Long-Term Outcomes of Endovascular Embolization in a Vein of Galen Aneurysmal Malformation: A Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Endovascular Embolization Procedures
2.3. Follow-Up
3. Results
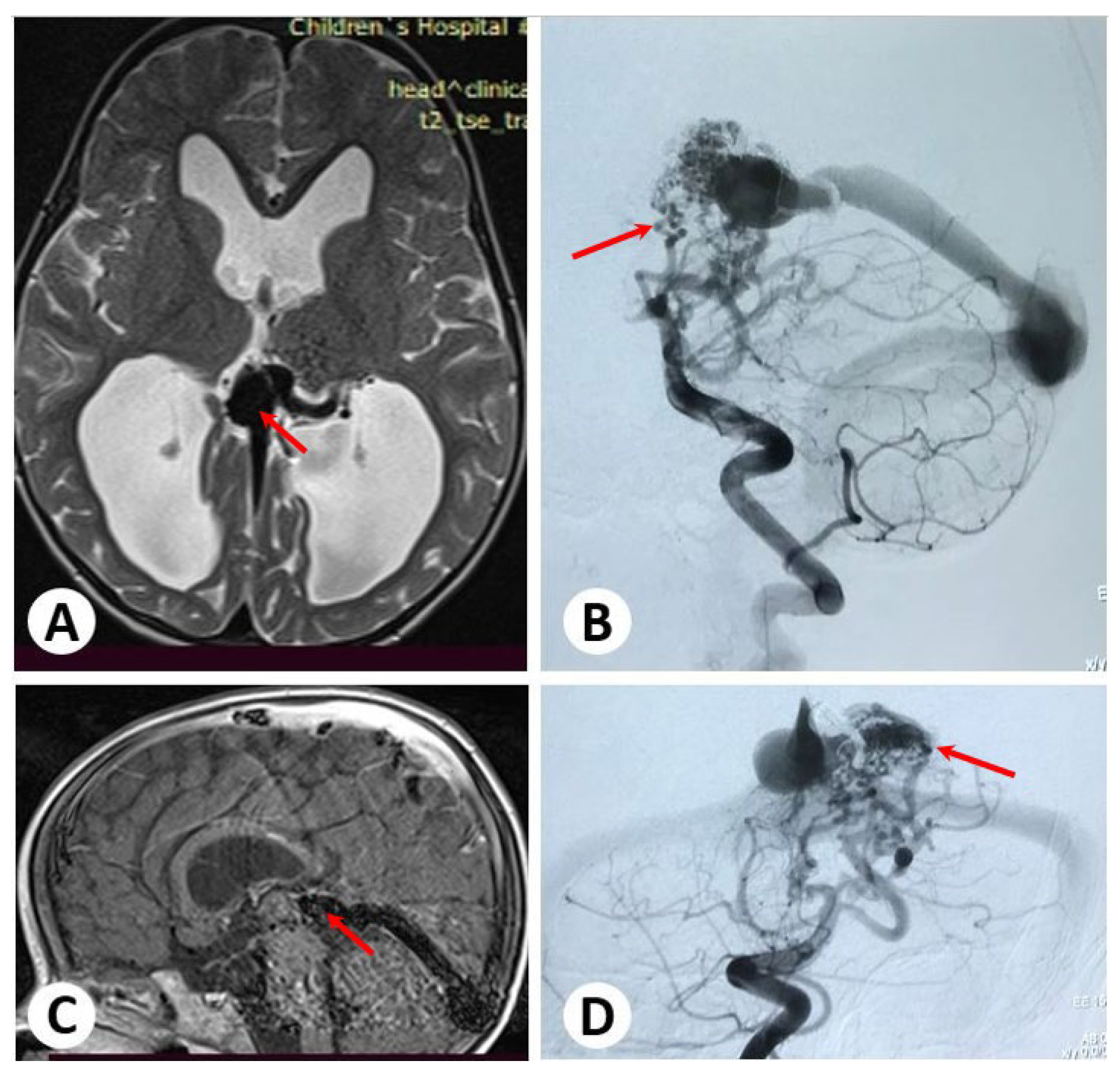
3.1. Illustrative Cases: Case 1
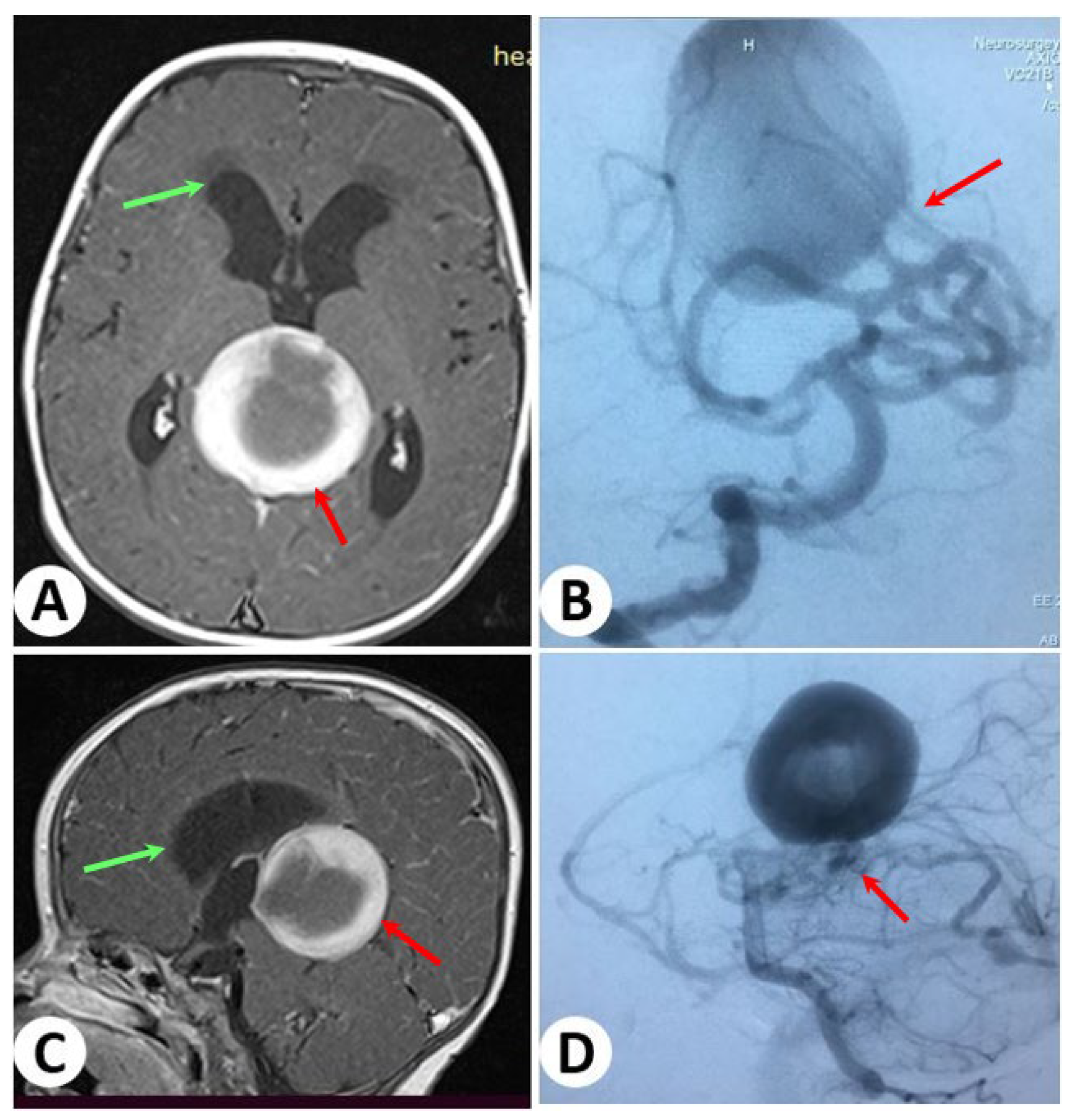
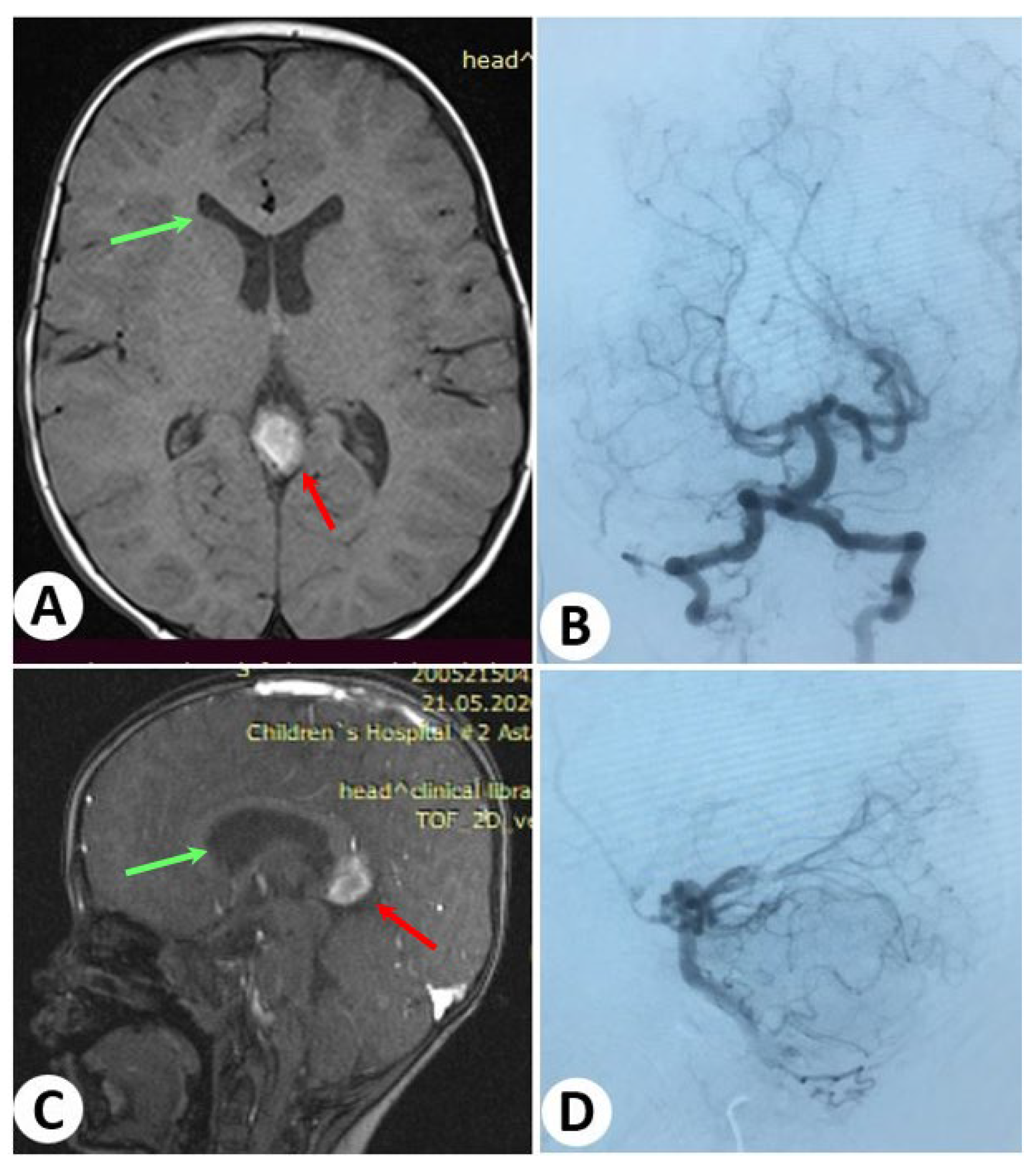
3.2. Illustrative Cases: Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaeger, R.; Forbes, R.P. Bilateral Congenital Arteriovenous Communications (Aneurysm) of the Cerebral Vessels. Arch. Neurol. Psychiatry 1946, 55, 591–599. [Google Scholar] [CrossRef]
- Brinjikji, W.; Krings, T.; Murad, M.H.; Rouchaud, A.; Meila, D. Endovascular Treatment of Vein of Galen Malformations: A Systematic Review and Meta-Analysis. AJNR Am. J. Neuroradiol. 2017, 38, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Raybaud, C.A.; Strother, C.M.; Hald, J.K. Aneurysms of the Vein of Galen: Embryonic Considerations and Anatomical Features Relating to the Pathogenesis of the Malformation. Neuroradiology 1989, 31, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Ustaszewski, A.; Janowska-Głowacka, J.; Wołyńska, K.; Pietrzak, A.; Badura-Stronka, M. Genetic Syndromes with Vascular Malformations—Update on Molecular Background and Diagnostics. Arch. Med. Sci. 2021, 17, 965–991. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, J. The Genetics of Vein of Galen Malformation and Assessment of Candidate Genes in Xenopus Tropicalis. Yale Med. Thesis Digit. Libr. 2019, 1, 11–13. [Google Scholar]
- Gold, A.; Ransohoff, J.; Carter, S. Vein of galen malformation. Acta Neurol. Scand. Suppl. 1964, 40 (Suppl. 11), 1–31. [Google Scholar]
- Lasjaunias, P. Vein of Galen Aneurysmal Malformation. In Vascular Diseases in Neonates, Infants and Children: Interventional Neuroradiology Management; Lasjaunias, P., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 67–202. ISBN 978-3-662-10740-9. [Google Scholar]
- Khullar, D.; Andeejani, A.M.I.; Bulsara, K.R. Evolution of Treatment Options for Vein of Galen Malformations. J. Neurosurg. Pediatr. 2010, 6, 444–451. [Google Scholar] [CrossRef]
- Baranoski, J.F.; Catapano, J.S.; Albuquerque, F.C.; Abruzzo, T.A. Rapid Ventricular Overdrive Pacing and Other Advanced Flow-Control Techniques for the Endovascular Embolization of Vein of Galen Malformations. Front. Pediatr. 2023, 11, 1082318. [Google Scholar] [CrossRef]
- Savage, C.; Hale, A.T.; Parr, M.S.; Hedaya, A.; Saccomano, B.W.; Tsemo, G.B.; Hafeez, M.U.; Tanweer, O.; Kan, P.; Solomon, L.J.; et al. Outcomes of Endovascular Embolization for Vein of Galen Malformations: An Individual Participant Data Meta-Analysis. Front. Pediatr. 2022, 10, 976060. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Zhang, X.; Ye, C.; Wang, S.; An, X. Development of PVA-Based Microsphere as a Potential Embolization Agent. Biomater. Adv. 2022, 135, 112677. [Google Scholar] [CrossRef]
- Yan, J.; Wen, J.; Gopaul, R.; Zhang, C.-Y.; Xiao, S. Outcome and Complications of Endovascular Embolization for Vein of Galen Malformations: A Systematic Review and Meta-Analysis. J. Neurosurg. 2015, 123, 872–890. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.V.; Ball, W.S.; Tomsick, T.A.; Millard, J.; Crone, K.R. Vein of Galen Aneurysmal Malformation: Diagnosis and Treatment of 13 Children with Extended Clinical Follow-Up. AJNR Am. J. Neuroradiol. 2002, 23, 1717–1724. [Google Scholar] [PubMed]
- Ciricillo, S.F.; Edwards, M.S.; Schmidt, K.G.; Hieshima, G.B.; Silverman, N.H.; Higashida, R.T.; Halbach, V.V. Interventional Neuroradiological Management of Vein of Galen Malformations in the Neonate. Neurosurgery 1990, 27, 22–27, discussion 27–28. [Google Scholar] [CrossRef]
- Boldrey, E.; Miller, E.R. Arteriovenous Fistula (Aneurysm) of the Great Cerebral Vein (of Galen) and the Circle of Willis; Report on Two Patients Treated by Ligation. Arch. Neurol. Psychiatry 1949, 62, 778–783. [Google Scholar] [CrossRef]
- Dowd, C.F.; Halbach, V.V.; Barnwell, S.L.; Higashida, R.T.; Edwards, M.S.; Hieshima, G.B. Transfemoral Venous Embolization of Vein of Galen Malformations. AJNR Am. J. Neuroradiol. 1990, 11, 643–648. [Google Scholar]
- Mickle, J.P.; Quisling, R.G. The Transtorcular Embolization of Vein of Galen Aneurysms. J. Neurosurg. 1986, 64, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Lasjaunias, P.; Terbrugge, K.; Piske, R.; Lopez Ibor, L.; Manelfe, C. Dilatation of the vein of Galen. Anatomoclinical forms and endovascular treatment apropos of 14 cases explored and/or treated between 1983 and 1986. Neurochirurgie 1987, 33, 315–333. [Google Scholar] [PubMed]
- Gupta, A.K.; Varma, D.R. Vein of Galen Malformations: Review. Neurol. India 2004, 52, 43–53. [Google Scholar]
- Zerah, M.; Garcia-Monaco, R.; Rodesch, G.; Terbrugge, K.; Tardieu, M.; de Victor, D.; Lasjaunias, P. Hydrodynamics in Vein of Galen Malformations. Childs Nerv. Syst. 1992, 8, 111–117, discussion 117. [Google Scholar] [CrossRef]
- Gailloud, P.; O’Riordan, D.P.; Burger, I.; Levrier, O.; Jallo, G.; Tamargo, R.J.; Murphy, K.J.; Lehmann, C.U. Diagnosis and Management of Vein of Galen Aneurysmal Malformations. J. Perinatol. 2005, 25, 542–551. [Google Scholar] [CrossRef]
- Lasjaunias, P.L.; Chng, S.M.; Sachet, M.; Alvarez, H.; Rodesch, G.; Garcia-Monaco, R. The Management of Vein of Galen Aneurysmal Malformations. Neurosurgery 2006, 59, S184–S194, discussion S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Cho, W.-S.; Kang, H.-S.; Kim, J.E.; Lee, S.J.; Han, M.H. Vein of Galen Aneurysmal Malformation: Endovascular Management of 6 Cases in a Single Institute. J. Korean Neurosurg. Soc. 2011, 50, 191–194. [Google Scholar] [CrossRef]
- Cory, M.J.; Durand, P.; Sillero, R.; Morin, L.; Savani, R.; Chalak, L.; Angelis, D. Vein of Galen Aneurysmal Malformation: Rationalizing Medical Management of Neonatal Heart Failure. Pediatr. Res. 2023, 93, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.B.; Jungreis, C.A.; Quisling, R.G.; Pollack, I. Vein of Galen Aneurysms: A Review and Current Perspective. AJNR Am. J. Neuroradiol. 1994, 15, 1486–1496. [Google Scholar] [PubMed]
- Sepulveda, W.; Platt, C.C.; Fisk, N.M. Prenatal Diagnosis of Cerebral Arteriovenous Malformation Using Color Doppler Ultrasonography: Case Report and Review of the Literature. Ultrasound Obstet. Gynecol. 1995, 6, 282–286. [Google Scholar] [CrossRef]
- Nikas, D.C.; Proctor, M.R.; Scott, R.M. Spontaneous Thrombosis of Vein of Galen Aneurysmal Malformation. Pediatr. Neurosurg. 1999, 31, 33–39. [Google Scholar] [CrossRef]
- Lomachinsky, V.; Taborsky, J.; Felici, G.; Charvat, F.; Benes Iii, V.; Liby, P. Endoscopic Third Ventriculostomy in an Infant with Vein of Galen Aneurysmal Malformation Treated by Endovascular Occlusion: Case Report and a Review of Literature. Neurochirurgie 2022, 68, 540–543. [Google Scholar] [CrossRef]
- Kuzeyli, K.; Cakir, E.; Karaarslan, G.; Ahmetoğlu, A.; Peksoylu, B.; Yazar, U.; Baykal, S. Spontaneous Thrombosis of Vein of Galen Aneurysmal Malformation after Ventriculoperitoneal Shunting. J. Clin. Neurosci. 2004, 11, 439–442. [Google Scholar] [CrossRef]
- Hassan, T.; Nassar, M.; Elghandour, M. Vein of Galen Aneurysms: Presentation and Endovascular Management. Pediatr. Neurosurg. 2010, 46, 427–434. [Google Scholar] [CrossRef]
- Berenstein, A.; Paramasivam, S.; Sorscher, M.; Molofsky, W.; Meila, D.; Ghatan, S. Vein of Galen Aneurysmal Malformation: Advances in Management and Endovascular Treatment. Neurosurgery 2019, 84, 469–479. [Google Scholar] [CrossRef]

| Points | Cardiac Function | Cerebral Function | Respiratory Function | Hepatic Function | Renal Function |
|---|---|---|---|---|---|
| 5 | Normal | Normal | Normal | - | - |
| 4 | Overload, no medical treatment | Subclinical, isolated EEG a abnormalities | Tachypnea finishes bottle | - | - |
| 3 | Failure, stable with medical treatment | Nonconvulsive intermittent neurologic signs | Tachypnea does not finish bottle | No hepatomegaly, normal hepatic function | Normal |
| 2 | Failure, not stable with medical treatment | Isolated convulsion | Assisted ventilation, normal saturation FiO2 b < 25% | Hepatomegaly, normal hepatic function | Transient anuria |
| 1 | Ventilation necessary | Seizures | Assisted ventilation. Normal saturation FiO2 > 25% | Moderate or transient hepatic insufficiency | Unstable diuresis with treatment |
| 0 | Resistant to medical therapy | Permanent neurological signs | Assisted ventilation, desaturation | Abnormal coagulation, elevated enzymes | Anuria |
| Patients | N = 22, (100%) |
|---|---|
| Age, mean ± SD | 37.85 months ± 58.96 |
| neonates (<1 month) | 2 (9.1%) |
| infant (≥1 month to <2 years) | 17 (77.27%) |
| child/adult (≥2 years) | 3 (13.63%) |
| Sex | |
| female | 3 (13.63%) |
| male | 19 (86.36%) |
| Comorbidities | |
| hydrocephalus | 12 (54.54%) |
| heart failure | 5 (22.73%) |
| pulmonary hypertension | 2 (9.1%) |
| hemorrhage | 3 (13.63%) |
| Number of endovascular sessions | |
| 1 | 6 (27.27%) |
| 2 | 9 (40.91%) |
| 3 and more | 7 (31.83%) |
| Occlusion Rate | |
| total | 8 (36.3%) |
| partial | 14 (63.7%) |
| Type of intervention | |
| liquid embolizing agents | 11 (50%) |
| coil | 4 (18.18) |
| combination of glue and coil | 7 (31.82%) |
| ventriculoperitoneal shunting | 3 (13.63%) |
| VGAM Type | |
| choroidal type | 9 (40.9%) |
| mural type | 13 (59.1%) |
| Long-Term Outcomes | |
| good outcome | 16 (72.7%) |
| poor outcome | 6 (27.3%) |
| Mortality | 2 (9.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurimanov, C.; Makhambetov, Y.; Menlibayeva, K.; Nurakay, N.; Makhambetov, N.; Zholdybayeva, E.; Akshulakov, S. Long-Term Outcomes of Endovascular Embolization in a Vein of Galen Aneurysmal Malformation: A Single-Center Experience. Diagnostics 2023, 13, 2704. https://doi.org/10.3390/diagnostics13162704
Nurimanov C, Makhambetov Y, Menlibayeva K, Nurakay N, Makhambetov N, Zholdybayeva E, Akshulakov S. Long-Term Outcomes of Endovascular Embolization in a Vein of Galen Aneurysmal Malformation: A Single-Center Experience. Diagnostics. 2023; 13(16):2704. https://doi.org/10.3390/diagnostics13162704
Chicago/Turabian StyleNurimanov, Chingiz, Yerbol Makhambetov, Karashash Menlibayeva, Nurtay Nurakay, Nursultan Makhambetov, Elena Zholdybayeva, and Serik Akshulakov. 2023. "Long-Term Outcomes of Endovascular Embolization in a Vein of Galen Aneurysmal Malformation: A Single-Center Experience" Diagnostics 13, no. 16: 2704. https://doi.org/10.3390/diagnostics13162704
APA StyleNurimanov, C., Makhambetov, Y., Menlibayeva, K., Nurakay, N., Makhambetov, N., Zholdybayeva, E., & Akshulakov, S. (2023). Long-Term Outcomes of Endovascular Embolization in a Vein of Galen Aneurysmal Malformation: A Single-Center Experience. Diagnostics, 13(16), 2704. https://doi.org/10.3390/diagnostics13162704







