Diagnosis of Migrainous Infarction: A Case Report and Analysis of Previously Published Cases
Abstract
1. Introduction
2. Case History
2.1. Patient Information
2.2. Clinical Findings
The First Visit
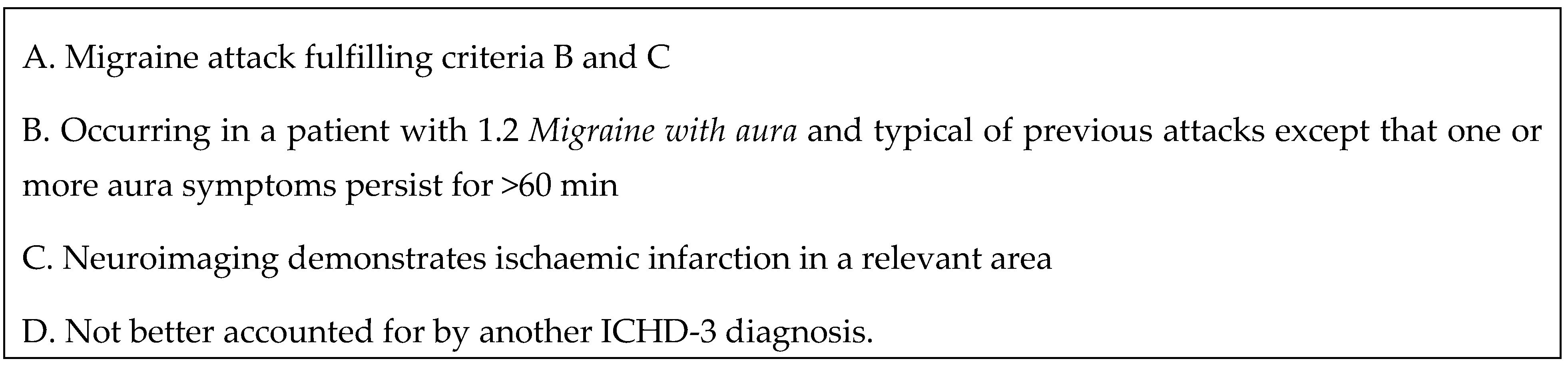
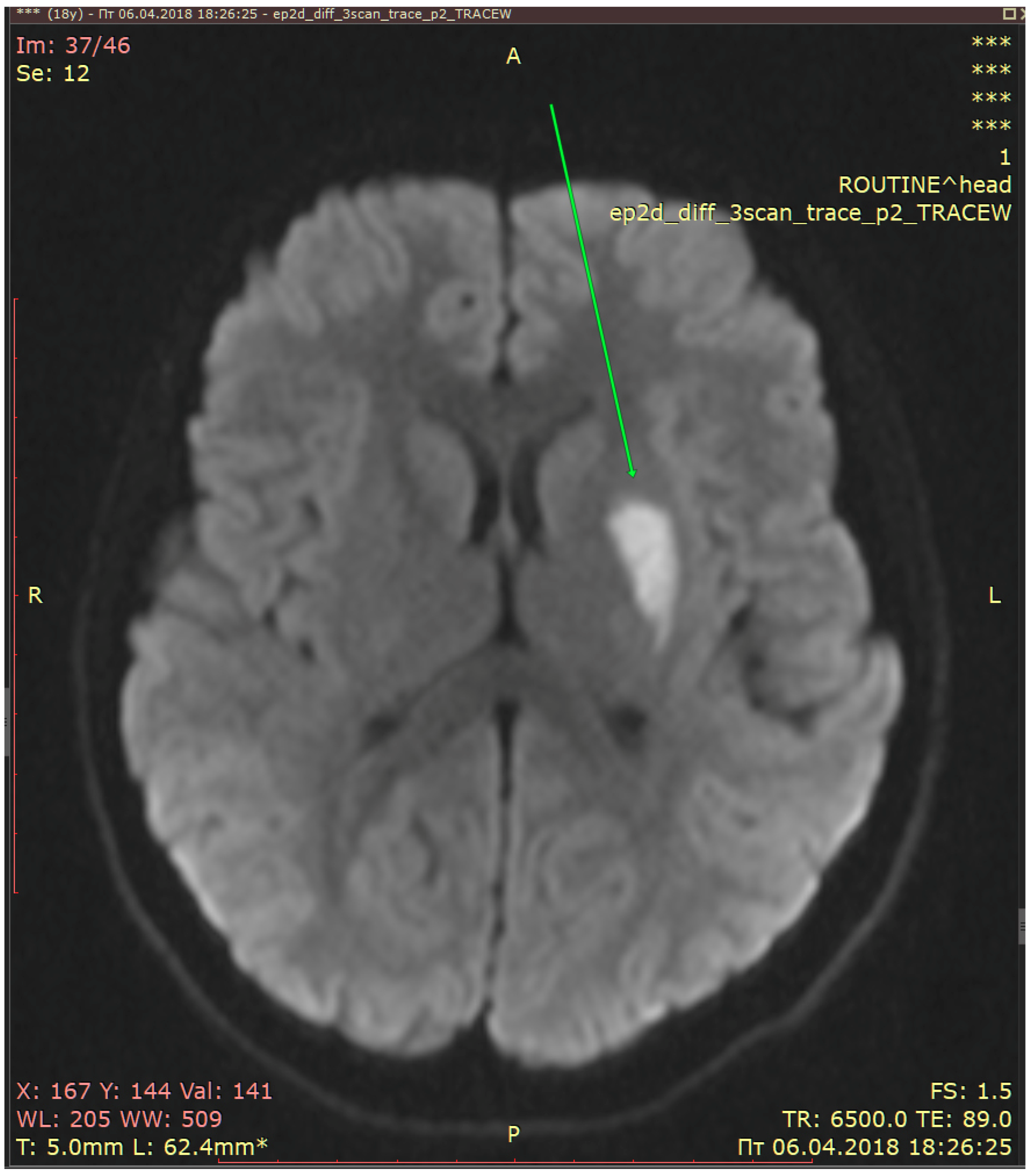
2.3. Diagnostic Assessment and Evaluation
2.4. Treatment
2.5. Follow-Up of the Patient
2.5.1. The Second Visit: 27 October 2018
2.5.2. The Third Visit: 23 August 2019
2.5.3. The Fourth Visit: 21 December 2020
2.5.4. The Fifth Visit: 27 January 2022
2.5.5. The Sixth Visit: 22 November 2022
2.5.6. Telephone Interview: 21 May 2023
3. Discussion
4. Diagnostic Recommendations for Migrainous Infarction
- (1)
- It is important to ask a patient who has a focal neurological deficit about present and previous headaches and their characteristics, and to classify headaches according to the International Classification of Headache Disorders.
- (2)
- It is necessary to know the duration of focal neurological symptoms and how they developed: sudden onset, all symptoms at the same time or gradually, one after the other. Were they positive or negative?
- (3)
- The sudden onset of negative focal neurological symptoms warns of TIA or stroke.
- (4)
- The gradual spread of focal neurological symptoms with positive/irritative signs and headache are diagnostic for migraine with aura except for cases with long duration of focal symptoms (>60 min) which require urgent neurological examinations, imaging including MRI with DWI, and other investigations for the early diagnosis of cerebral infarcts.
- (5)
- A neurologist must compare current and previous clinical characteristics of headache and aura symptoms at stroke onset, as well as their site/localization in relation to the site/localization of acute cerebral infarct, in order to differentiate migrainous infarction and ischemic stroke from aura-like symptoms.
- (6)
- It is necessary to perform neuroimaging in patients with migraine with aura when they have abnormal neurological status, atypical features, or “red flags”, and this should be used for the early detection of migrainous infarction.
- (7)
- The diagnostic criteria of migrainous infarction of the ICHD should be used by neurologists in daily practice for improvement in diagnoses.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinciguerra, L.; Cantone, M.; Lanza, G.; Bramanti, A.; Santalucia, P.; Puglisi, V.; Pennisi, G.; Bella, R. Migrainous Infarction and Cerebral Vasospasm: Case Report And Literature Review. J. Pain Res. 2019, 12, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- The International Classification of Headache Disorders, 1st ed.; (ICHD-1); SAGE Publications: Thousand Oaks, CA, USA, 1988; Volume 8, pp. 1–96.
- The International Classification of Headache Disorders, 3rd ed.; (ICHD-3); SAGE Publications: Thousand Oaks, CA, USA, 2018; Volume 38, pp. 1–211.
- Sacco, S.; Harriott, A.M.; Ayata, C.; Ornello, R.; Bagur, R.; Jimenez-Ruiz, A.; Sposato, L.A. Microembolism and Other Links Between Migraine and Stroke: Clinical and Pathophysiologic Update. Neurology 2023, 100, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Singh, U.; Sarmah, D.; Datta, A.; Baidya, F.; Shah, B.; Bohra, M.; Jagtap, P.; Sarkar, A.; Kalia, K.; et al. Migraine and Ischemic Stroke: Deciphering the Bidirectional Pathway. ACS Chem. Neurosci. 2020, 11, 1525–1538. [Google Scholar] [CrossRef] [PubMed]
- Scutelnic, A.; Kreis, L.A.; Beyeler, M.; Heldner, M.R.; Meinel, T.R.; Kaesmacher, J.; Hakim, A.; Arnold, M.; Fischer, U.; Mattle, H.P.; et al. Migraine aura-like symptoms at onset of stroke and stroke-like symptoms in migraine with aura. Front. Neurol. 2022, 13, 1004058. [Google Scholar] [CrossRef]
- Scutelnic, A.; Mattle, H.P.; Branca, M.; Jung, S.; Reichlin, T.; Fischer, U.; Schankin, C.J. Migraine and atrial fibrillation: A systematic review. Eur. J. Neurol. 2022, 29, 910–920. [Google Scholar] [CrossRef]
- Gollion, C.; Gazagnes, J.; Fabry, V.; Barbieux-Guillot, M.; Lerebours, F.; Larrue, V. Atrial fibrillation and migraine with aura in young adults with ischemic stroke. Cephalalgia 2021, 41, 375–382. [Google Scholar] [CrossRef]
- Olesen, J.; Friberg, L.; Olsen, T.S.; Andersen, A.R.; Lassen, N.A.; Hansen, P.E.; Karle, A. Ischaemia-induced (symptomatic) migraine attacks may be more frequent than migraine-induced ischaemic insults. Brain 1993, 116 Pt 1, 187–202. [Google Scholar] [CrossRef]
- Lebedeva, E.R.; Tsypushkina, T.S.; Gurary, N.M.; Toporkova, M.G.; Olesen, J. Migrainous stroke (two cases). Ural. Med. J. 2015, 10, 45–48. [Google Scholar]
- Iftikhar, W.; Cheema, F.F.; Khanal, S.; Khan, Q.U. Migrainous Infarction and Cortical Spreading Depression. Discoveries 2020, 8, e112. [Google Scholar] [CrossRef]
- Mancini, V.; Mastria, G.; Frantellizzi, V.; Troiani, P.; Zampatti, S.; Carboni, S.; Giardina, E.; Campopiano, R.; Gambardella, S.; Turchi, F.; et al. Migrainous infarction in a patient with sporadic hemiplegic migraine and cystic fibrosis: A 99mTc-HMPAO brain SPECT study. Headache 2019, 59, 253–258. [Google Scholar] [CrossRef]
- Campagna, G.; Vickers, A.; Ponce, C.M.P.; Lee, A.G. Homonymous hemianopsia as the presenting sign of migrainous infarction. Can. J. Ophthalmol. 2018, 53, e229–e232. [Google Scholar] [CrossRef]
- Khardenavis, V.; Karthik, D.K.; Kulkarni, S.; Deshpande, A. Cortical laminar necrosis in a case of migrainous cerebral infarction. BMJ Case Rep. 2018, 2018, bcr2017221483. [Google Scholar] [CrossRef]
- Morais, R.; Sobral, F.; Cunha, G.; Brito, O.; Santana, I. Advanced MRI study of migrainous infarction presenting as cortical laminar necrosis—Case report and literature review. Clin. Neurol. Neurosurg. 2018, 167, 82–85. [Google Scholar] [CrossRef]
- Serrano, F.; Arauz, A.; Uribe, R.; Becerra, L.C.; Mantilla, K.; Zermenño, F. Long-term follow-up of patients with migrainous infarction. Clin. Neurol. Neurosurg. 2018, 165, 7–9. [Google Scholar] [CrossRef]
- Kreling, G.A.D.; Neuro Rodrigues de Almeida, N.; Pedro José dos Santos, N. Migrainous infarction: A rare and often overlooked diagnosis. Autops. Case Rep. 2017, 7, 61–68. [Google Scholar] [CrossRef]
- Renard, D.; Nerrant, E.; Freitag, C. Early recurrence of migrainous infarction. Acta Neurol. Belg. 2015, 115, 675–676. [Google Scholar] [CrossRef]
- Parks, N.E.; Rigby, H.B.; Gubitz, G.J.; Shankar, J.J.; Purdy, R.A. Dysmetropsia and Cotard’s syndrome due to migrainous infarction—Or not? Cephalalgia Int. J. Headache 2014, 34, 717–720. [Google Scholar] [CrossRef]
- Thissen, S.; Koehler, P.J. Persistent aura with small occipital cortical infarction: Implications for migraine pathophysiology. Case Rep. Neurol. 2014, 6, 217–221. [Google Scholar] [CrossRef]
- Arboix, A.; González-Peris, S.; Grivé, E.; Sánchez, M.J.; Comes, E. Cortical laminar necrosis related to migrainous cerebral infarction. World J. Clin. Cases 2013, 1, 256–259. [Google Scholar] [CrossRef]
- Lai, T.H.; Hong, C.T. Prolonged symptoms in sporadic hemiplegic migraine: Aura or migrainous infarction? Acta Neurol Taiwan 2012, 21, 129–132. [Google Scholar]
- Wolf, M.E.; Szabo, K.; Griebe, M.; Förster, A.; Gass, A.; Hennerici, M.G.; Kern, R. Clinical and MRI characteristics of acute migrainous infarction. Neurology 2011, 76, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Laurell, K.; Artto, V.; Bendtsen, L.; Hagen, K.; Kallela, M.; Meyer, E.L.; Putaala, J.; Tronvik, E.; Zwart, J.A.; Linde, M. Migrainous infarction: A Nordic multicenter study. Eur. J. Neurol. 2011, 18, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Chen, C.C.; Wang, S.C.; Yip, P.K. Reversible vasospasm in migrainous infarction: A transcranial Doppler follow-up study. J. Ultrasound Med. 2010, 29, 481–484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Decima, D.; Cavallo, M.; Leotta, M.R.; Gaballo, A. Migrainous infarction: Association with vascular risk factors in a male subject. Neurol. Sci. 2009, 30 (Suppl. 1), S145–S146. [Google Scholar] [CrossRef]
- Caballero, P.E.J. Migrainous cerebral infarction after postcoital contra- ception. Cephalalgia Int. J. Headache 2009, 29, 691–693. [Google Scholar] [CrossRef]
- Schulz, U.G.; Blamire, A.M.; Davies, P.; Styles, P.; Rothwell, P.M. Normal cortical energy metabolism in migrainous stroke: A 31P-MR spectroscopy study. Stroke 2009, 40, 3740–3744. [Google Scholar] [CrossRef]
- Arai, S.; Utsunomiya, H.; Arihiro, S.; Arakawa, S. Migrainous infarction in an adult: Evaluation with serial diffusion-weighted images and cerebral blood flow studies. Radiat. Med. 2008, 26, 313–317. [Google Scholar] [CrossRef]
- Marshall, N.; Maclaurin, W.A.; Koulouris, G. MRA captures vasospasm in fatal migrainous infarction. Headache 2007, 47, 280–283. [Google Scholar] [CrossRef]
- Liang, Y.; Scott, T.F. Migrainous infarction with appearance of laminar necrosis on MRI. Clin. Neurol. Neurosurg. 2007, 109, 592–596. [Google Scholar] [CrossRef]
- Tzoulis, C.H.; Naess, H.; Thomassen, L. Migrainous cerebral infarction in a previously healthy 93-year-old female patient with no risk factors for stroke. Cephalalgia Int. J. Headache 2006, 26, 894–895. [Google Scholar] [CrossRef]
- Matsuo, S.; Suzuki, Y.; Hashimoto, M.; Ohtsuka, K. Migrainous cerebral infarction triggered by alcohol intake under the burning sun. Jpn. J. Ophthalmol. 2006, 50, 395–397. [Google Scholar] [CrossRef]
- Frigerio, R.; Santoro, P.; Ferrarese, C.; Agostoni, E. Migrainous cerebral infarction: Case reports. Neurol. Sci. 2004, 25 (Suppl. 3), S300–S301. [Google Scholar] [CrossRef]
- Tang, S.C.; Jeng, J.S.; Liu, H.M.; Yip, P.K. Migrainous infarction involving two different arterial territories: Report of two cases. Acta Neurol. Taiwanica 2004, 13, 20–23. [Google Scholar]
- Lee, H.; Whitman, G.T.; Lim, J.G.; Yi, S.D.; Cho, Y.W.; Ying, S.; Baloh, R.W. Hearing symptoms in migrainous infarction. Arch. Neurol. 2003, 60, 113–116. [Google Scholar] [CrossRef]
- Arboix, A.; Massons, J.; Garcia-Eroles, L.; Oliveres, M.; Balcells, M.; Targa, C. Migrainous cerebral infarction in the Sagrat Cor Hospital of Barcelona stroke registry. Cephalalgia Int. J. Headache 2003, 23, 389–394. [Google Scholar] [CrossRef]
- Linetsky, E.; Leker, R.R.; Ben-Hur, T. Headache characteristics in patients after migrainous stroke. Neurology 2001, 57, 130–132. [Google Scholar] [CrossRef]
- Demirkaya, S.; Odabasi, Z.; Gokcil, Z.; Ozdag, F.; Kutukcu, Y.; Vural, O. Migrainous stroke causing bilateral anterior cerebral artery territory infarction. Headache 1999, 39, 513–516. [Google Scholar] [CrossRef][Green Version]
- Meschia, J.F.; Malkoff, M.D.; Biller, J. Reversible segmental cerebral arterial vasospasm and cerebral infarction: Possible association with excessive use of sumatriptan and Midrin. Arch. Neurol. 1998, 55, 712–714. [Google Scholar] [CrossRef]
- Mendizabal, J.E.; Greiner, F.; Hamilton, W.J.; Rothrock, J.F. Migrainous stroke causing thalamic infarction and amnesia during treatment with propranolol. Headache 1997, 37, 594–596. [Google Scholar] [CrossRef]
- Sanin, L.C.; Mathew, N.T. Severe diffuse intracranial vasospasm as a cause of extensive migrainous cerebral infarction. Cephalalgia Int. J. Headache 1993, 13, 289–292. [Google Scholar] [CrossRef]
- Gomez, C.R.; Gomez, S.M.; Puricelli, M.S.; Malik, M.M. Transcranial Doppler in reversible migrainous vasospasm causing cerebellar infarction: Report of a case. Angiology 1991, 42, 152–156. [Google Scholar] [CrossRef] [PubMed]
| N | Authors, Year | Number, Age, and Sex | Diagnosis Fulfils the Criteria of MI in the ICHD (Yes or No) | Comments and Expert Diagnosis |
|---|---|---|---|---|
| 1 | Lebedeva et al. (2023, present publication) | 1/female/44 y.o. | Yes | Migrainous infarction |
| 2 | Vinciguerra et al. (2019) [1] | 1/female/44 y.o. | Yes | Migrainous infarction |
| 3 | Mancini et al. (2019) [12] | 1/male/32 y.o. | Yes | Migrainous infarction |
| 4 | Campagna et al. (2018) [13] | 1/female/47 y.o. | Yes | The exact duration of the migrainous aura is not indicated in migrainous infarction |
| 5 | Khardenavis et al. (2018) [14] | 1/female/27 y.o. | Yes | Migrainous infarction |
| 6 | Morais et al. (2018) [15] | 1/female/37 y.o. | Yes | Migrainous infarction |
| 7 | Serrano et al. (2018) [16] | 8 females and 7 males/18–55 y.o. | Uncertain | Absence of detailed characteristics and number of migrainous auras in the past and at the onset of infarction in all cases, absence of full description of all infarcts |
| 8 | Kreling et al. (2017) [17] | 1/female/16 y.o. | No | Migrainous aura was not similar to the previous migraine with visual aura and had features of basilar aura |
| 9 | Renard et al. (2015) [18] | 1/male/47 | Yes | Migrainous infarction |
| 10 | Lebedeva at all (2015) [10] | 2/1 female 53 y.o, 1 male 54 y.o. | Yes | Migrainous infarction |
| 11 | Parks et al. (2014) [19] | 1/female/59 y.o. | No | Absence of infarction upon MRI with DWI |
| 12 | Thissen et al. (2014) [20] | 1/female/74 y.o. | No | Persistent aura with infarction |
| 13 | Arboix et al. (2013) [21] | 1/female/29 y.o. | No | Focal neurological symptoms (hemiparesis, left hemihypesthesia, dysarthria) were not similar to the previous migraine with visual aura and had features of hemiplegic aura |
| 14 | Lai e Hong (2012) [22] | 1/male/60 y.o. | Yes | Migrainous infarction |
| 15 | Wolf et al. (2011) [23] | 4 males and 13 females/20–71 y.o. | No | Not enough information about the characteristics of aura |
| 16 | Laurell et al. (2011) [24] | 13 males and 20 females/16–76 y.o. | No | Not enough information about the characteristics of aura |
| 17 | Tsai et al. (2010) [25] | 1/female/42 y.o. | No | The patient had a migraine without aura before the ischemic stroke |
| 18 | Decima et al. (2009) [26] | 1/male/41 y.o. | No | Not enough information about the characteristics of aura |
| 19 | Caballero (2009) [27] | 1/female/21 y.o. | No | Focal neurological symptoms (left homonymous hemianopia, metamorphopsias, dysarthria, left hemi-paraesthesias) were not similar to the previous attacks of migraine with visual aura |
| 20 | Schulz et al. (2009) [28] | 3 males and 2 females/21–58 y.o. | Uncertain | Not enough information about the comparison of characteristics of aura and focal symptoms, absence of description of localization of infarcts |
| 21 | Arai et al. (2008) [29] | 1/male/64 y.o. | Yes | Migrainous infarction |
| 22 | Marshall et al. (2007) [30] | 1/female/57 y.o. | No | Neurological symptoms at the onset of stroke (delirium, right arm weakness, near total visual loss) were not similar to the previous attacks of migraine with visual aura |
| 23 | Liang e Scott (2007) [31] | 1/female/57 y.o. | Yes | Migrainous infarction |
| 24 | Tzoulis et al. (2006) [32] | 1/female/93 y.o. | Yes | Migrainous infarction |
| 25 | Matsuo et al. (2006) [33] | 1/female/35 y.o. | No | The patient had a migraine without aura before the ischemic stroke |
| 26 | Frigerio et al. (2004) [34] | 1 male and 5 females/23–40 y.o. | No | Several patients had focal neurological symptoms which were not similar to the previous attacks of migraine with aura |
| 27 | Tang et al. (2004) [35] | 1 male, 29 y.o. and 1 female 47 y.o. | No | Not enough information about the characteristics of aura |
| 28 | Lee et al. (2003) [36] | 1 male 40 y.o. and 1 female 25 y.o. | No | MRI and MR angiography did not reveal an infarct in a male. Neurological symptoms at the onset of stroke in a woman (vertigo, subjective right-sided hearing loss, diplopia, quadriparesis, right-sided hemihypesthesia) were not similar to the previous attacks of migraine with visual aura |
| 29 | Arboix et al. (2003) [37] | 3 males and 6 females/24–60 y.o. | Uncertain | Absence of detailed characteristics and information about the number of migrainous auras in the past and no comparison of previous aura symptoms and symptoms at the onset of infarction |
| 30 | Linetsky et al. (2001) [38] | 1 male and 5 females/15–46 y.o. | Not all cases were migrainous infarctions | Several patients had focal neurological symptoms at the onset of stroke which were not similar to the previous attacks of migraine with visual aura and their stroke manifested mainly as motor and sensory deficits and only one patient had evident hemianopsia upon bedside examination |
| 31 | Demirkaya et al. (1999) [39] | 1/male/38 y.o. | No | The patient had a migraine without aura before the ischemic stroke |
| 32 | Meschia et al. (1998) [40] | 1/male/43 y.o. | No | The patient had a migraine without aura before the ischemic stroke |
| 33 | Mendizabal et al. (1997) [41] | 1/female/47 y.o. | No | Focal symptoms at stroke onset were not typical of previous attacks of migraine with basilar aura since neurological examination showed severe stupor without obvious focal findings |
| 34 | Sanin et al. (1993) [42] | 1/female/47 y.o. | No | Focal symptoms at stroke onset (cortical blindness and left-sided hemiparesis and hemineglect) were different from previous attacks of migraine with visual aura |
| 35 | Gomez et al. (1991) [43] | 1/female/33 | No | Not enough information about all characteristics of aura |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedeva, E.R.; Gurary, N.M.; Olesen, J. Diagnosis of Migrainous Infarction: A Case Report and Analysis of Previously Published Cases. Diagnostics 2023, 13, 2502. https://doi.org/10.3390/diagnostics13152502
Lebedeva ER, Gurary NM, Olesen J. Diagnosis of Migrainous Infarction: A Case Report and Analysis of Previously Published Cases. Diagnostics. 2023; 13(15):2502. https://doi.org/10.3390/diagnostics13152502
Chicago/Turabian StyleLebedeva, Elena R., Natalia M. Gurary, and Jes Olesen. 2023. "Diagnosis of Migrainous Infarction: A Case Report and Analysis of Previously Published Cases" Diagnostics 13, no. 15: 2502. https://doi.org/10.3390/diagnostics13152502
APA StyleLebedeva, E. R., Gurary, N. M., & Olesen, J. (2023). Diagnosis of Migrainous Infarction: A Case Report and Analysis of Previously Published Cases. Diagnostics, 13(15), 2502. https://doi.org/10.3390/diagnostics13152502







