Prognosis Predictive Markers in Patients with Chronic Obstructive Pulmonary Disease and COVID-19
Abstract
1. Introduction
2. Materials and Methods
- mild disease: mild symptoms without dyspnea or pneumonia;
- moderate disease: evidence of lower respiratory disease by clinical assessment or imaging and a saturation of oxygen (SaO2) ≥ 94 percent in room air at sea level;
- severe disease: tachypnea (respiratory rate > 30 breaths/minute), hypoxia (oxygen saturation ≤93% in room air or PaO2/FiO2 < 300 mmHg), or >50% lung involvement on imaging;
- critical care: involving respiratory failure, shock, or multiorgan dysfunction.
3. Results
3.1. Markers (Clinical, Imaging, or Blood Tests) That Could Predict the Outcomes in Patients with Both COPD and COVID-19
3.1.1. Risk Factors for Non-Invasive Ventilation Prognosis
3.1.2. Risk Factors for ICU and Invasive Mechanical Ventilation Prognosis
3.1.3. Risk Factors for Death Prognosis
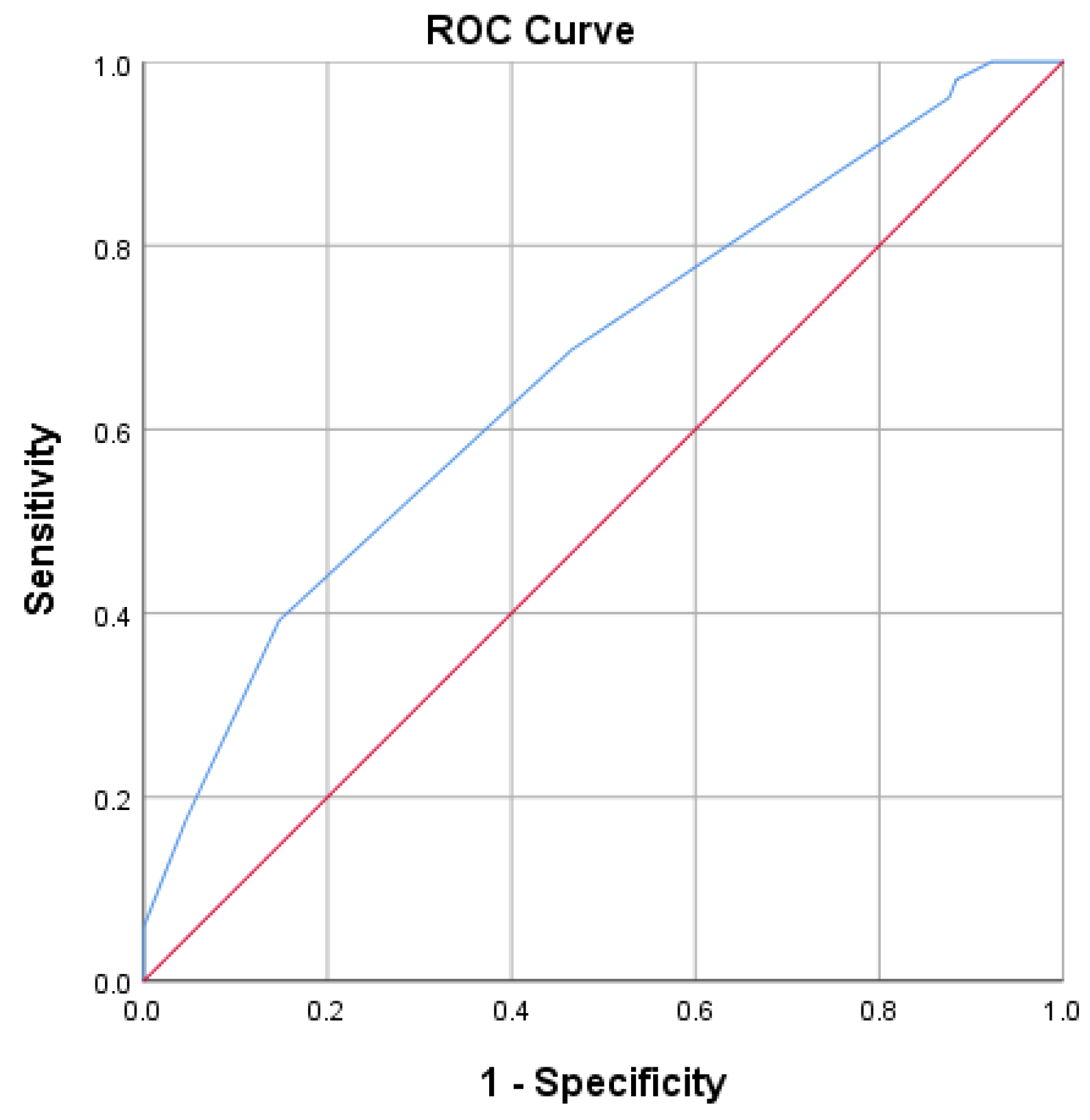
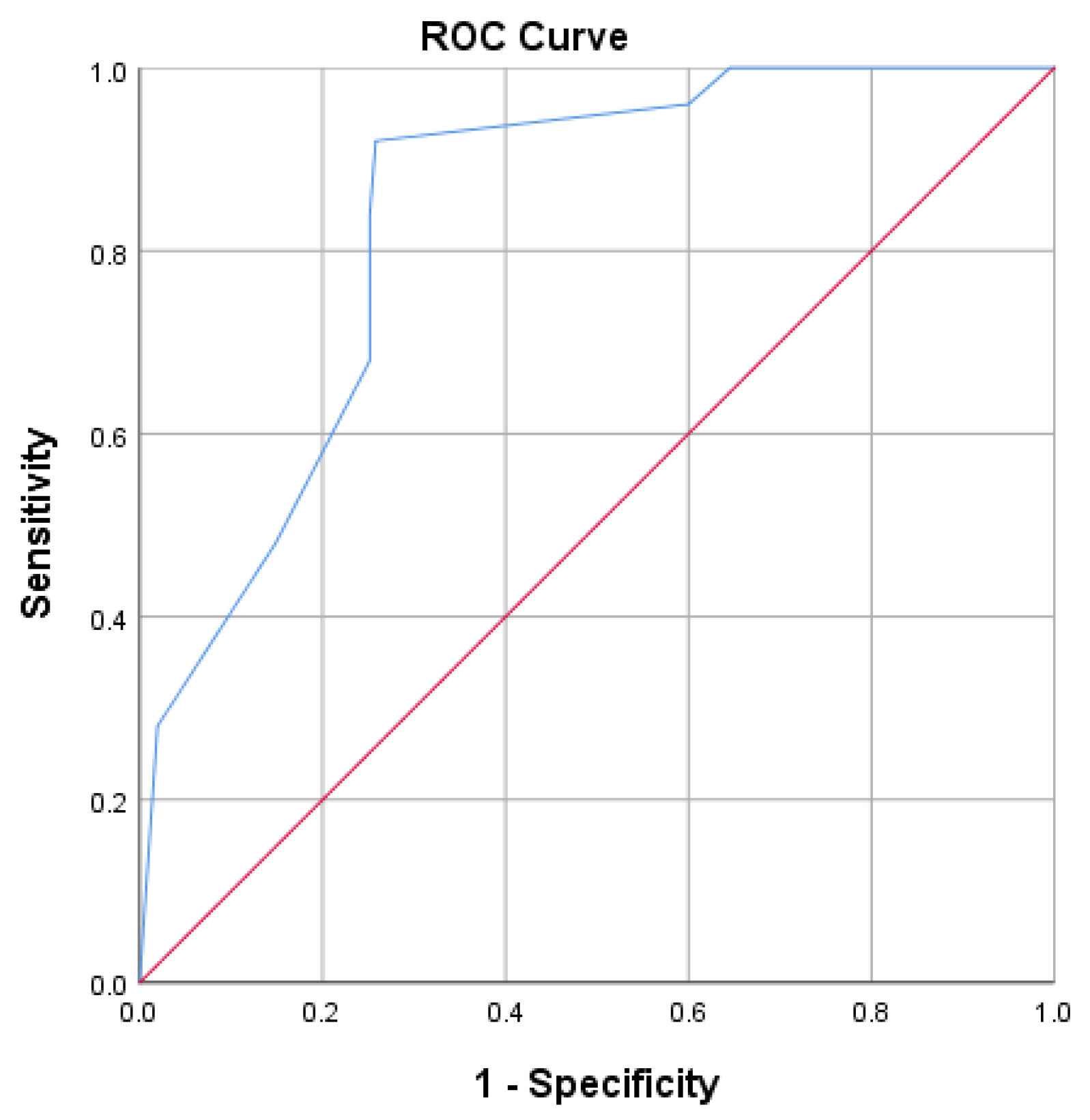
- Non-invasive ventilation score = −4.87 (power of predictive model 61.7%, chance of non-invasive ventilation), which was lower than −4.5; thus, he had an 85% chance of being placed on non- invasive ventilation.
- Invasive ventilation score = −0.3 (power of predictive model 78%, chance of invasive mechanical ventilation), which was greater than −7.15, and he had a 74.2% chance of being transferred to the ICU and being placed on invasive ventilation.
- Mortality score = 0.27, which was higher than −2.80. Since there were only 25 deaths in the sample, this model had low precision and only 38.7% of the variance of death was explained by the above markers.
3.2. Good versus Poor Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, C.C.Y.; Ng, Y.N.C.; Jain, R.; Chung, B.H.Y. A thematic study: Impact of COVID-19 pandemic on rare disease organisations and patients across ten jurisdictions in the Asia Pacific region. Orphanet J. Rare Dis. 2021, 16, 119. [Google Scholar] [CrossRef]
- Limongelli, G.; Iucolano, S.; Monda, E.; Elefante, P.; De Stasio, C.; Lubrano, I.; Caiazza, M.; Mazzella, M.; Fimiani, F.; Galdo, M.; et al. Diagnostic issues faced by a rare disease healthcare network during COVID-19 outbreak: Data from the Campania Rare Disease Registry. J. Public Health 2022, 44, 586–594. [Google Scholar] [CrossRef]
- Higham, A.; Mathioudakis, A.; Vestbo, J.; Singh, D. COVID-19 and COPD: A narrative review of the basic science and clinical outcomes. Eur. Respir. Rev. 2020, 29, 200199. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with High Risk for Severe COVID-19: Information for Healthcare Providers [Internet]; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021; [updated date—9 February 2023]. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 1 March 2022).
- Centers for Disease Control and Prevention. Science Brief: Evidence Used to Update the List of Underlying Medical Conditions That Increase a Person’s Risk of Severe Illness from COVID-19 [Internet]; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021; [updated date 9 February 2023]. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html#anchor_1618433687270 (accessed on 1 March 2022).
- Centers for Disease Control and Prevention. Risk for COVID-19 Infection, Hospitalization, and Death by Age Group [Internet]; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021; [updated date—25 April 2023]. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html (accessed on 16 June 2022).
- McIntosh, K. COVID-19: Clinical Features [Internet]; Up to Date: Alphen aan den Rijn, The Netherlands, 2023; [updated date—27 March 2023]; Available online: https://www.uptodate.com/contents/covid-19-clinical-features?search=covid%2019%20risk%20factors§ionRank=1&usage_type=default&anchor=H2249070035&source=machineLearning&selectedTitle=1~150&display_rank=1#H2249070035 (accessed on 16 January 2022).
- Guan, W.J.; Liang, W.H.; Shi, Y.; Gan, L.X.; Wang, H.B.; He, J.X.; Zhong, N.S. Chronic Respiratory Diseases and the Outcomes of COVID-19: A Nationwide Retrospective Cohort Study of 39,420 Cases. J. Allergy Clin. Immunol. Pract. 2021, 9, 2645–2655. [Google Scholar] [CrossRef]
- Bloom, C.I.; Drake, T.M.; Docherty, A.B.; Lipworth, B.J.; Johnston, S.L.; Nguyen-Van-Tam, J.S.; Carson, G.; Dunning, J.; Harrison, E.M.; Baillie, J.K.; et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: A national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir. Med. 2021, 9, 699–711. [Google Scholar] [CrossRef]
- Aveyard, P.; Gao, M.; Lindson, N.; Hartmann-Boyce, J.; Watkinson, P.; Young, D.; Coupland, C.A.; San Tan, P.; Clift, A.K.; Harrison, D.; et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: A population cohort study. Lancet Respir. Med. 2021, 9, 909–923. [Google Scholar] [CrossRef]
- Signes-Costa, J.; Nunez-Gil, I.J.; Soriano, J.B.; Arroyo-Espliguero, R.; Eid, C.M.; Romero, R.; Uribarri, A.; Fernandez-Rozas, I.; Aguado, M.G.; Becerra-Muñoz, V.M.; et al. Prevalence and 30-Day Mortality in Hospitalized Patients with COVID-19 and Prior Lung Diseases. Arch. Bronconeumol. 2021, 57, 13–20. [Google Scholar] [CrossRef]
- Schultze, A.; Walker, A.J.; MacKenna, B.; Morton, C.E.; Bhaskaran, K.; Brown, J.P.; Rentsch, C.T.; Williamson, E.; Drysdale, H.; Croker, R.; et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: An observational cohort study using the OpenSAFELY platform. Lancet Respir. Med. 2020, 8, 1106–1120. [Google Scholar] [CrossRef]
- Liu, S.; Cao, Y.; Du, T.; Zhi, Y. Prevalence of Comorbid Asthma and Related Outcomes in COVID-19: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2021, 9, 693–701. [Google Scholar] [CrossRef]
- Peters, M.C.; Sajuthi, S.; Deford, P.; Christenson, S.; Rios, C.L.; Montgomery, M.T.; Woodruff, P.G.; Mauger, D.T.; Erzurum, S.C.; Johansson, M.W.; et al. COVID-19-related Genes in Sputum Cells in Asthma. Relationship to Demographic Features and Corticosteroids. Am. J. Respir. Crit. Care Med. 2020, 202, 83–90. [Google Scholar] [CrossRef]
- He, Z.F.; Zhong, N.S.; Guan, W.J. Impact of Chronic Respiratory Diseases on the Outcomes of COVID-19. Arch. Bronconeumol. 2022, 58, 5–7. [Google Scholar] [CrossRef]
- Polverino, F.; Kheradmand, F. COVID-19, COPD, and AECOPD: Immunological, Epidemiological, and Clinical Aspects. Front. Med. 2021, 7, 627278. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Niikura, M.; Yang, C.W.T.; Sin, D.D. COVID-19 and COPD. Eur. Respir. J. 2020, 56, 2002108. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R., Jr.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of COVID-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef] [PubMed]
- Berghella, V.; Hughes, B.L. Classification of Disease Severity [Internet]. Up to Date: Alphen aan den Rijn, The Netherlands, 2023; [updated date—28 April 2023]; Available online: https://www.uptodate.com/contents/covid-19-overview-of-pregnancy-issues?search=covid19%20classification§ionRank=1&usage_type=default&anchor=H750940065&source=machineLearning&selectedTitle=1~150&display_rank=1#H750940065 (accessed on 16 January 2022).
- Li, K.; Fang, Y.; Li, W.; Pan, C.; Qin, P.; Zhong, Y.; Liu, X.; Huang, M.; Liao, Y.; Li, S. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur. Radiol. 2020, 30, 4407–4416. [Google Scholar] [CrossRef]
- Mihaltan, F.; Ulmean, R.; Nemes, R.; Nedelcu, R. Chronic obstructive pulmonary disease day—A traditional strategy of the Romanian Society of Pneumology. Pneumologia 2019, 68, 200–201. [Google Scholar] [CrossRef]
- Huang, K.; Yang, T.; Xu, J.; Yang, L.; Zhao, J.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; et al. Prevalence, risk factors, and management of asthma in China: A national cross-sectional study. Lancet 2019, 394, 407–418. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Au Yeung, S.L.; Li, A.M.; He, B.; Kwok, K.O.; Schooling, C.M. Association of smoking, lung function and COPD in COVID-19 risk: A two-step Mendelian randomization study. Addiction 2022, 117, 2027–2036. [Google Scholar] [CrossRef]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef]
- Monserrat, J.; Gómez-Lahoz, A.; Ortega, M.A.; Sanz, J.; Muñoz, B.; Arévalo-Serrano, J.; Rodríguez, J.M.; Gasalla, J.M.; Gasulla, Ó.; Arranz, A.; et al. Role of Innate and Adaptive Cytokines in the Survival of COVID-19 Patients. Int. J. Mol. Sci. 2022, 23, 10344. [Google Scholar] [CrossRef]
- Cai, G.; Bossé, Y.; Xiao, F.; Kheradmand, F.; Amos, C.I. Tobacco Smoking Increases the Lung Gene Expression of ACE2, the Receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 201, 1557–1559. [Google Scholar] [CrossRef]
- Lee, S.C.; Son, K.J.; Han, C.H.; Park, S.C.; Jung, J.Y. Impact of COPD on COVID-19 prognosis: A nationwide population-based study in South Korea. Sci. Rep. 2021, 11, 3735. [Google Scholar] [CrossRef]
- Gomez Antunez, M.; Muino Miguez, A.; Bendala Estrada, A.D.; Maestro de la Calle, G.; Monge Monge, D.; Boixeda, R.; Ena, J.; Mella Perez, C.; Anton Santos, J.M.; Lumbreras Bermejo, C.; et al. Clinical Characteristics and Prognosis of COPD Patients Hospitalized with SARS-CoV-2. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 15, 3433–3445. [Google Scholar] [CrossRef]
- Bellou, V.; Tzoulaki, I.; van Smeden, M.; Moons, K.G.M.; Evangelou, E.; Belbasis, L. Prognostic factors for adverse outcomes in patients with COVID-19: A field-wide systematic review and meta-analysis. Eur. Respir. J. 2022, 59, 2002964. [Google Scholar] [CrossRef]
- Razek, A.; Fouda, N.; Fahmy, D.; Tanatawy, M.S.; Sultan, A.; Bilal, M.; Zaki, M.; Abdel-Aziz, M.; Sobh, D. Computed tomography of the chest in patients with COVID-19: What do radiologists want to know? Pol. J. Radiol. 2021, 86, e122–e135. [Google Scholar] [CrossRef]
- Hefeda, M.M. CT chest findings in patients infected with COVID-19: Review of literature. Egypt. J. Radiol. Nucl. Med. 2020, 51, 239. [Google Scholar] [CrossRef]
- Okoye, C.; Finamore, P.; Bellelli, G.; Coin, A.; Del Signore, S.; Fumagalli, S.; Gareri, P.; Malara, A.; Mossello, E.; Trevisan, C.; et al. Computed tomography findings and prognosis in older COVID-19 patients. BMC Geriatr. 2022, 22, 166. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- Papi, A.; Bellettato, C.M.; Braccioni, F.; Romagnoli, M.; Casolari, P.; Caramori, G.; Fabbri, L.M.; Johnston, S.L. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 2006, 173, 1114–1121. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef]
- Leung, J.M.; Tiew, P.Y.; Mac Aogáin, M.; Budden, K.F.; Yong, V.F.; Thomas, S.S.; Pethe, K.; Hansbro, P.M.; Chotirmall, S.H. The role of acute and chronic respiratory colonization and infections in the pathogenesis of COPD. Respirology 2017, 22, 634–650. [Google Scholar] [CrossRef]
- Ortega, M.A.; García-Montero, C.; Fraile-Martinez, O.; Colet, P.; Baizhaxynova, A.; Mukhtarova, K.; Alvarez-Mon, M.; Kanatova, K.; Asúnsolo, A.; Sarría-Santamera, A. Recapping the Features of SARS-CoV-2 and Its Main Variants: Status and Future Paths. J. Pers. Med. 2022, 12, 995. [Google Scholar] [CrossRef]

| Factors n = 165 | n (%) | Factors n = 165 | n (%) |
|---|---|---|---|
Location
| 82 (49.7) 83 (50.3) | Smoking status
| 40 (24.3) 42 (25.4) 83 (50.3) |
Gender
| 127 (77) 38 (23) | Comorbidities present n(%)
| 136 (82.4) 136 (82.4) 61 (36.9) 22 (13.3) 55 (33.3) 118 (71.52) |
Age
| 42 (25.45) 123 (74.5) | ||
| Spirometry parameters | m ± SD | 50% (25–75%) | |
| FVC% | 73.98 ± 22.68 | 72.4 (61.9–89.1) | |
| FEV1% | 66.89 ± 26.49 | 63.9 (50.15–83.58) | |
| FEV1 (L) | 1.78 ± 0.53 | 1.77 (1.42–2.07) | |
| MEF 50 | 43.71 ± 30.99 | 36.2 (19.73–60.23) | |
| Non-Invasive Ventilation | ||||
|---|---|---|---|---|
| Qualitative Markers | Present (n = 42) n (%) | Absent (n = 123) n (%) | p-Value | |
| COVID-19 severity | Severe | 39 (92.9%) | 79 (64.2%) | 0.025 a |
| moderate | 2 (4.8%) | 25 (20.3%) | ||
| light | 1 (2.4%) | 19 (15.4%) | ||
| Consolidation | 28 (66.7%) | 51 (41.5%) | 0.005 a | |
| Number of affected lobes = 0 | 17 (40.5%) | 76 (61.8%) | <0.001 a | |
| Cardiovascular disease present | 34 (81%) | 102 (82.9%) | 0.468 b | |
| Arterial hypertension present | 37 (88.1%) | 99 (80.5%) | 0.263 a | |
| Diabetes present | 22 (52.4%) | 39 (31.7%) | 0.017 a | |
| Renal failure present | 10 (23.8%) | 12 (9.8%) | 0.021 a | |
| Respiratory failure present | 16 (38.1%) | 39 (31.7%) | 0.448 a | |
| Pre-existing treatment present | 31 (73.8%) | 87 (70.7%) | 0.703 a | |
| O2-therapy type at admission | No O2 | 3 (7.2%) | 21 (17.1%) | <0.01 a |
| NC | 0 (0%) | 32 (26.1%) | ||
| SM | 3 (7.2%) | 19 (15.5%) | ||
| VM | 8 (19.1%) | 25 (20.4%) | ||
| NRM | 25 (59.6%) | 24 (19.6%) | ||
| HFOT | 3 (7.2%) | 2 (1.7%) | ||
| Smoker status | Non-smoker | 27 (64.3%) | 56 (45.5%) | 0.044 a |
| Former smoker | 5 (11.9%) | 37 (30.1%) | ||
| Active smoker | 10 (23.8%) | 30 (24.4%) | ||
| Non-smoker and former smoker | 32 (76.2%) | 93 (75.6%) | 0.940 a | |
| Active smoker | 10 (23.8%) | 30 (24.4%) | ||
| Non-smoker | 27 (64.3%) | 56 (45.5%) | 0.036 a | |
| Active smoker and former smoker | 15 (35.7%) | 67 (54.5%) | ||
| ICU and invasive mechanical ventilation present | 13 (31%) | 5 (4.1%) | <0.001 b | |
| Death | 13 (31%) | 12 (9.8%) | 0.001 a | |
| Quantitative Markers | Present (n = 42) Median (Q1–Q3) | Absent (n = 123) Median (Q1–Q3) | Mann–Whitney U: p-Value |
|---|---|---|---|
| Age | 72 (65.75–75) | 70 (64–78) | 0.752 |
| LDH | 425 (326.5–695) | 336 (240–482) | 0.003 |
| PCR | 60.1 (9.88–111.33) | 15.88 (6.5–57) | 0.004 |
| Eosinophile | 0 (0–0.01) | 0.01 (0–0.08) | 0.002 |
| Lymphocytes | 0.75 (0.54–1.13) | 0.98 (0.72–1.35) | 0.017 |
| Leucocytes | 8.73 (5.68–11.72) | 7.66 (5.92–10.12) | 0.429 |
| Thrombocytes | 202.5 (157.75–286) | 225 (174–300) | 0.483 |
| Neutrophiles | 7.08 (4.43–9.71) | 6.12 (4.32–8.27) | 0.346 |
| PLR | 257.13 (171.03–452.69) | 221.54 (162.1–321.84) | 0.109 |
| NLR | 10.37 (4.44–14.85) | 6.12 (4.07–9.88) | 0.020 |
| Invasive Ventilation | ||||
|---|---|---|---|---|
| Qualitative Markers | Present (n = 18) n (%) | Absent (n = 147) n (%) | p-Value | |
| COVID-19 severity | severe | 14 (77.8%) | 104 (70.7%) | >0.05 b |
| moderate | 2 (11.1%) | 25 (17%) | ||
| light | 2 (11.1%) | 18 (12.2%) | ||
| Consolidation | 12 (66.7%) | 67 (45.6%) | 0.091 a | |
| Number of affected lobes = 0 | 8 (44.8%) | 85 (57.8%) | ||
| Number of affected lobes ≥ 3 | 8 (44.8%) | 18 (12.3%) | 0.002 b | |
| Cardio-vascular disease present | 16 (88.9%) | 120 (81.6%) | 0.742 b | |
| Arterial hypertension present | 14 (77.8%) | 122 (83%) | 0.526 b | |
| Diabetes present | 7 (38.9%) | 54 (36.7%) | 0.858 a | |
| Renal failure present | 4 (22.2%) | 18 (12.2%) | 0.267 b | |
| Respiratory failure present | 6 (33.3%) | 49 (33.3%) | >0.05 b | |
| Pre-existing treatment present | 6 (33.3%) | 112 (76.2%) | <0.001 a | |
| O2-therapy type at admission | No O2 | 4 (22.2%) | 20 (13.6%) | >0.05 b |
| NC | 0 (0%) | 32 (21.8%) | ||
| SM | 4 (22.2%) | 18 (12.2%) | ||
| VM | 1 (5.6%) | 32 (21.8%) | ||
| NRM | 7 (38.9%) | 42 (28.6%) | ||
| HFOT | 2 (11.1%) | 3 (2%) | ||
| Smoker status | Non-smoker | 13 (72.2%) | 70 (47.6%) | >0.05 b |
| Former smoker | 1 (5.6%) | 41 (27.9%) | ||
| Active smoker | 4 (22.2%) | 36 (24.5%) | ||
| Non-smoker and former smoker | 14 (77.8%) | 111 (75.5%) | >0.05 b | |
| Active smoker | 4 (22.2%) | 36 (24.5%) | ||
| Non-smoker | 13 (72.2%) | 70 (47.6%) | 0.049 a | |
| Active-smoker and former smoker | 5 (27.8%) | 77 (52.4%) | ||
| Non-invasive ventilation present | 13 (72.2%) | 29 (19.7%) | <0.001 b | |
| Quantitative Markers | Present (n = 18) Median (Q1–Q3) | Absent (n = 147) Median (Q1–Q3) | Mann–Whitney U: p-Value |
|---|---|---|---|
| Age | 69 (62.25–74.5) | 71 (64–78) | 0.430 |
| LDH | 425 (326.5–717.25) | 349 (244–506) | 0.053 |
| PCR | 16.44 (7.78–93.03) | 20.6 (7.19–70) | 0.576 |
| Eosinophile | 0 (0–0.01) | 0.01 (0–0.07) | 0.031 |
| Lymphocytes | 0.69 (0.57–0.91) | 0.98 (0.71–1.34) | 0.012 |
| Leucocytes | 8.88 (6.28–14.67) | 7.77 (5.84–10.12) | 0.234 |
| Thrombocytes | 222 (152.25–324) | 222 (172–298) | 0.724 |
| Neutrophiles | 7.86 (5.67–13.06) | 6.29 (4.31–8.35) | 0.083 |
| PLR | 291.89 (212.83–476.66) | 232.97 (162.6–354.08) | 0.123 |
| NLR | 11.73 (6.73–17.2) | 6.13 (4.07–10.33) | 0.006 |
| Death | ||||
|---|---|---|---|---|
| Qualitative Markers | Present (n = 25) n (%) | Absent (n = 140) n (%) | p-Value | |
| COVID-19 severity | Severe | 21 (84%) | 97 (69.3%) | >0.05 b |
| moderate | ||||
| light | 2 (8%) | 25 (17.9%) | ||
| Consolidation | 15 (60%) | 64 (45.7%) | 0.188 a | |
| Number of affected lobes ≥ 3 | 9 (36%) | 17 (12.1%) | <0.01 b | |
| Cardio-vascular disease present | 23 (92%) | 113 (80.7%) | 0.255 b | |
| Arterial hypertension present | 20 (80%) | 116 (82.9%) | 0.776 b | |
| Diabetes present | 10 (40%) | 51 (36.4%) | 0.733 a | |
| Renal failure present | 7 (28%) | 15(10.7%) | 0.048 b | |
| Respiratory failure present | 11 (44%) | 44 (31.4%) | 0.219 a | |
| Pre-existing treatment present | 15 (60%) | 103 (73.6%) | 0.166 a | |
| O2-therapy type at admission | No O2 | 4 (16%) | 20 (14.3%) | ≥0.05 b |
| NC | 0 (0%) | 32 (22.9%) | ||
| SM | 3 (12%) | 19 (13.6%) | ||
| VM | 3 (12%) | 30 (21.4%) | ||
| NRM | 12 (48%) | 37 (26.4%) | ||
| HFOT | 3 (12%) | 2 (1.4%) | ||
| Smoker status | Non-smoker | 16 (64%) | 67 (47.9%) | 0.308 a |
| Former smoker | 4 (16%) | 38 (27.1%) | ||
| Active smoker | 5 (20%) | 35 (25%) | ||
| Non-smoker and former smoker | 20 (80%) | 105 (75%) | 0.591 a | |
| Active smoker | 5 (20%) | 35 (25%) | ||
| Non-smoker | 16 (64%) | 67 (47.9%) | 0.137 a | |
| Active smoker and former smoker | 9 (36%) | 73 (52.1%) | ||
| ICU and Invasive mechanical ventilation present | 13 (52%) | 5 (3.6%) | <0.001 b | |
| Non-invasive ventilation present | 13 (52%) | 29 (20.7%) | 0.001 a | |
| Quantitative Markers | Present (n = 25) Median (Q1–Q3) | Absent (n = 140) Median (Q1–Q3) | Mann–Whitney U: p-Value |
|---|---|---|---|
| Age | 71 (66.5–76.5) | 71 (64–77.75) | 0.457 |
| LDH | 427 (351–737.5) | 343.5 (244–503.75) | 0.031 |
| PCR | 24 (9.18–95.3) | 20.25 (6.53–65.88) | 0.233 |
| Eosinophile | 0 (0–0.03) | 0.01 (0–0.06) | 0.221 |
| Lymphocytes | 0.77 (0.6–0.99) | 0.99 (0.68–1.34) | 0.036 |
| Leucocytes | 8.61 (5.15–11.82) | 7.68 (5.88–10.33) | 0.757 |
| Thrombocytes | 198 (133.5–277) | 225 (174–299.5) | 0.139 |
| Neutrophiles | 6.47 (3.65–8.75) | 6.36 (4.5–9.01) | 0.849 |
| PLR | 232.97 (157.79–418.86) | 239.78 (167.85–359.88) | 0.794 |
| NLR | 8.61 (4.45–12.54) | 6.44 (4.03–10.88) | 0.304 |
| Ventilation | ICU and Invasive Mechanical Ventilation | Total | |||||
|---|---|---|---|---|---|---|---|
| Mechanical Ventilation | Mechanical Ventilation Total | No Mechanical Ventilation | No Mechanical Ventilation Total | ||||
| Death | Survival | Death | Survival | ||||
| Non-invasive ventilation | 9 | 4 | 13 | 4 | 25 | 29 | 42 |
| No ventilation | 4 | 1 | 5 | 8 | 110 | 118 | 123 |
| Total | 13 | 5 | 18 | 12 | 133 | 147 | 165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motoc, N.Ș.; Făgărășan, I.; Urda-Cîmpean, A.E.; Todea, D.A. Prognosis Predictive Markers in Patients with Chronic Obstructive Pulmonary Disease and COVID-19. Diagnostics 2023, 13, 2597. https://doi.org/10.3390/diagnostics13152597
Motoc NȘ, Făgărășan I, Urda-Cîmpean AE, Todea DA. Prognosis Predictive Markers in Patients with Chronic Obstructive Pulmonary Disease and COVID-19. Diagnostics. 2023; 13(15):2597. https://doi.org/10.3390/diagnostics13152597
Chicago/Turabian StyleMotoc, Nicoleta Ștefania, Iulia Făgărășan, Andrada Elena Urda-Cîmpean, and Doina Adina Todea. 2023. "Prognosis Predictive Markers in Patients with Chronic Obstructive Pulmonary Disease and COVID-19" Diagnostics 13, no. 15: 2597. https://doi.org/10.3390/diagnostics13152597
APA StyleMotoc, N. Ș., Făgărășan, I., Urda-Cîmpean, A. E., & Todea, D. A. (2023). Prognosis Predictive Markers in Patients with Chronic Obstructive Pulmonary Disease and COVID-19. Diagnostics, 13(15), 2597. https://doi.org/10.3390/diagnostics13152597







