Mater. 2022, 10, 2200205. [Google Scholar] [CrossRef]
- Eduardo Cuevas, L.; Al-Sonboli, N.; Lawson, L.; Ahmed Yassin, M.; Arbide, I.; Al-Aghbari, N.; Bahadur Sherchand, J.; Al-Absi, A.; Nnamdi Emenyonu, E.; Merid, Y.; et al. LED fluorescence microscopy for the diagnosis of pulmonary tuberculosis: A multi–country cross–sectional evaluation. PLoS Med. 2011, 8, e1001057. [Google Scholar]
- Repetto, L.; Piano, E.; Pontiggia, C. Lensless digital holographic microscope with light–emitting diode illumination. Opt. Lett. 2004, 29, 1132–1134. [Google Scholar] [CrossRef]
- Carl Teich, M.; Saleh, B.E.A. Fundamentals of Photonics, 2nd ed.; Wiley—Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Dong, J.T.; Lu, R.S.; Shi, Y.Q.; Xia, R.X.; Li, Q.; Xu, Y. Optical design of color light–emitting diode ring light for machine vision inspection. Opt. Eng. 2011, 50, 043001. [Google Scholar] [CrossRef]
- Wang, X. LED ring array light source design and uniform illumination properties analysis. Optik 2017, 140, 273–281. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, G.; You, Z. Large–aperture kHz operating frequency Ti–alloy based optical micro scanning mirror for LiDAR application. Micromachines 2017, 8, 120. [Google Scholar] [CrossRef]
- Kallmann, U.; Lootze, M.; Mescheder, U. Simulative and experimental characterization of an adaptive astigmatic membrane mirror. Micromachines 2021, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Nylean, C.O. The microscope in aural surgery, its first use and later development. Acta Otolaryngol. Suppl. 1954, 116, 226–240. [Google Scholar] [CrossRef]
- Ma, P.; Chen, Y.; Amotchkina, T.; Trubetskov, M.; Pervak, V.; Li, L. Design, fabrication and measurement of highly–dispersive mirrors with total internal reflection. Opt. Express 2020, 28, 29230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Miyamoto, T. Optimization for compact and high output LED–based Optical wireless power transmission system. Photonics 2022, 9, 14. [Google Scholar] [CrossRef]
- Schaafsma, B.E.; Verbeek, F.P.; Rietbergen, D.D.; Hiel, V.B.D.; Vorst, J.R.V.D.; Liefers, G.L.; Frangioni, J.V.; Velde, C.J.H.V.D.; Leeuwen, F.W.B.V.; Vahrmeijer, A.L. Clinical trial of combined radio–and fluorescence–guided sentinel lymph node biopsy in breast cancer. Br. J. Surg. 2013, 100, 1037–1044. [Google Scholar] [CrossRef]
- Mathéron, H.; Van Den Berg, N.; Brouwer, O.; Kleinjan, G.H.; Driel, W.J.V.; Trum, J.W.; Vegt, E.; Kenter, G.; Leeuwen, F.W.B.V.; Valdés Olmos, R.A. Multimodal surgical guidance towards the sentinel node in vulvar cancer. Gynecol. Oncol. 2013, 131, 720–725. [Google Scholar] [CrossRef]
- Ma, L.; Fei, B. Comprehensive review of surgical microscopes: Technology development and medical applications. J. Biomed. Opt. 2021, 26, 010901. [Google Scholar] [CrossRef]
- Yuan, W.; Xu, C.; Xue, L.; Pang, H.; Cao, A.; Fu, Y.; Deng, Q. Integrated double–sided random microlens array used for laser beam homogenization. Micromachines 2021, 12, 673. [Google Scholar] [CrossRef]
- Belykh, E.; Miller, E.J.; Patel, A.A.; Bozkurt, B.; Yağmurlu, K.; Robinson, T.R.; Nakaji, P.; Spetzler, R.F.; Lawton, M.T.; Nelson, L.Y.; et al. Optical characterization of neurosurgical operating microscopes: Quantitative fluorescence and assessment of PpIX photobleaching. Sci. Rep. 2018, 8, 12543. [Google Scholar] [CrossRef] [PubMed]
- Katsuura, T.; Ochiai, Y.; Senoo, T.; Lee, S.; Takahashi, Y.; Shimomura, Y. Effects of blue pulsed light on human physiological functions and subjective evaluation. J. Physiol. Anthropol. 2012, 31, 23. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, A.V.; Lin, H.; Henderson, E.R.; Samkoe, K.S.; Pogue, B.W. Review of fluorescence guided surgery systems: Identification of key performance capabilities beyond indocyanine green imaging. J. Biomed. Opt. 2016, 21, 080901. [Google Scholar] [CrossRef] [PubMed]
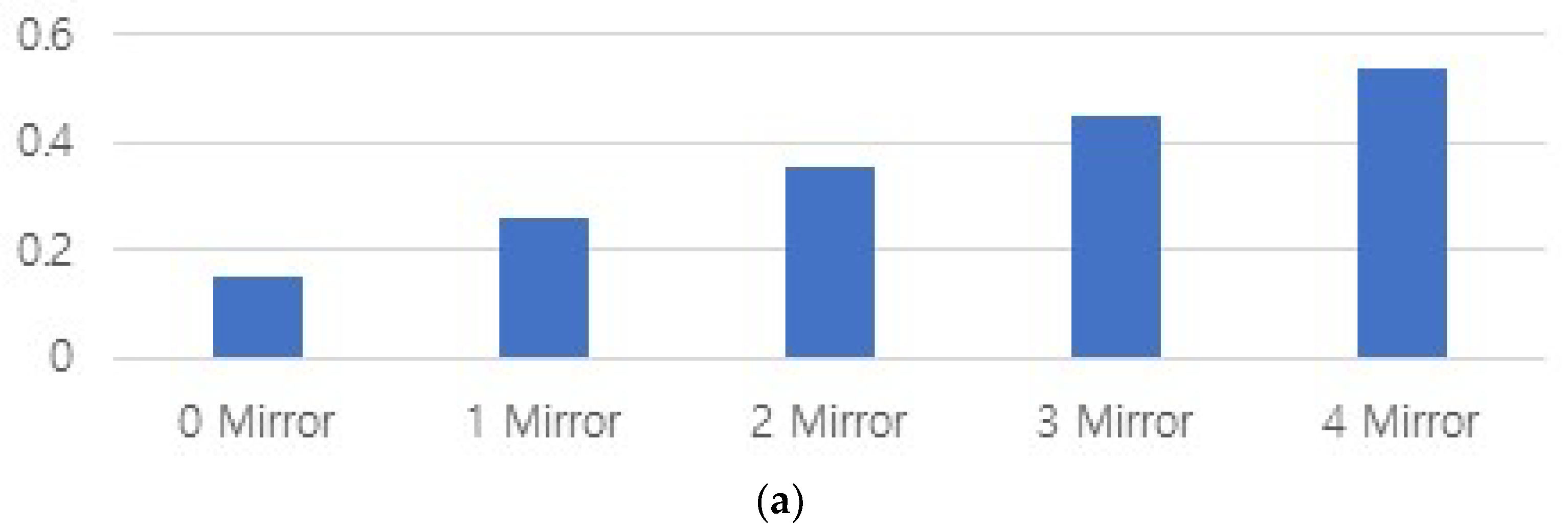
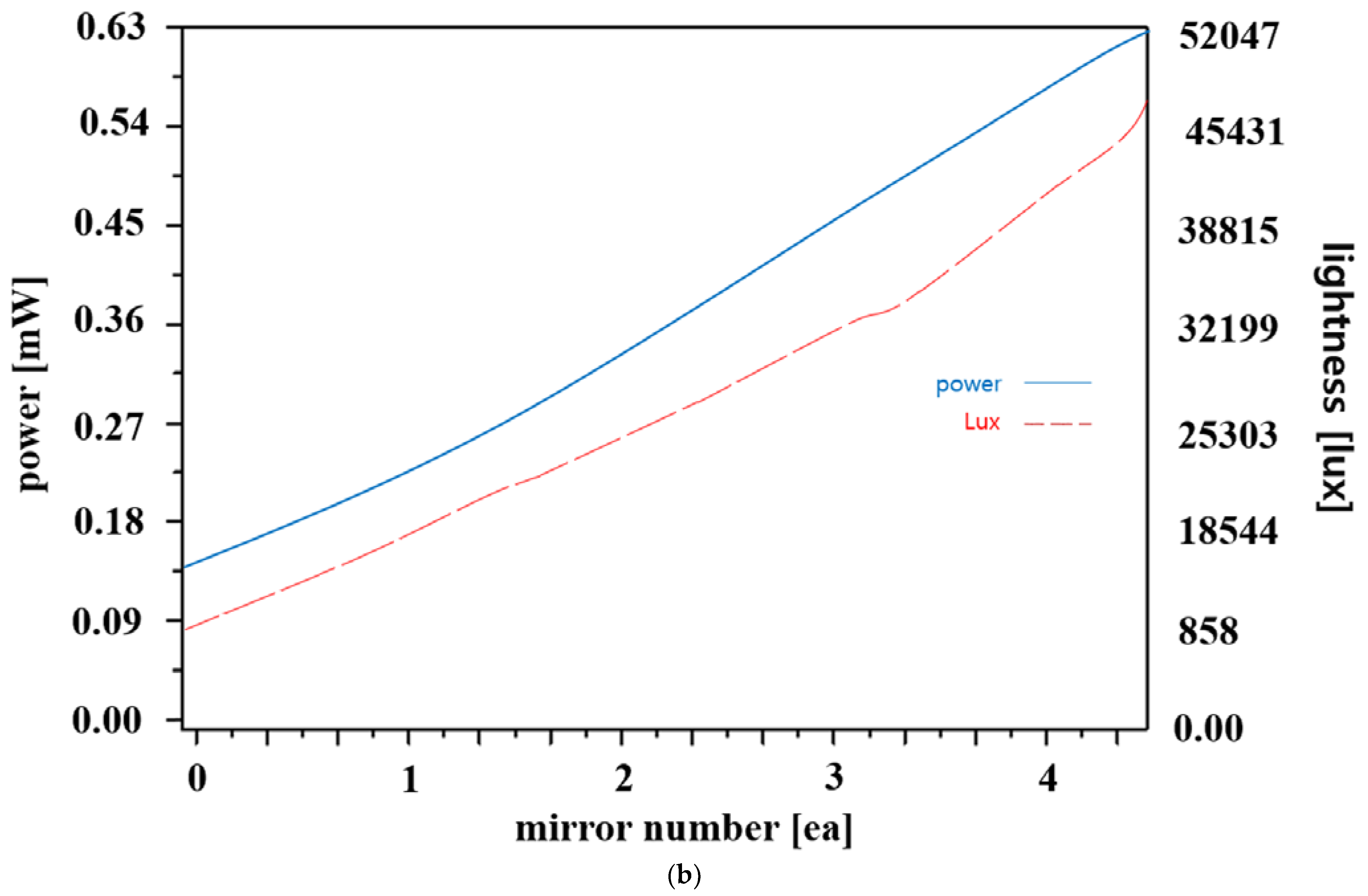
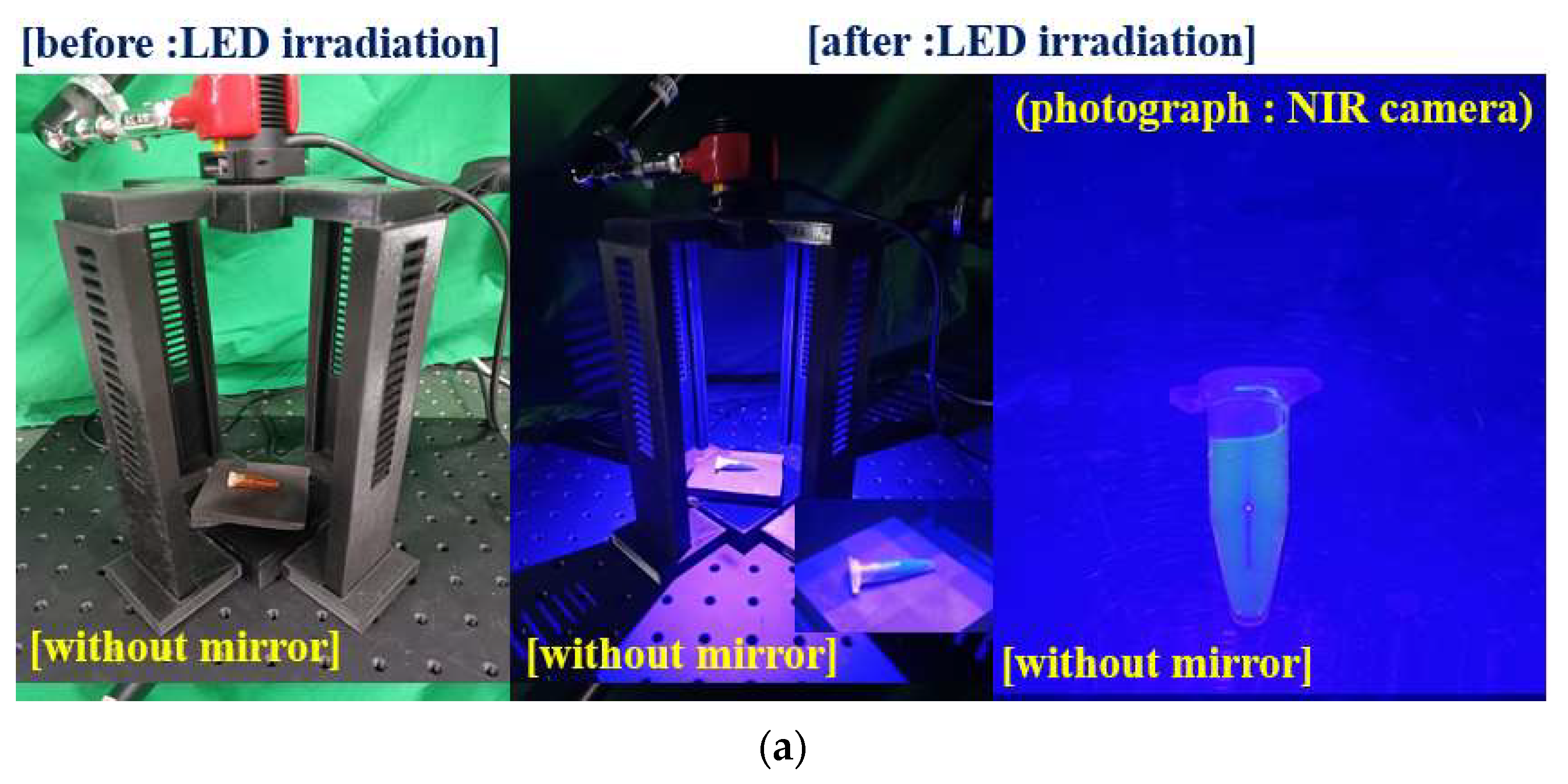

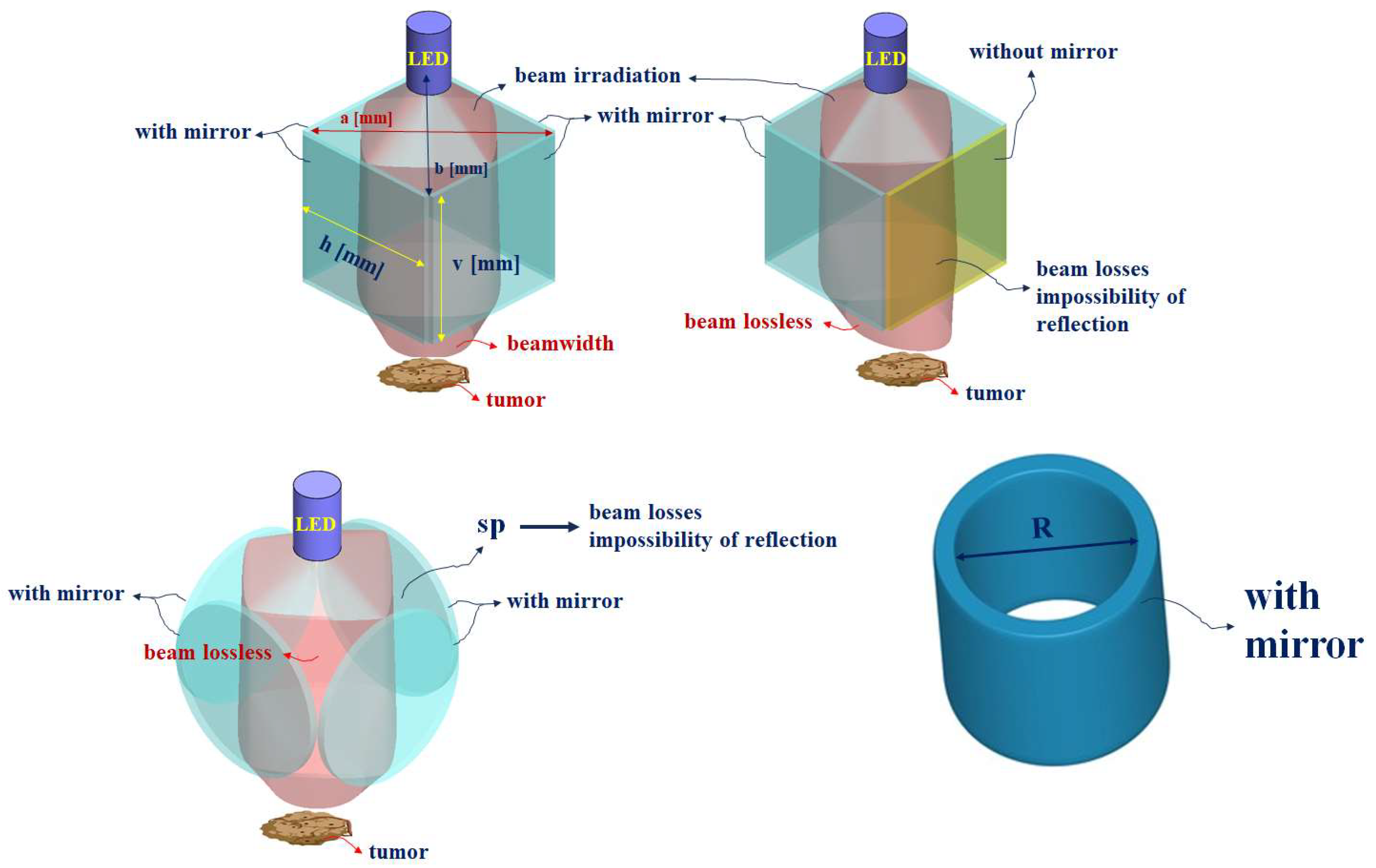
| Mirror Quantity | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Light brightness change |  |  |  |  |  |
| Power (Po) [mW] | 0.15 | 0.258 | 0.352 | 0.448 | 0.600 |
| Illuminance intensity [lux] | 858 | 18,544 | 25,303 | 32,199 | 41,144 |
| Ref. [#] | λext [nm] | WD [cm] | Pmax [mW] | Po [mW] | θw [degree] | Characteristic |
|---|---|---|---|---|---|---|
| [32] | 467 | 6.17 | 100 | 6.10 | 30.0 | LED |
| [40] | 405 | 0.25 | 40 | 12.3 | 6.0 | laser |
| [41] | 405 | 0.0133 | 30 | 13.2 | 26 | laser |
| [42] | 405 | 35.0 | 4.70 | 6.00 | 42 | laser |
| [43] | 460 | 100 | 1540 | 12 µW | 26.6 | LED |
| this work | 405 | 20.0 | 18.0 | 0.6 | 43 | LED |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, M.; Yoon, K.; Lee, S.; Kim, K.G. Single Quasi–Symmetrical LED with High Intensity and Wide Beam Width Using Diamond–Shaped Mirror Refraction Method for Surgical Fluorescence Microscope Applications. Diagnostics 2023, 13, 2763. https://doi.org/10.3390/diagnostics13172763
Ju M, Yoon K, Lee S, Kim KG. Single Quasi–Symmetrical LED with High Intensity and Wide Beam Width Using Diamond–Shaped Mirror Refraction Method for Surgical Fluorescence Microscope Applications. Diagnostics. 2023; 13(17):2763. https://doi.org/10.3390/diagnostics13172763
Chicago/Turabian StyleJu, Minki, Kicheol Yoon, Sangyun Lee, and Kwang Gi Kim. 2023. "Single Quasi–Symmetrical LED with High Intensity and Wide Beam Width Using Diamond–Shaped Mirror Refraction Method for Surgical Fluorescence Microscope Applications" Diagnostics 13, no. 17: 2763. https://doi.org/10.3390/diagnostics13172763
APA StyleJu, M., Yoon, K., Lee, S., & Kim, K. G. (2023). Single Quasi–Symmetrical LED with High Intensity and Wide Beam Width Using Diamond–Shaped Mirror Refraction Method for Surgical Fluorescence Microscope Applications. Diagnostics, 13(17), 2763. https://doi.org/10.3390/diagnostics13172763






