Evaluating Headache and Facial Pain in a Headache Diagnostic Laboratory: Experiences from the Danish Headache Center
Abstract
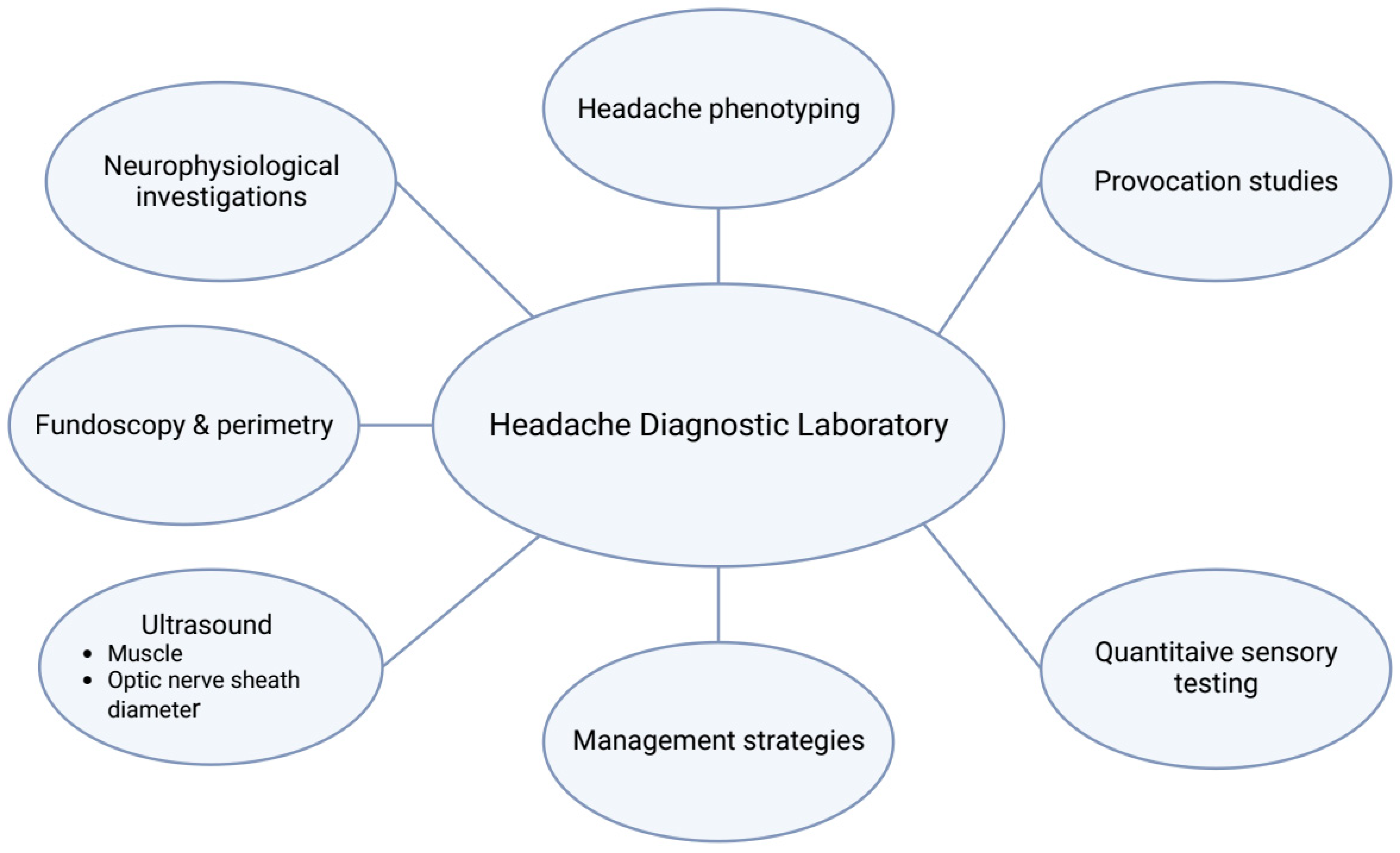
1. Introduction
2. Methods
3. Neurophysiological Studies—The Blink Reflex
4. Headache Phenotyping Using Quantitative Sensory Testing
5. Ultrasound Investigations of Pericranial Muscles
6. Headache Provocation Models
7. Semi-Structured Interviews
8. Laboratory Investigations in Intracranial Hypertension
9. Headache Diagnostic Laboratory to Assess Management Strategies
10. Discussion
11. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Do, T.P.; Remmers, A.; Schytz, H.W.; Schankin, C.; Nelson, S.E.; Obermann, M.; Hansen, J.M.; Sinclair, A.J.; Gantenbein, A.R.; Schoonman, G.G. Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology 2019, 92, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Schytz, H.W.; Olesen, J. Laboratory tests of headache disorders—Dawn of a new era? Cephalalgia 2016, 36, 1268–1290. [Google Scholar] [CrossRef] [PubMed]
- Mouaanaki, S.A.; Carlsen, L.N.; Bendtsen, L.; Jensen, R.H.; Schytz, H.W. Treatment experiences and clinical characteristics in migraine and tension-type headache patients before the first visit to a tertiary headache center. Cephalalgia 2022, 42, 1265–1273. [Google Scholar] [CrossRef]
- Magis, D.; Vigano, A.; Sava, S.; D’elia, T.S.; Schoenen, J.; Coppola, G. Pearls and pitfalls: Electrophysiology for primary headaches. Cephalalgia 2013, 33, 526–539. [Google Scholar] [CrossRef]
- Obermann, M.; Yoon, M.-S.; Ese, D.; Maschke, M.; Kaube, H.; Diener, H.-C.; Katsarava, Z. Impaired trigeminal nociceptive processing in patients with trigeminal neuralgia. Neurology 2007, 69, 835–841. [Google Scholar] [CrossRef]
- Bjerring, B.; Maarbjerg, S.; Heinskou, T.; Bendtsen, L.; Nikolic, M.; Grillo, V.; De Icco, R.; Schytz, H.W. Comparison of the blink reflex in classical and idiopathic trigeminal neuralgia. Cephalalgia 2023, 43, 03331024231191136. [Google Scholar] [CrossRef]
- Iljazi, A.; Ashina, H.; Lipton, R.B.; Chaudhry, B.; Al-Khazali, H.M.; Naples, J.G.; Schytz, H.W.; Cvetkovic, V.V.; Burstein, R.; Ashina, S. Dizziness and vertigo during the prodromal phase and headache phase of migraine: A systematic review and meta-analysis. Cephalalgia 2020, 40, 1095–1103. [Google Scholar] [CrossRef]
- Eigenbrodt, A.K.; Christensen, R.H.; Ashina, H.; Iljazi, A.; Christensen, C.E.; Steiner, T.J.; Lipton, R.B.; Ashina, M. Premonitory symptoms in migraine: A systematic review and meta-analysis of observational studies reporting prevalence or relative frequency. J. Headache Pain 2022, 23, 140. [Google Scholar] [CrossRef]
- Hvedstrup, J.; Kolding, L.T.; Younis, S.; Ashina, M.; Schytz, H.W. Ictal neck pain investigated in the interictal state—A search for the origin of pain. Cephalalgia 2020, 40, 614–624. [Google Scholar] [CrossRef]
- Ashina, S.; Bendtsen, L.; Lyngberg, A.C.; Lipton, R.B.; Hajiyeva, N.; Jensen, R. Prevalence of neck pain in migraine and tension-type headache: A population study. Cephalalgia 2015, 35, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ashina, S.; Mitsikostas, D.D.; Lee, M.J.; Yamani, N.; Wang, S.J.; Messina, R.; Ashina, H.; Buse, D.C.; Pozo-Rosich, P.; Jensen, R.H.; et al. Tension-type headache. Nat. Rev. Dis. Primers 2021, 7, 24. [Google Scholar] [CrossRef]
- Christensen, M.; Bendtsen, L.; Ashina, M.; Jensen, R. Experimental Induction of Muscle Tenderness and Headache in Tension-Type Headache Patients. Cephalalgia 2005, 25, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Ashina, S.; Bendtsen, L.; Burstein, R.; Iljazi, A.; Jensen, R.H.; Lipton, R.B. Pain sensitivity in relation to frequency of migraine and tension-type headache with or without coexistent neck pain: An exploratory secondary analysis of the population study. Scand. J. Pain 2023, 23, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Al-Khazali, H.M.; Younis, S.; Al-Sayegh, Z.; Ashina, S.; Ashina, M.; Schytz, H.W. Prevalence of neck pain in migraine: A systematic review and meta-analysis. Cephalalgia 2022, 42, 663–673. [Google Scholar] [CrossRef]
- Maier, C.; Baron, R.; Tölle, T.R.; Binder, A.; Birbaumer, N.; Birklein, F.; Gierthmühlen, J.; Flor, H.; Geber, C.; Huge, V.; et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 2010, 150, 439–450. [Google Scholar] [CrossRef]
- Rolke, R.; Magerl, W.; Campbell, K.A.; Schalber, C.; Caspari, S.; Birklein, F.; Treede, R.-D. Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur. J. Pain 2006, 10, 77. [Google Scholar] [CrossRef]
- Burstein, R.; Collins, B.; Jakubowski, M. Defeating migraine pain with triptans: A race against the development of cutaneous allodynia. Ann. Neurol. 2004, 55, 19–26. [Google Scholar] [CrossRef]
- Bendtsen, L.; Jensen, R.; Jensen, N.; Olesen, J. Pressure-Controlled Palpation: A New Technique Which Increases the Reliability of Manual Palpation. Cephalalgia 1995, 15, 205–210. [Google Scholar] [CrossRef]
- Defrin, R.; Riabinin, M.; Feingold, Y.; Schreiber, S.; Pick, C.G. Deficient Pain Modulatory Systems in Patients with Mild Traumatic Brain and Chronic Post-Traumatic Headache: Implications for its Mechanism. J. Neurotrauma 2015, 32, 28–37. [Google Scholar] [CrossRef]
- Defrin, R.; Gruener, H.; Schreiber, S.; Pick, C.G. Quantitative somatosensory testing of subjects with chronic post-traumatic headache: Implications on its mechanisms. Eur. J. Pain 2010, 14, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Ashina, H.; Al-Khazali, H.M.; Iljazi, A.; Ashina, S.; Amin, F.M.; Schytz, H.W. Total tenderness score and pressure pain thresholds in persistent post-traumatic headache attributed to mild traumatic brain injury. J. Headache Pain 2022, 23, 96. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Burstein, R.; Ashina, M.; Tfelt-Hansen, P. Origin of pain in migraine: Evidence for peripheral sensitisation. Lancet Neurol. 2009, 8, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Hvedstrup, J.; Kolding, L.T.; Ashina, M.; Schytz, H.W. Increased neck muscle stiffness in migraine patients with ictal neck pain: A shear wave elastography study. Cephalalgia 2020, 40, 565–574. [Google Scholar] [CrossRef]
- Davis, L.C.; Baumer, T.G.; Bey, M.J.; van Holsbeeck, M.T. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography 2019, 38, 2–12. [Google Scholar] [CrossRef]
- Kolding, L.T.; Do, T.P.; Ewertsen, C.; Schytz, H.W. Muscle stiffness in tension-type headache patients with pericranial tenderness. Cephalalgia Rep. 2018, 1, 2515816318760293. [Google Scholar] [CrossRef]
- Ashina, H.; Schytz, H.W.; Ashina, M. CGRP in human models of primary headaches. Cephalalgia 2018, 38, 353–360. [Google Scholar] [CrossRef]
- Ashina, H.; Iljazi, A.; Al-Khazali, H.M.; Do, T.P.; Eigenbrodt, A.K.; Larsen, E.L.; Andersen, A.M.; Hansen, K.J.; Bräuner, K.B.; Chaudhry, B.A.; et al. CGRP-induced migraine-like headache in persistent post-traumatic headache attributed to mild traumatic brain injury. J. Headache Pain 2022, 23, 135. [Google Scholar] [CrossRef]
- Ashina, H.; Iljazi, A.; Al-Khazali, H.M.; Christensen, C.E.; Amin, F.M.; Ashina, M.; Schytz, H.W. Hypersensitivity to Calcitonin Gene–Related Peptide in Post-Traumatic Headache. Ann. Neurol. 2020, 88, 1220–1228. [Google Scholar] [CrossRef]
- Ashina, H.; Schytz, H.W.; Ashina, M. CGRP in Human Models of Migraine. Handb. Exp. Pharmacol. 2019, 255, 109–120. [Google Scholar] [CrossRef]
- Iljazi, A.; Ashina, H.; Zhuang, Z.A.; Lopez, C.L.; Snellman, J.; Ashina, M.; Schytz, H.W. Hypersensitivity to calcitonin gene-related peptide in chronic migraine. Cephalalgia 2021, 41, 701–710. [Google Scholar] [CrossRef]
- Al-Hassany, L.; Boucherie, D.M.; Creeney, H.; van Drie, R.W.A.; Farham, F.; Favaretto, S.; Gollion, C.; Grangeon, L.; Lyons, H.; Marschollek, K.; et al. Future targets for migraine treatment beyond CGRP. J. Headache Pain 2023, 24, 76. [Google Scholar] [CrossRef]
- Ashina, H.; Eigenbrodt, A.K.; Seifert, T.; Sinclair, A.J.; I Scher, A.; Schytz, H.W.; Lee, M.J.; De Icco, R.; Finkel, A.G.; Ashina, M. Post-traumatic headache attributed to traumatic brain injury: Classification, clinical characteristics, and treatment. Lancet Neurol. 2021, 20, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Ashina, H.; Porreca, F.; Anderson, T.; Amin, F.M.; Ashina, M.; Schytz, H.W.; Dodick, D.W. Post-traumatic headache: Epidemiology and pathophysiological insights. Nat. Rev. Neurol. 2019, 15, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Ashina, H.; Iljazi, A.; Al-Khazali, H.M.; Ashina, S.; Jensen, R.H.; Amin, F.M.; Ashina, M.; Schytz, H.W. Persistent post-traumatic headache attributed to mild traumatic brain injury: Deep phenotyping and treatment patterns. Cephalalgia 2020, 40, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Lampl, C.; Sacco, S.; Martelletti, P. Narrative-based medicine in headache disorders. J. Headache Pain 2022, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.I.; Liu, G.T.; Digre, K.B. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013, 81, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Mollan, S.P.; Davies, B.; Silver, N.C.; Shaw, S.; Mallucci, C.L.; Wakerley, B.R.; Krishnan, A.; Chavda, S.V.; Ramalingam, S.; Edwards, J.; et al. Idiopathic intracranial hypertension: Consensus guidelines on management. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1088–1100. [Google Scholar] [CrossRef]
- Fisayo, A.; Bruce, B.B.; Newman, N.J.; Biousse, V. Overdiagnosis of idiopathic intracranial hypertension. Neurology 2016, 86, 341–350. [Google Scholar] [CrossRef]
- Aletreby, W.; Alharthy, A.; Brindley, P.G.; Kutsogiannis, D.J.; Faqihi, F.; Alzayer, W.; Balhahmar, A.; Soliman, I.; Hamido, H.; A Alqahtani, S.; et al. Optic Nerve Sheath Diameter Ultrasound for Raised Intracranial Pressure. J. Ultrasound Med. 2022, 41, 585–595. [Google Scholar] [CrossRef]
- Lochner, P.; Brigo, F.; Zedde, M.L.; Sanguigni, S.; Coppo, L.; Nardone, R.; Naldi, A.; Sola, D.; Stolz, E. Feasibility and usefulness of ultrasonography in idiopathic intracranial hypertension or secondary intracranial hypertension. BMC Neurol. 2016, 16, 85. [Google Scholar]
- Chen, L.M.; Wang, L.J.; Hu, Y.; Jiang, X.H.; Wang, Y.Z.; Xing, Y.Q. Ultrasonic measurement of optic nerve sheath diameter: A non-invasive surrogate approach for dynamic, real-time evaluation of intracranial pressure. Br. J. Ophthalmol. 2019, 103, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Korsbæk, J.J.; Hagen, S.M.; Schytz, H.W.; Vukovic-Cvetkovic, V.; Wibroe, E.A.; Hamann, S.; Jensen, R.H. Transorbital sonography: A non-invasive bedside screening tool for detection of pseudotumor cerebri syndrome. Cephalalgia 2022, 42, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.D.; Garza, P.S.; Bruce, B.B.; Newman, N.J.; Biousse, V. The demise of direct ophthalmoscopy. Neurol. Clin. Pract. 2015, 5, 150–157. [Google Scholar] [CrossRef]
- Rissan, Z.; Hansen, N.S.; Carlsen, L.N.; Korsbæk, J.J.; Jensen, R.H.; Hamann, S.; Schytz, H.W. Fundus imaging and perimetry in patients with idiopathic intracranial hypertension—An intermethod and interrater validity study. Eur. J. Neurol. 2023, 30, 1973–1982. [Google Scholar] [CrossRef]
- Bigio, J.; MacLean, E.; Vasquez, N.A.; Huria, L.; Kohli, M.; Gore, G.; Hannay, E.; Pai, M.; Adam, P. Most common reasons for primary care visits in low- and middle-income countries: A systematic review. PLoS Glob. Public Health 2022, 2, e0000196. [Google Scholar] [CrossRef]
- Carlsen, L.N.; Bendtsen, L.; Jensen, R.H.; Schytz, H.W. Telephone follow-up on treatment and patient satisfaction at a Danish tertiary headache center: A prospective study: A prospective study. Headache J. Head Face Pain 2022, 62, 1312–1321. [Google Scholar] [CrossRef]
- Karlsson, W.K.; Ashina, H.; Cullum, C.K.; Christensen, R.H.; Al-Khazali, H.M.; Amin, F.M.; Ashina, M.; Iljazi, A.; Thomsen, A.V.; Chaudhry, B.A.; et al. The Registry for Migraine (REFORM) study: Methodology, demographics, and baseline clinical characteristics. J. Headache Pain 2023, 24, 70. [Google Scholar] [CrossRef]
- Marie, B.S.; Perkhounkova, Y.; Gedney-Lose, A.; Jimmerson, A.; Porter, B.; Herr, K.; Nadkarni, P. Testing the Decision Support Tool for Responsible Pain Management for Headache and Facial Pain Diagnosis with Opioid-Risk-Stratified Treatment. SN Compr. Clin. Med. 2023, 5, 91. [Google Scholar] [CrossRef]
- Pohl, H.; Gantenbein, A.R.; Sandor, P.S.; Andrée, C. A Survey on Probable and Improbable Decisions About Headache Treatment. SN Compr. Clin. Med. 2020, 2, 2245–2252. [Google Scholar] [CrossRef]
- Han, X.; Wan, D.; Zhang, S.; Yin, Z.; Huang, S.; Xie, F.; Guo, J.; Qu, H.; Yao, Y.; Xu, H.; et al. Verification of a clinical decision support system for the diagnosis of headache disorders based on patient–computer interactions: A multi-center study. J. Headache Pain 2023, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Messina, R.; Christensen, R.H.; Cetta, I.; Ashina, M.; Filippi, M. Imaging the brain and vascular reactions to headache treatments: A systematic review. J. Headache Pain 2023, 24, 58. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schytz, H.W.; Hvedstrup, J. Evaluating Headache and Facial Pain in a Headache Diagnostic Laboratory: Experiences from the Danish Headache Center. Diagnostics 2023, 13, 2671. https://doi.org/10.3390/diagnostics13162671
Schytz HW, Hvedstrup J. Evaluating Headache and Facial Pain in a Headache Diagnostic Laboratory: Experiences from the Danish Headache Center. Diagnostics. 2023; 13(16):2671. https://doi.org/10.3390/diagnostics13162671
Chicago/Turabian StyleSchytz, Henrik Winter, and Jeppe Hvedstrup. 2023. "Evaluating Headache and Facial Pain in a Headache Diagnostic Laboratory: Experiences from the Danish Headache Center" Diagnostics 13, no. 16: 2671. https://doi.org/10.3390/diagnostics13162671
APA StyleSchytz, H. W., & Hvedstrup, J. (2023). Evaluating Headache and Facial Pain in a Headache Diagnostic Laboratory: Experiences from the Danish Headache Center. Diagnostics, 13(16), 2671. https://doi.org/10.3390/diagnostics13162671




